

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine kinase (Human herpesvirus 2) | BDBM50101068 (4-Chloro-10,10-dioxo-9,10-dihydro-10lambda*6*-thio...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101051 (4,5-Dichloro-9H-xanthene-9-carboxylic acid [(2R,3R...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101069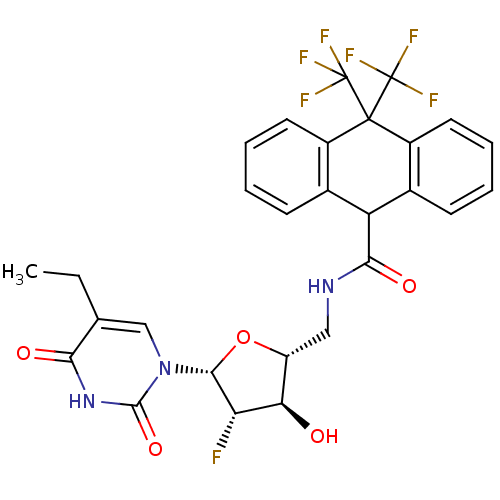 (10,10-Bis-trifluoromethyl-9,10-dihydro-anthracene-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101045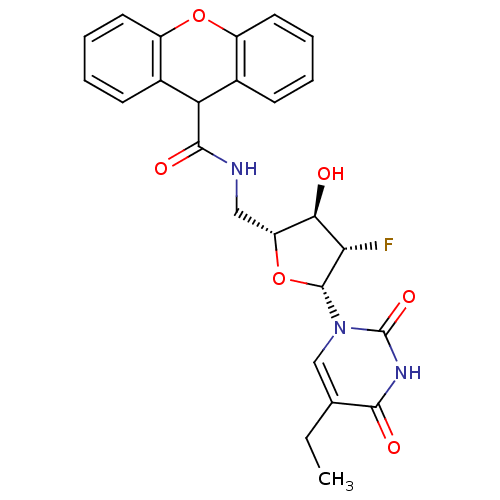 (9H-Xanthene-9-carboxylic acid [(2R,3R,4S,5R)-5-(5-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101050 (10,10-Dioxo-9,10-dihydro-10lambda*6*-thioxanthene-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101071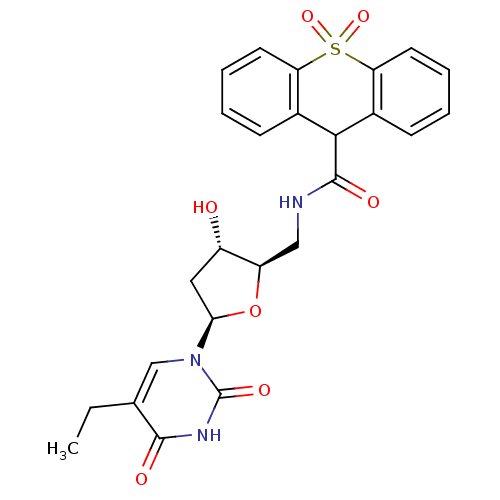 (10,10-Dioxo-9,10-dihydro-10lambda*6*-thioxanthene-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101053 (9H-Xanthene-9-carboxylic acid [(2R,3S,5R)-5-(5-eth...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101048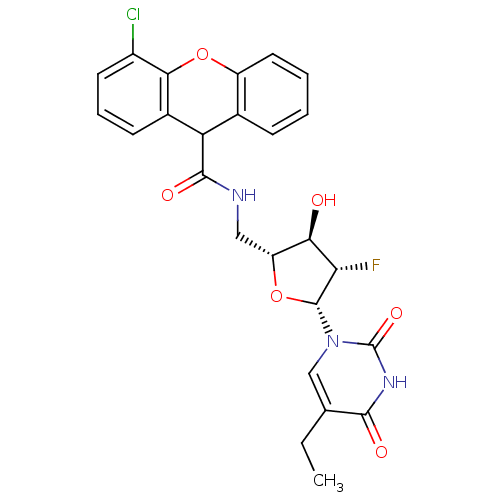 (4-Chloro-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101058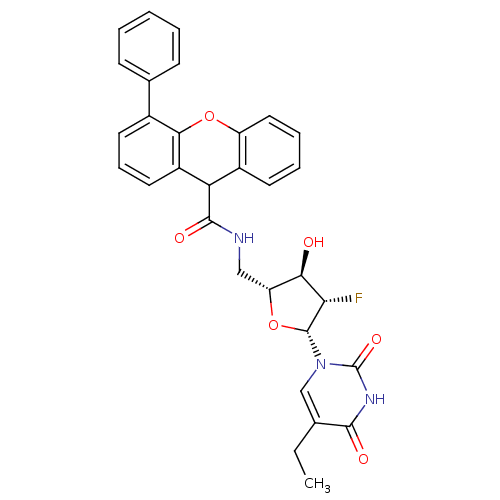 (4-Phenyl-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101060 (4-Trifluoromethyl-9H-xanthene-9-carboxylic acid [(...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101047 (4-Methyl-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101069 (10,10-Bis-trifluoromethyl-9,10-dihydro-anthracene-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101067 ((S)-2-(2,4-Dichloro-5-methoxy-phenoxy)-N-[(2R,3R,4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101062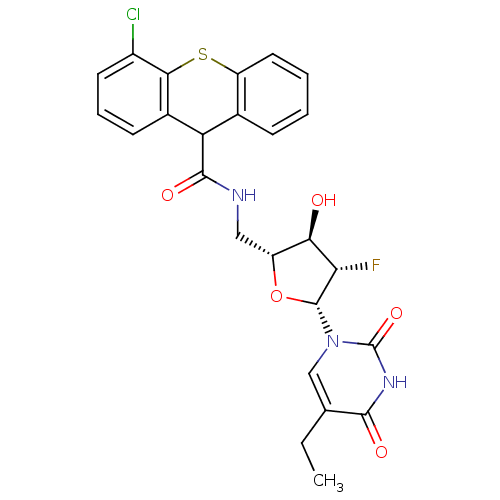 (4-Chloro-9H-thioxanthene-9-carboxylic acid [(2R,3R...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101074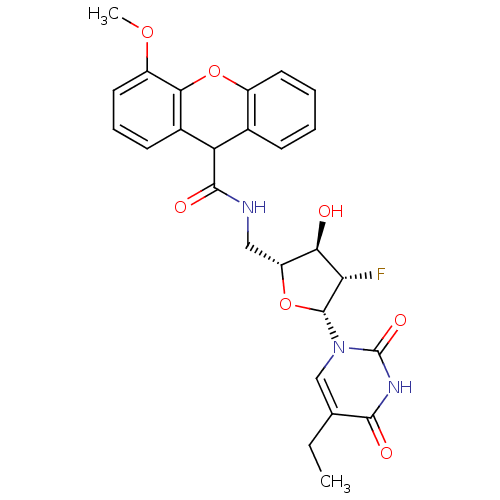 (4-Methoxy-9H-xanthene-9-carboxylic acid [(2R,3R,4S...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101068 (4-Chloro-10,10-dioxo-9,10-dihydro-10lambda*6*-thio...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101049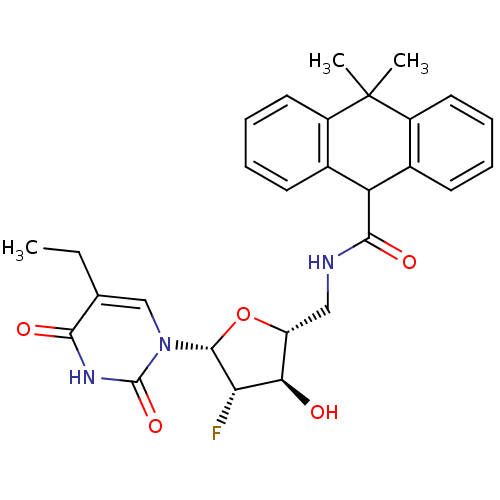 (10,10-Dimethyl-9,10-dihydro-anthracene-9-carboxyli...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101051 (4,5-Dichloro-9H-xanthene-9-carboxylic acid [(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101073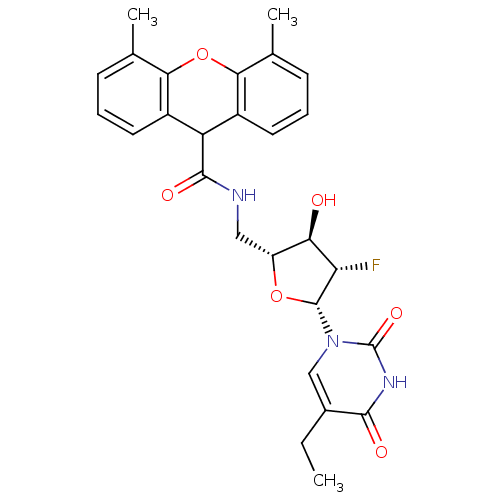 (4,5-Dimethyl-9H-xanthene-9-carboxylic acid [(2R,3R...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364171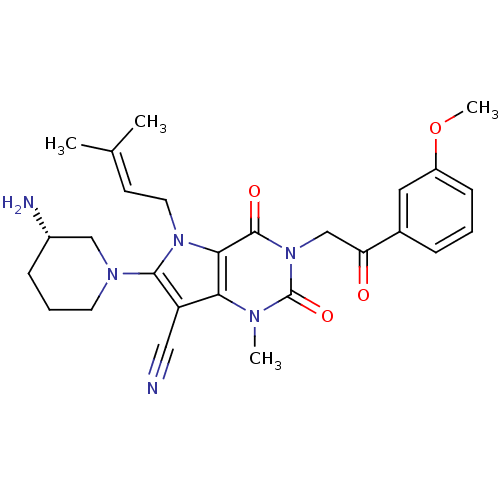 (CHEMBL1951598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364156 (CHEMBL1951432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101058 (4-Phenyl-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101043 (9H-Thioxanthene-9-carboxylic acid [(2R,3R,4S,5R)-5...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101048 (4-Chloro-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101050 (10,10-Dioxo-9,10-dihydro-10lambda*6*-thioxanthene-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101071 (10,10-Dioxo-9,10-dihydro-10lambda*6*-thioxanthene-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101072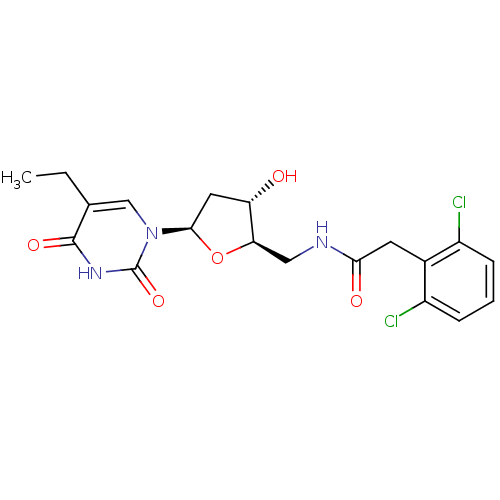 (2-(2,6-Dichloro-phenyl)-N-[(2R,3S,5R)-5-(5-ethyl-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364184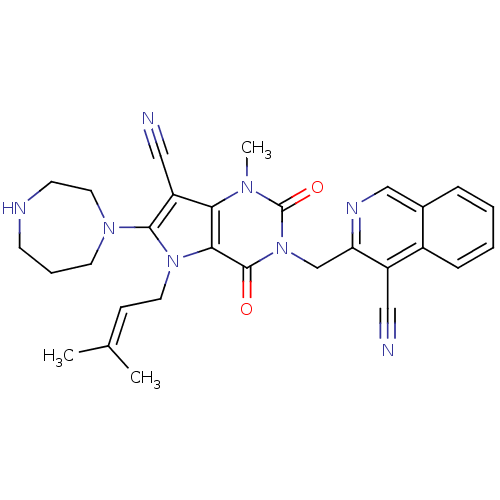 (CHEMBL1951611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364173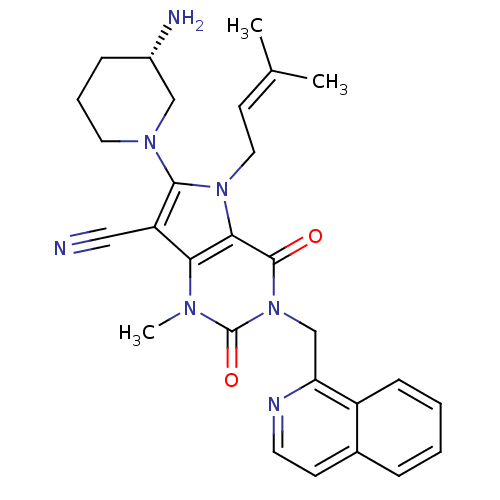 (CHEMBL1951599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364158 (CHEMBL1951430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101060 (4-Trifluoromethyl-9H-xanthene-9-carboxylic acid [(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364187 (CHEMBL1951416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101044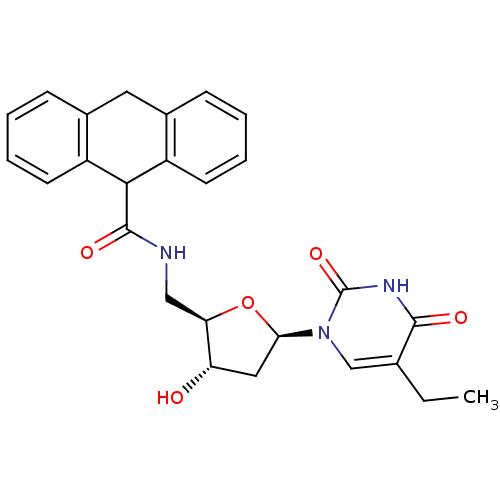 (9,10-Dihydro-anthracene-9-carboxylic acid [(2R,3S,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101064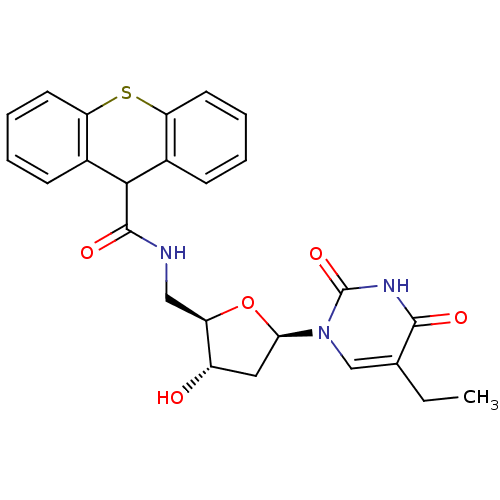 (9H-Thioxanthene-9-carboxylic acid [(2R,3S,5R)-5-(5...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101049 (10,10-Dimethyl-9,10-dihydro-anthracene-9-carboxyli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364147 (CHEMBL1951595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101062 (4-Chloro-9H-thioxanthene-9-carboxylic acid [(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101047 (4-Methyl-9H-xanthene-9-carboxylic acid [(2R,3R,4S,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101074 (4-Methoxy-9H-xanthene-9-carboxylic acid [(2R,3R,4S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101067 ((S)-2-(2,4-Dichloro-5-methoxy-phenoxy)-N-[(2R,3R,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364183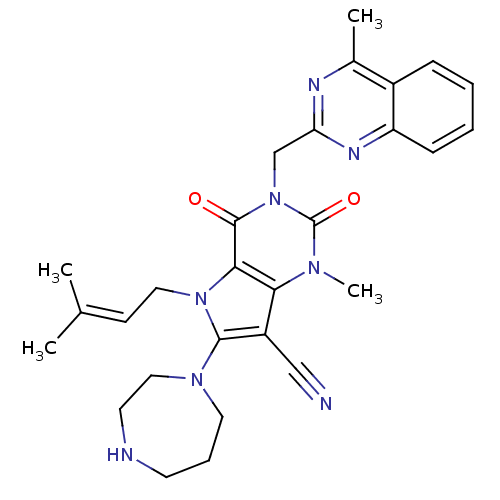 (CHEMBL1951609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364179 (CHEMBL1951607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364177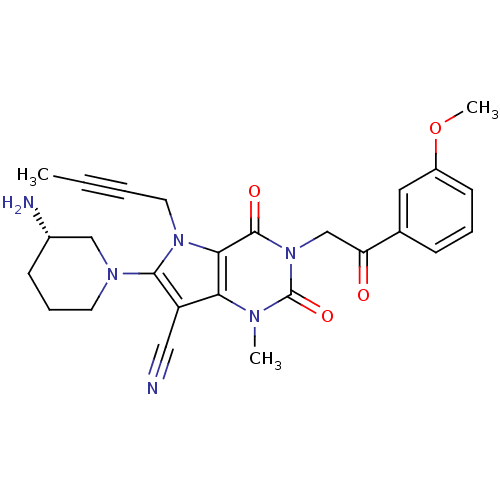 (CHEMBL1951603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364167 (CHEMBL1951600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364186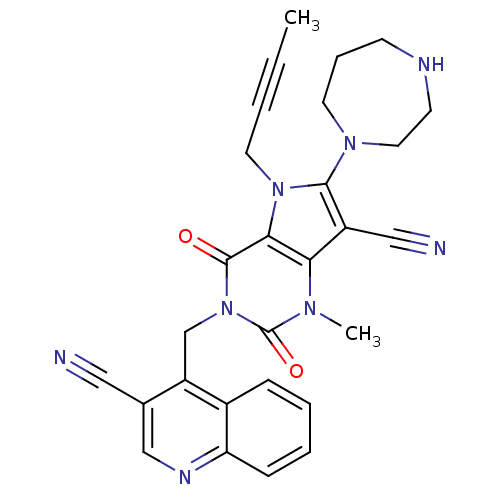 (CHEMBL1951614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101045 (9H-Xanthene-9-carboxylic acid [(2R,3R,4S,5R)-5-(5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101073 (4,5-Dimethyl-9H-xanthene-9-carboxylic acid [(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 2) | BDBM50101046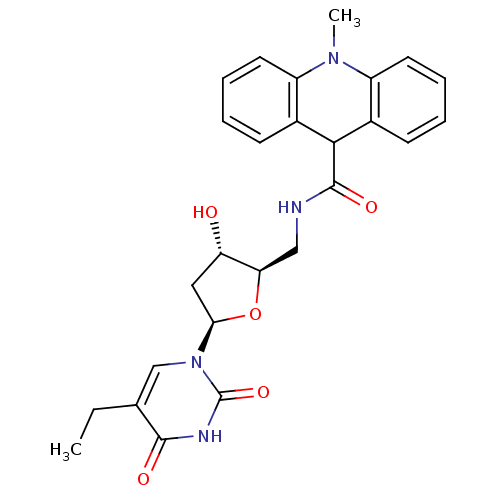 (10-Methyl-9,10-dihydro-acridine-9-carboxylic acid ...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-2 Thymidine Kinase (HSV-2 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50101053 (9H-Xanthene-9-carboxylic acid [(2R,3S,5R)-5-(5-eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine Kinase (HSV-1 TK) | Bioorg Med Chem Lett 11: 1655-8 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 186 total ) | Next | Last >> |