

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16318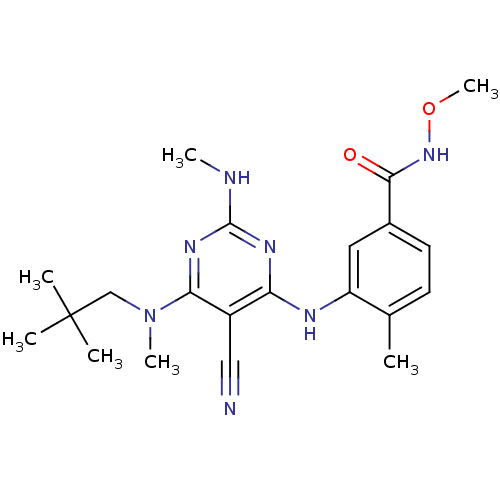 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16319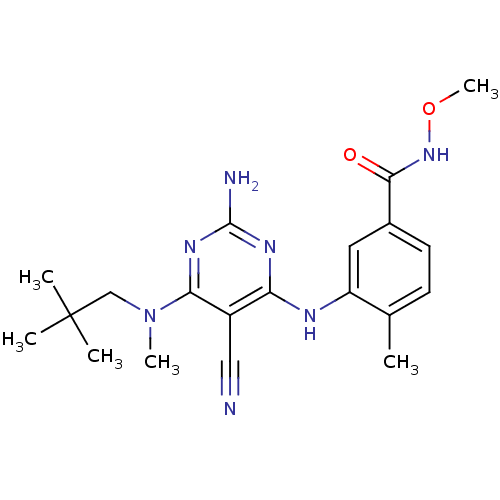 (3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16317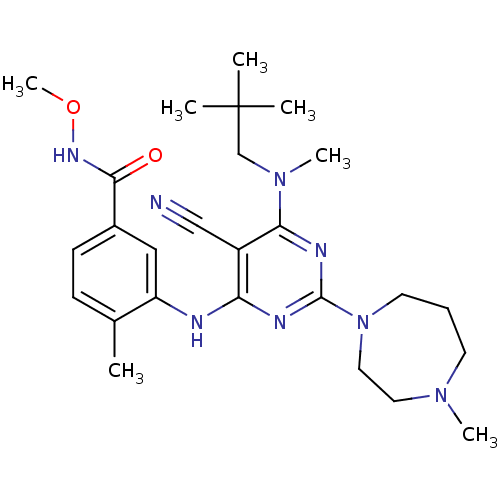 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16329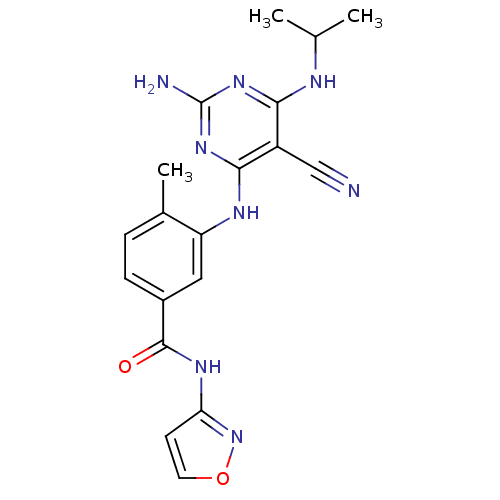 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16320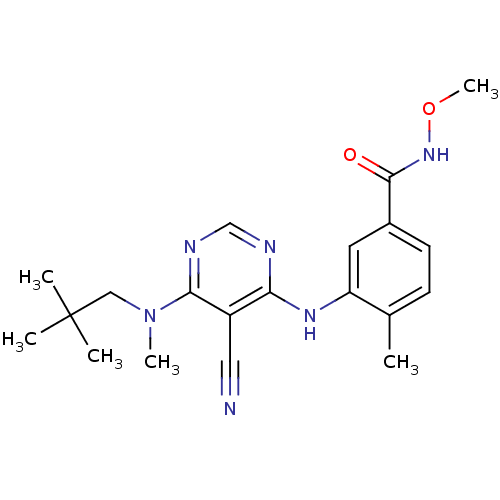 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16330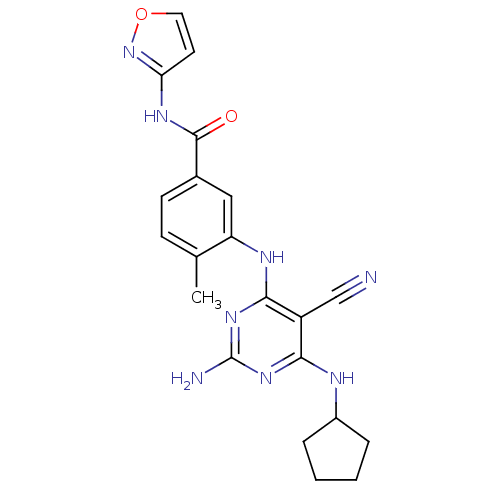 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16325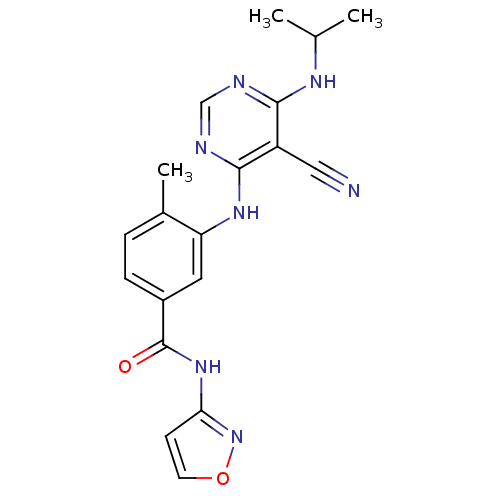 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16324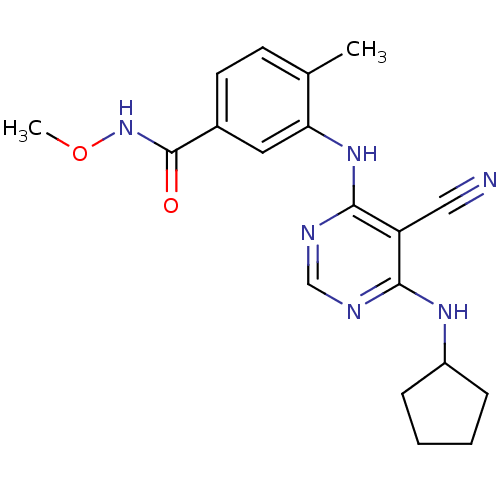 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16323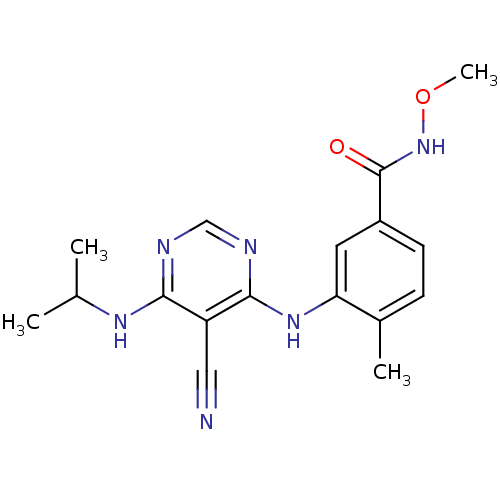 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16322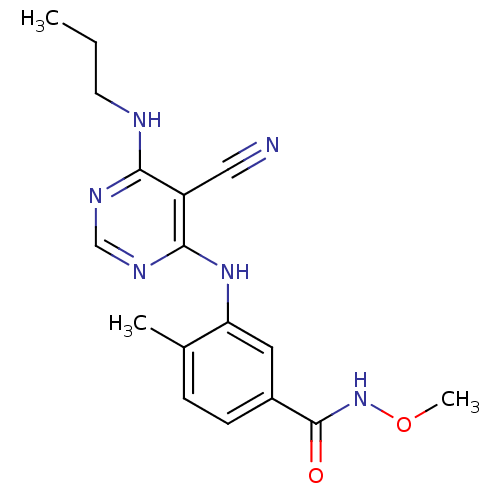 (3-{[5-cyano-6-(propylamino)pyrimidin-4-yl]amino}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.970 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16331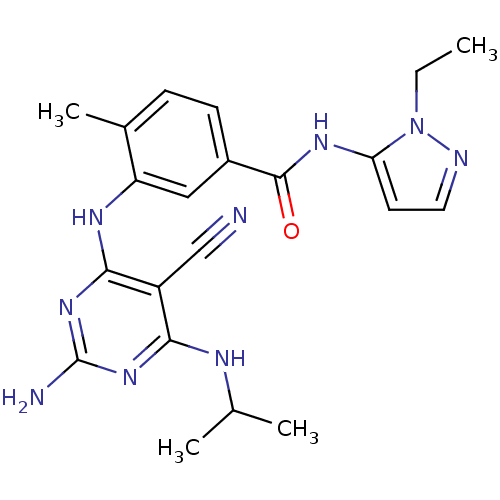 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16326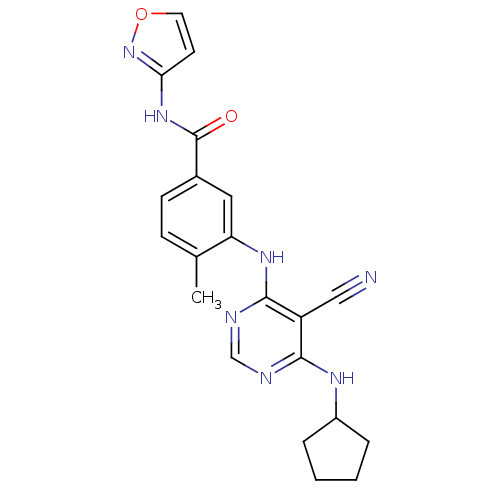 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16332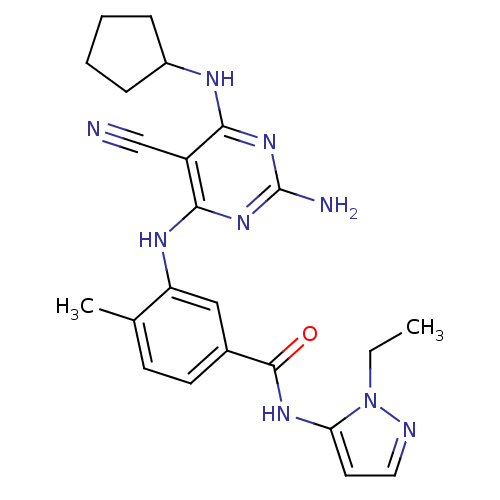 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16316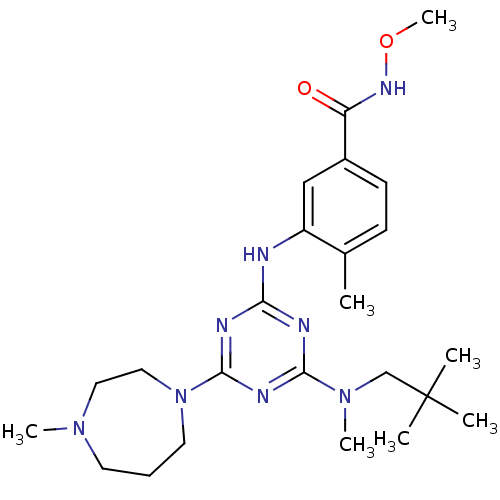 (3-({4-[(2,2-dimethylpropyl)(methyl)amino]-6-(4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16328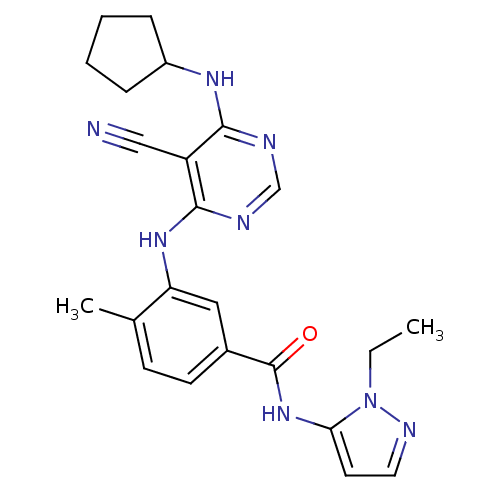 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16327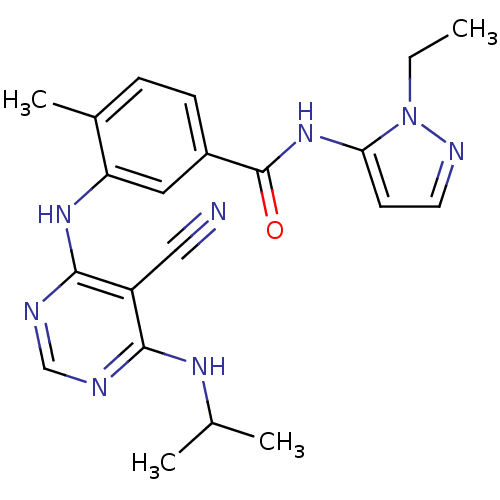 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16321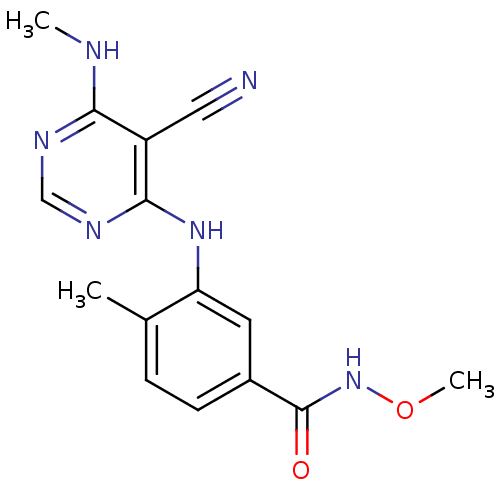 (3-{[5-cyano-6-(methylamino)pyrimidin-4-yl]amino}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311657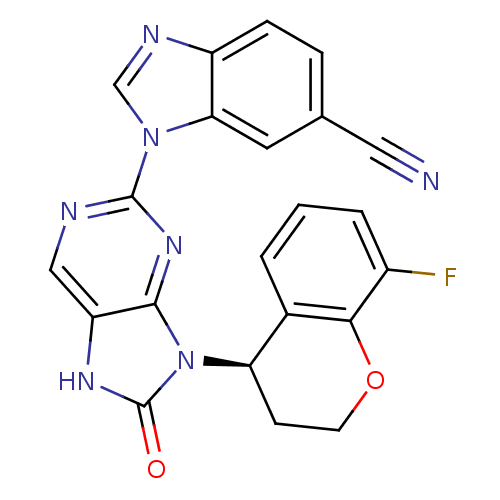 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454459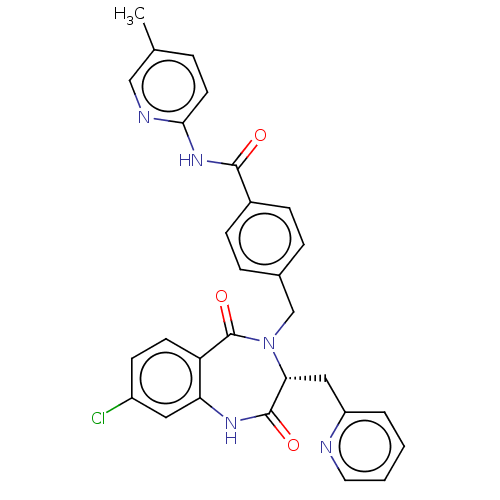 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498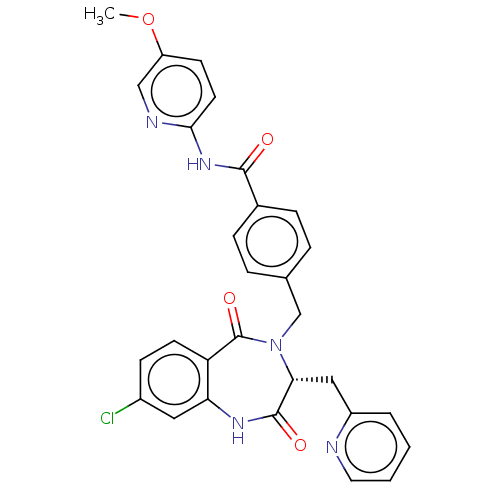 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311656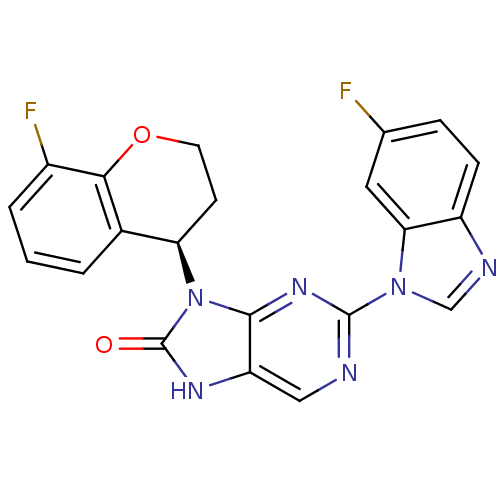 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454500 (CHEMBL4212258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311657 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311644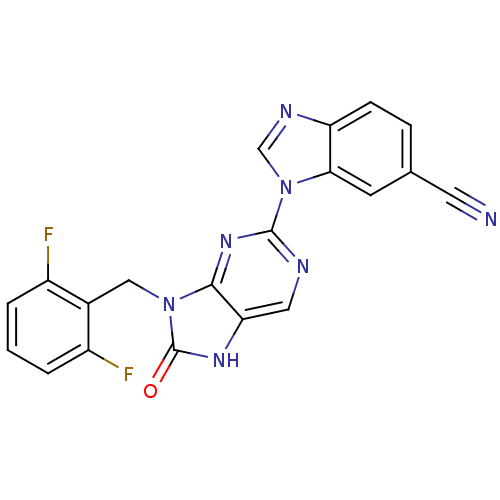 (1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311640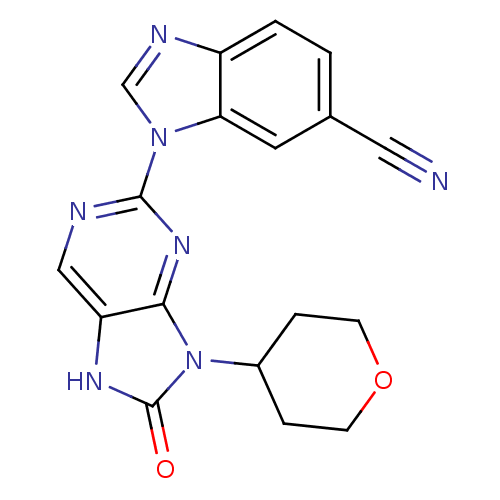 (1-(8-oxo-9-(tetrahydro-2H-pyran-4-yl)-8,9-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156754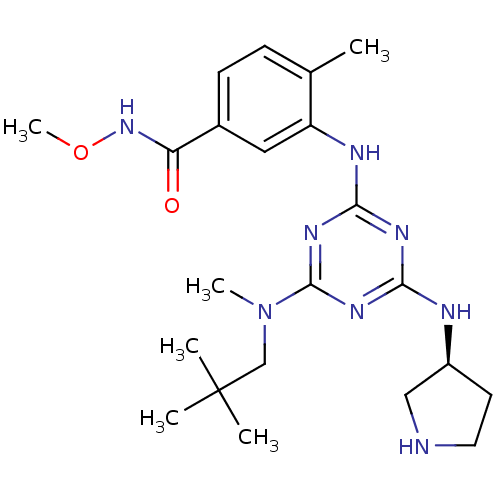 ((S)-N-methoxy-4-methyl-3-(4-(methyl(neopentyl)amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454486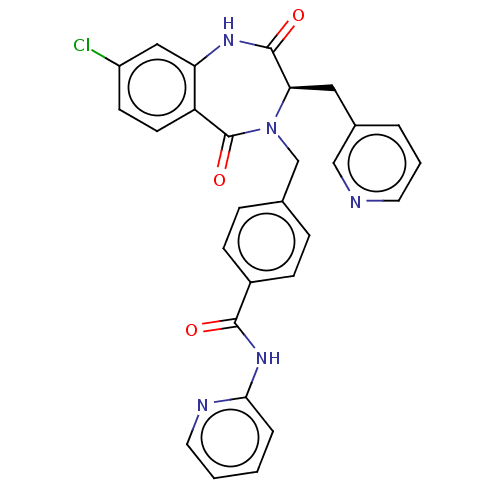 (CHEMBL4203819) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311639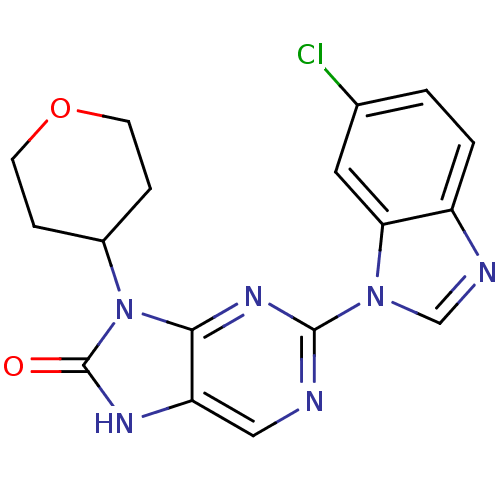 (2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(tetrahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311655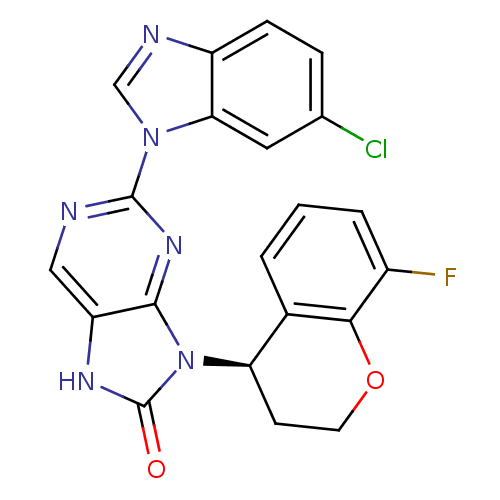 (2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156743 ((S)-N-hydroxy-4-methyl-3-(4-(methyl(neopentyl)amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311642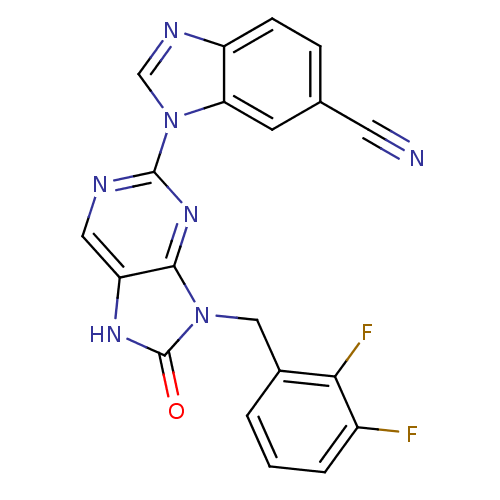 (1-(9-(2,3-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454504 (CHEMBL4210388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156750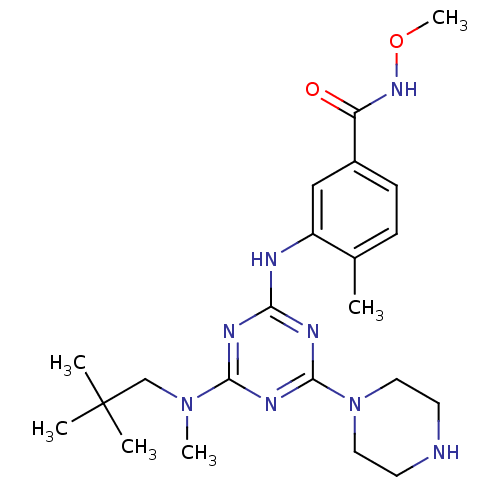 (CHEMBL376506 | DEL-A, 5 | N-methoxy-4-methyl-3-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM16316 (3-({4-[(2,2-dimethylpropyl)(methyl)amino]-6-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38beta | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156757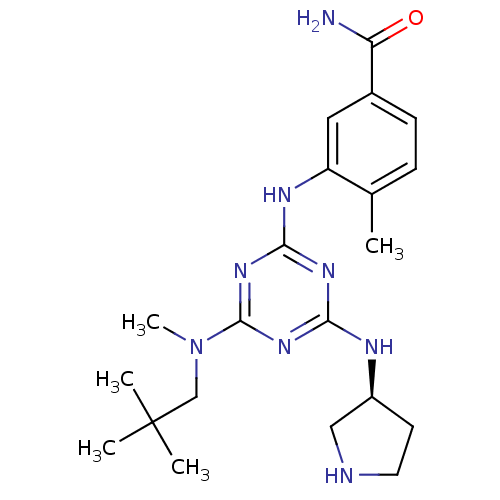 ((S)-4-methyl-3-(4-(methyl(neopentyl)amino)-6-(pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311656 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311644 (1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156741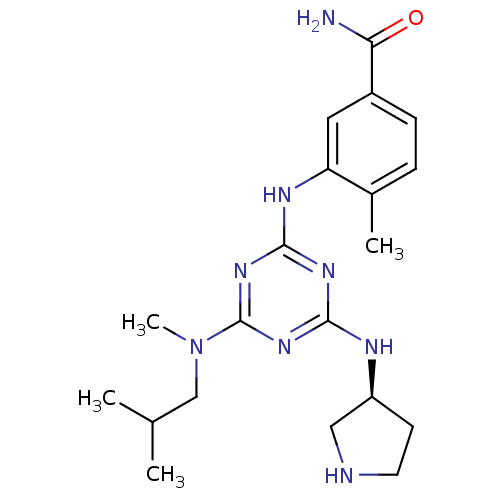 ((S)-3-(4-(isobutyl(methyl)amino)-6-(pyrrolidin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311648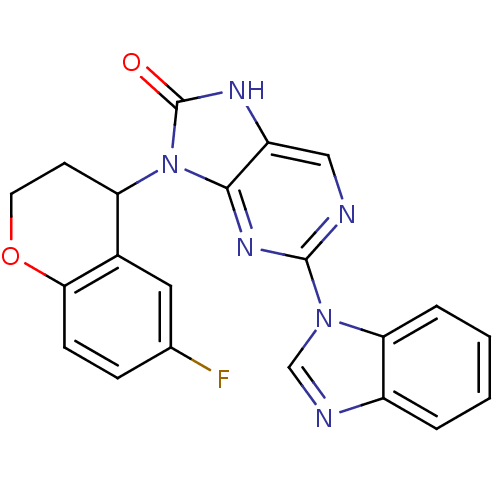 ((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(6-fluorochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311653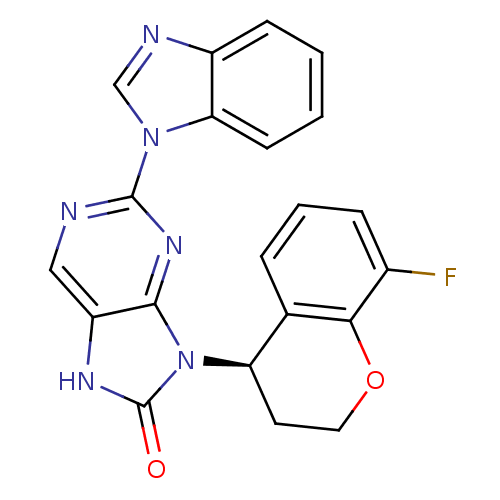 ((R)-2-(1H-benzo[d]imidazol-1-yl)-9-(8-fluorochroma...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311651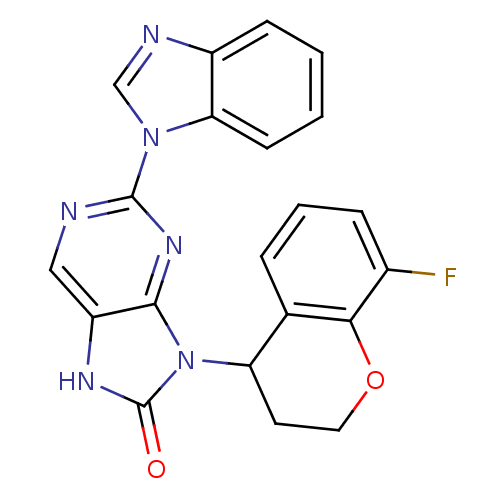 ((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(8-fluorochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156755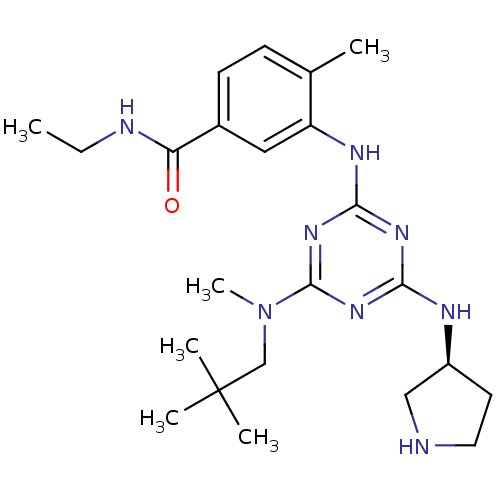 ((S)-N-ethyl-4-methyl-3-(4-(methyl(neopentyl)amino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50075780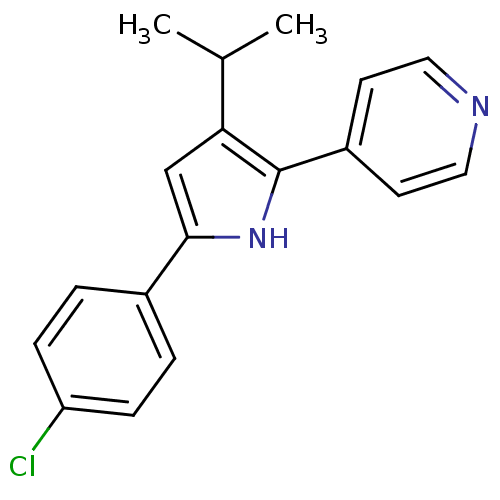 (4-[5-(4-Chloro-phenyl)-3-isopropyl-1H-pyrrol-2-yl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc. Curated by ChEMBL | Assay Description Antagonist activity against glucagon receptor | J Med Chem 48: 6980-90 (2005) Article DOI: 10.1021/jm050563r BindingDB Entry DOI: 10.7270/Q2RV0N72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311638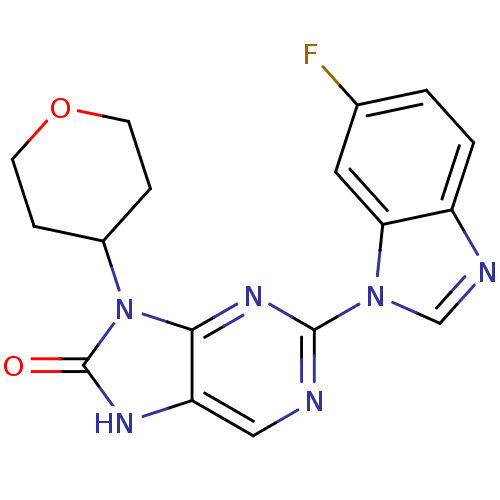 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(tetrahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454503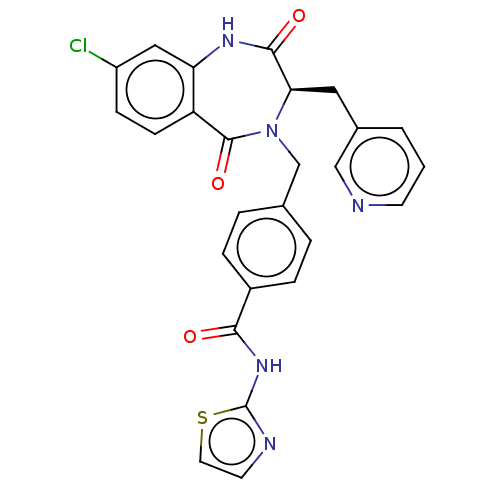 (CHEMBL4211420) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16316 (3-({4-[(2,2-dimethylpropyl)(methyl)amino]-6-(4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human p38-alpha expressed in Escherichia coli | J Med Chem 47: 6283-91 (2004) Article DOI: 10.1021/jm049521d BindingDB Entry DOI: 10.7270/Q2WS8SR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311647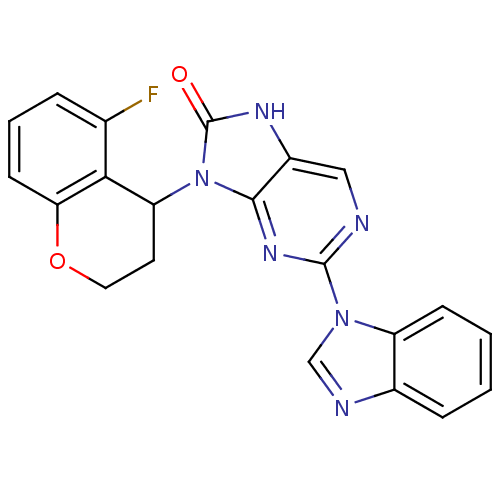 ((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(5-fluorochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Luciferin 4-monooxygenase (Photinus pyralis) | BDBM50478348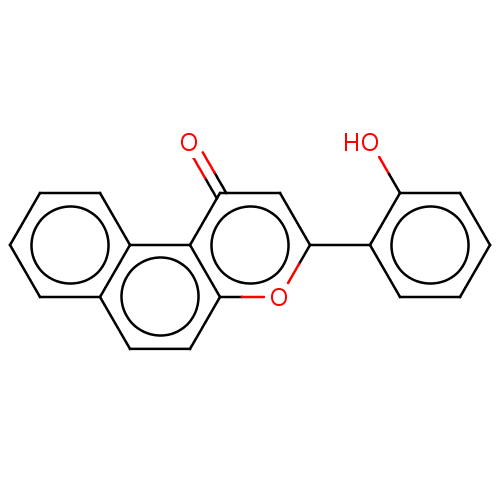 (CHEMBL406835) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Photinus pyralis luciferase | J Med Chem 51: 2372-86 (2008) Article DOI: 10.1021/jm701302v BindingDB Entry DOI: 10.7270/Q2F192HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 615 total ) | Next | Last >> |