 Found 465 hits with Last Name = 'eaves' and Initial = 'r'
Found 465 hits with Last Name = 'eaves' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440788
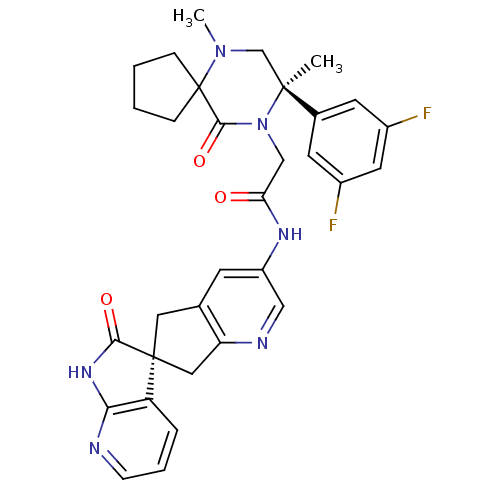
(CHEMBL2431249)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-30(20-11-21(33)13-22(34)12-20)18-39(2)32(7-3-4-8-32)29(43)40(30)17-26(41)37-23-10-19-14-31(15-25(19)36-16-23)24-6-5-9-35-27(24)38-28(31)42/h5-6,9-13,16H,3-4,7-8,14-15,17-18H2,1-2H3,(H,37,41)(H,35,38,42)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440782
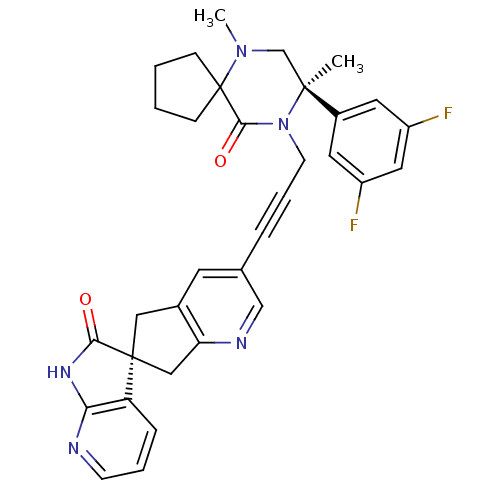
(CHEMBL2431246)Show SMILES CN1C[C@](C)(N(CC#Cc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H31F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440784
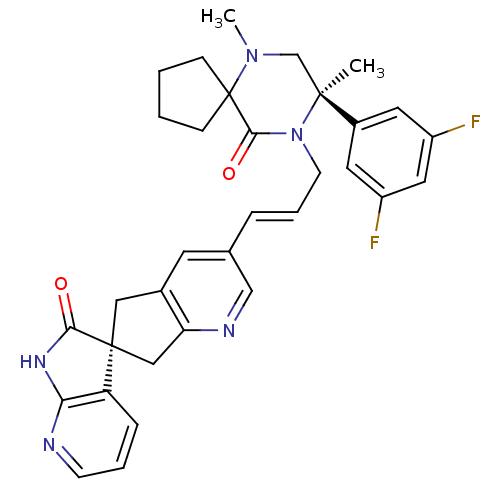
(CHEMBL2431253)Show SMILES CN1C[C@](C)(N(C\C=C\c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5-8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/b7-6+/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
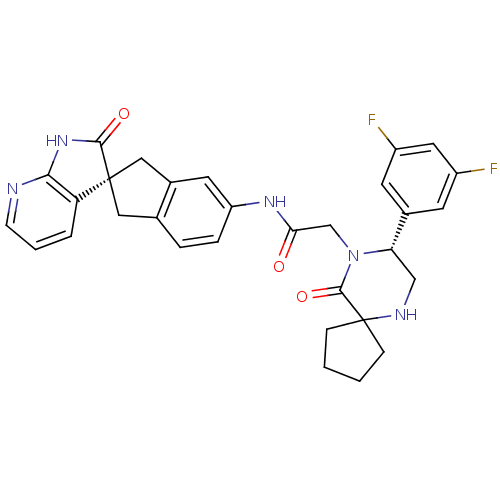
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440791
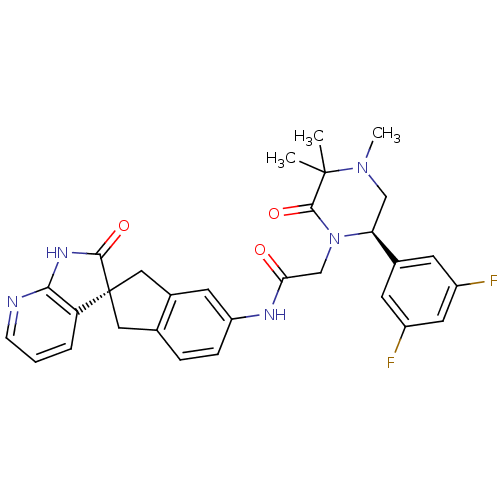
(CHEMBL2431256)Show SMILES CN1C[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-29(2)28(40)37(24(15-36(29)3)18-9-20(31)12-21(32)10-18)16-25(38)34-22-7-6-17-13-30(14-19(17)11-22)23-5-4-8-33-26(23)35-27(30)39/h4-12,24H,13-16H2,1-3H3,(H,34,38)(H,33,35,39)/t24-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440793
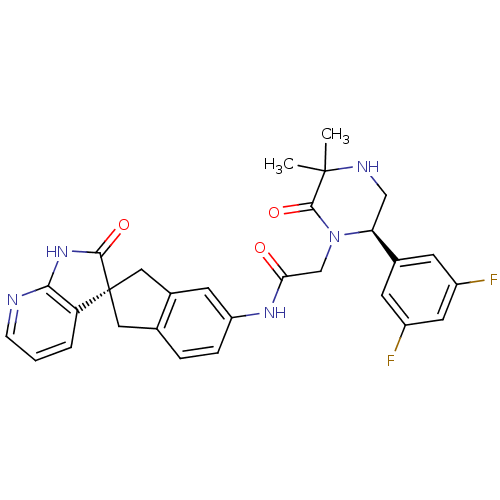
(CHEMBL2431254)Show SMILES CC1(C)NC[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H27F2N5O3/c1-28(2)27(39)36(23(14-33-28)17-8-19(30)11-20(31)9-17)15-24(37)34-21-6-5-16-12-29(13-18(16)10-21)22-4-3-7-32-25(22)35-26(29)38/h3-11,23,33H,12-15H2,1-2H3,(H,34,37)(H,32,35,38)/t23-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385314
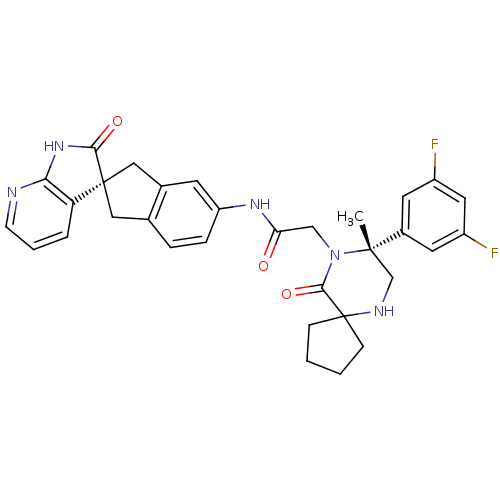
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440786
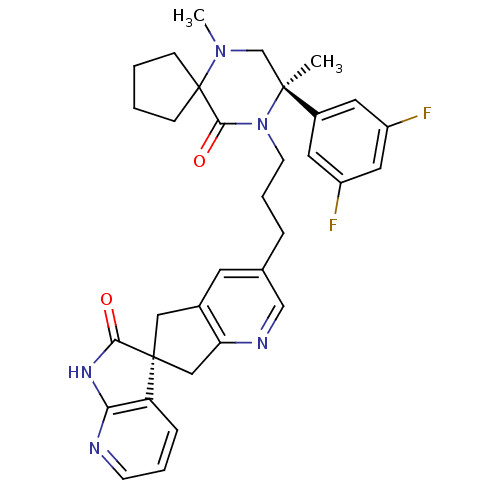
(CHEMBL2431251)Show SMILES CN1C[C@](C)(N(CCCc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H35F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,6-7,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440790
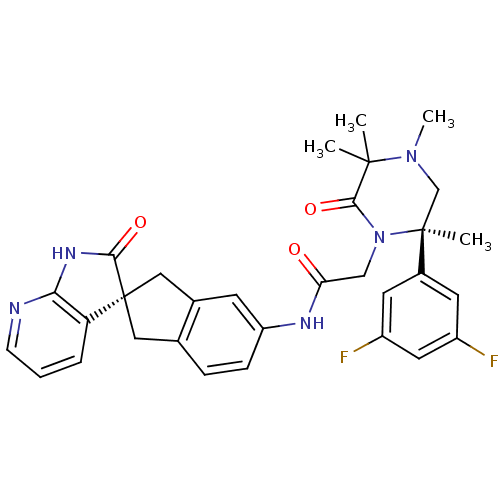
(CHEMBL2431247)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H31F2N5O3/c1-29(2)28(41)38(30(3,17-37(29)4)20-11-21(32)13-22(33)12-20)16-25(39)35-23-8-7-18-14-31(15-19(18)10-23)24-6-5-9-34-26(24)36-27(31)40/h5-13H,14-17H2,1-4H3,(H,35,39)(H,34,36,40)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440785
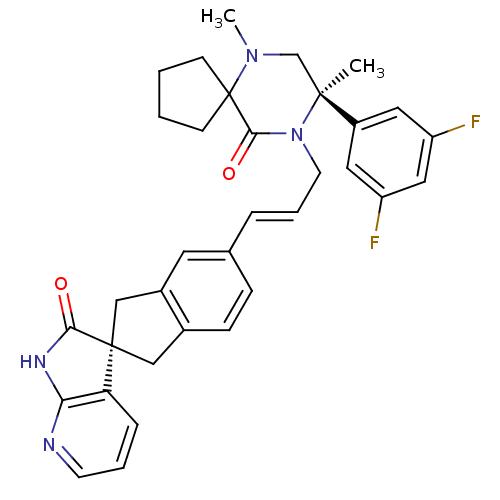
(CHEMBL2431252)Show SMILES CN1C[C@](C)(N(C\C=C\c2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H34F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/b7-6+/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50040671
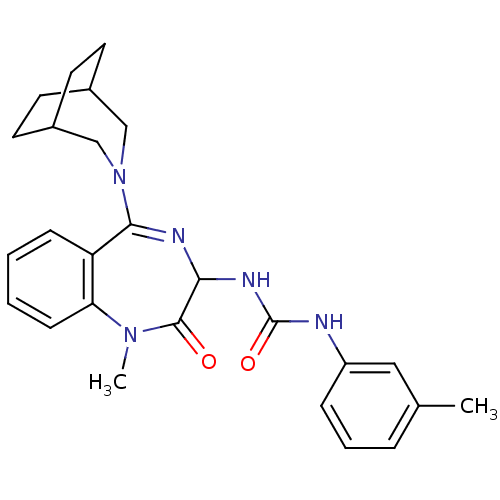
(1-[5-(3-Aza-bicyclo[3.2.2]non-3-yl)-1-methyl-2-oxo...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)N1CC2CCC(CC2)C1 |c:9,(7.22,-6.97,;7.67,-8.45,;6.69,-9.39,;5.36,-8.63,;4.02,-9.39,;4.02,-10.93,;5.36,-11.69,;6.71,-10.96,;7.63,-11.85,;9.14,-11.6,;9.86,-10.19,;11.4,-10.21,;12.13,-11.53,;11.35,-12.86,;13.67,-11.57,;14.48,-10.26,;13.72,-8.91,;14.5,-7.6,;16.04,-7.62,;16.81,-8.95,;18.33,-8.98,;16.01,-10.29,;9.18,-8.72,;10.1,-7.49,;7.17,-13.34,;5.55,-13.5,;4.73,-14.54,;5.14,-16.05,;6.65,-16.64,;8.09,-15.8,;7.22,-14.74,;6.1,-14.2,;8.22,-14.23,)| Show InChI InChI=1S/C26H31N5O2/c1-17-6-5-7-20(14-17)27-26(33)29-23-25(32)30(2)22-9-4-3-8-21(22)24(28-23)31-15-18-10-11-19(16-31)13-12-18/h3-9,14,18-19,23H,10-13,15-16H2,1-2H3,(H2,27,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440783
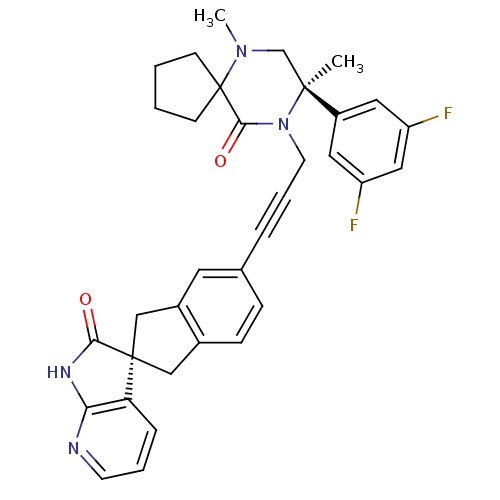
(CHEMBL2429882)Show SMILES CN1C[C@](C)(N(CC#Cc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H32F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440792

(CHEMBL2431255)Show SMILES CC1(C)NC[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-28(2)27(40)37(29(3,16-34-28)19-10-20(31)12-21(32)11-19)15-24(38)35-22-7-6-17-13-30(14-18(17)9-22)23-5-4-8-33-25(23)36-26(30)39/h4-12,34H,13-16H2,1-3H3,(H,35,38)(H,33,36,39)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440787
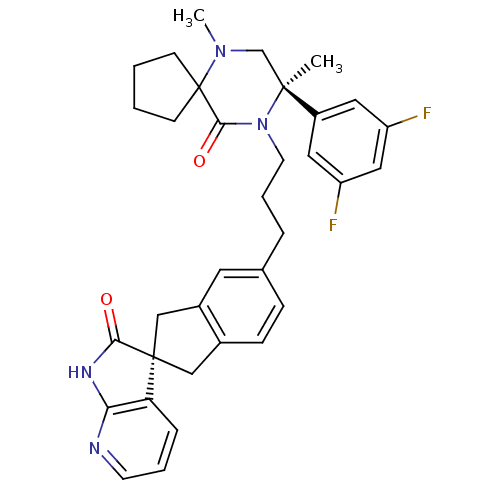
(CHEMBL2431250)Show SMILES CN1C[C@](C)(N(CCCc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H36F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,6-7,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224431
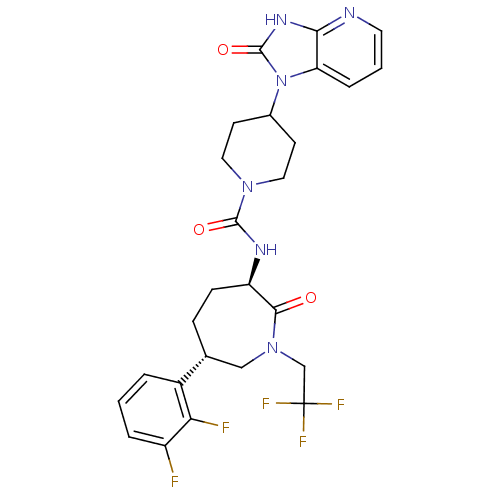
(CHEMBL236593 | MK-0974 | N-[(3R,6S)-6-(2,3-difluor...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CC(F)(F)F)C2)c1F Show InChI InChI=1S/C26H27F5N6O3/c27-18-4-1-3-17(21(18)28)15-6-7-19(23(38)36(13-15)14-26(29,30)31)33-24(39)35-11-8-16(9-12-35)37-20-5-2-10-32-22(20)34-25(37)40/h1-5,10,15-16,19H,6-9,11-14H2,(H,33,39)(H,32,34,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholecystokinin
(GUINEA PIG) | BDBM82514
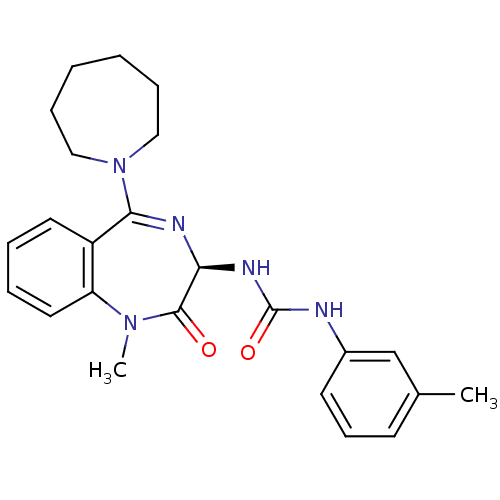
(1-[[(3R)-2,3-Dihydro-1-methyl-2-oxo-5-[(hexahydro-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)N1CCCCCC1 |c:9| Show InChI InChI=1S/C24H29N5O2/c1-17-10-9-11-18(16-17)25-24(31)27-21-23(30)28(2)20-13-6-5-12-19(20)22(26-21)29-14-7-3-4-8-15-29/h5-6,9-13,16,21H,3-4,7-8,14-15H2,1-2H3,(H2,25,27,31)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK3 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK2 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50061220
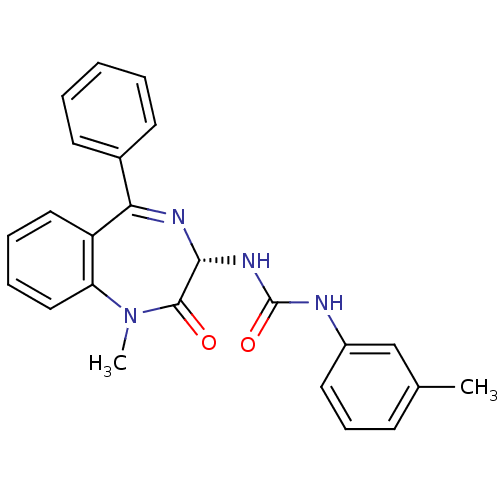
(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040934
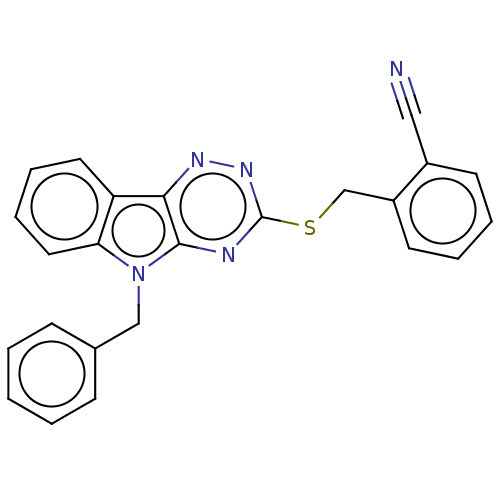
(CHEMBL3353833)Show SMILES N#Cc1ccccc1CSc1nnc2c(n1)n(Cc1ccccc1)c1ccccc21 Show InChI InChI=1S/C24H17N5S/c25-14-18-10-4-5-11-19(18)16-30-24-26-23-22(27-28-24)20-12-6-7-13-21(20)29(23)15-17-8-2-1-3-9-17/h1-13H,15-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040901

(CHEMBL3353822)Show InChI InChI=1S/C17H10BrN5S/c18-12-6-3-7-13-14(12)15-16(20-13)21-17(23-22-15)24-9-11-5-2-1-4-10(11)8-19/h1-7H,9H2,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040944
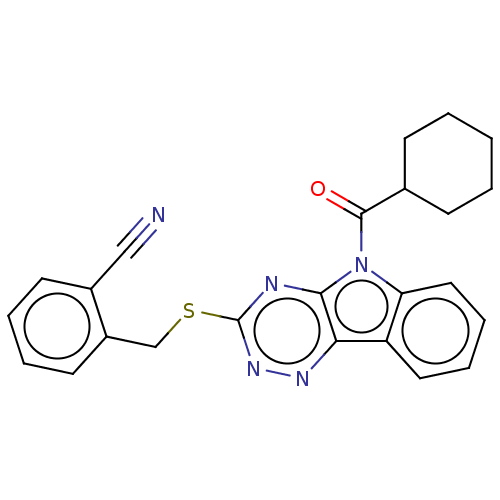
(CHEMBL3354395)Show SMILES O=C(C1CCCCC1)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C24H21N5OS/c25-14-17-10-4-5-11-18(17)15-31-24-26-22-21(27-28-24)19-12-6-7-13-20(19)29(22)23(30)16-8-2-1-3-9-16/h4-7,10-13,16H,1-3,8-9,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040934

(CHEMBL3353833)Show SMILES N#Cc1ccccc1CSc1nnc2c(n1)n(Cc1ccccc1)c1ccccc21 Show InChI InChI=1S/C24H17N5S/c25-14-18-10-4-5-11-19(18)16-30-24-26-23-22(27-28-24)20-12-6-7-13-21(20)29(23)15-17-8-2-1-3-9-17/h1-13H,15-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2C
(RAT-Rattus norvegicus (Rat)) | BDBM50419775
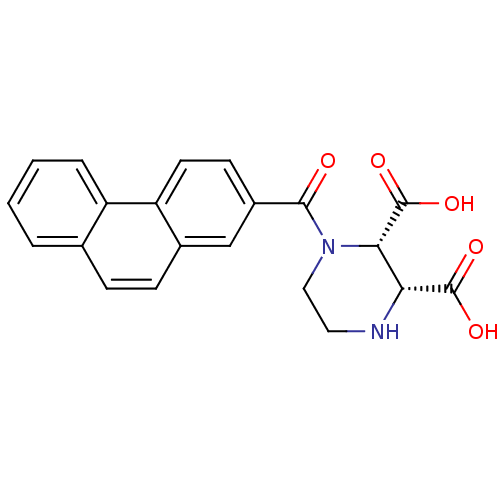
(CHEMBL1950808)Show SMILES OC(=O)[C@@H]1NCCN([C@@H]1C(O)=O)C(=O)c1ccc2c(ccc3ccccc23)c1 |r| Show InChI InChI=1S/C21H18N2O5/c24-19(23-10-9-22-17(20(25)26)18(23)21(27)28)14-7-8-16-13(11-14)6-5-12-3-1-2-4-15(12)16/h1-8,11,17-18,22H,9-10H2,(H,25,26)(H,27,28)/t17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2C receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2C
(RAT-Rattus norvegicus (Rat)) | BDBM50164549
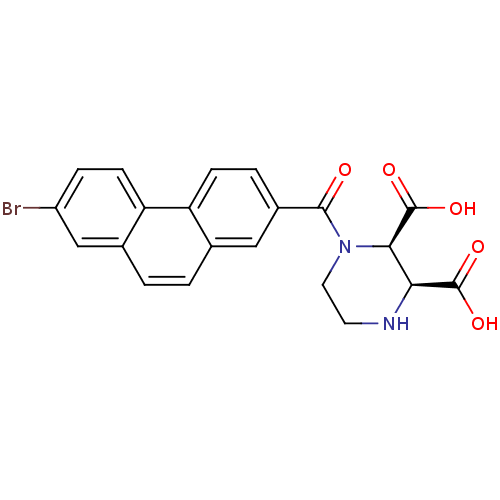
((2R,3S)-1-(7-Bromo-phenanthrene-2-carbonyl)-pipera...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc2c(ccc3cc(Br)ccc23)c1 Show InChI InChI=1S/C21H17BrN2O5/c22-14-4-6-16-12(10-14)2-1-11-9-13(3-5-15(11)16)19(25)24-8-7-23-17(20(26)27)18(24)21(28)29/h1-6,9-10,17-18,23H,7-8H2,(H,26,27)(H,28,29)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2C receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040891

(CHEMBL3353812)Show SMILES [O-][N+](=O)c1ccccc1CSc1nnc2c(n1)[nH]c1ccccc21 Show InChI InChI=1S/C16H11N5O2S/c22-21(23)13-8-4-1-5-10(13)9-24-16-18-15-14(19-20-16)11-6-2-3-7-12(11)17-15/h1-8H,9H2,(H,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2A
(Rattus norvegicus (Rat)-RAT) | BDBM50419775

(CHEMBL1950808)Show SMILES OC(=O)[C@@H]1NCCN([C@@H]1C(O)=O)C(=O)c1ccc2c(ccc3ccccc23)c1 |r| Show InChI InChI=1S/C21H18N2O5/c24-19(23-10-9-22-17(20(25)26)18(23)21(27)28)14-7-8-16-13(11-14)6-5-12-3-1-2-4-15(12)16/h1-8,11,17-18,22H,9-10H2,(H,25,26)(H,27,28)/t17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity at rat recombinant GluN1/GluN2A receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040935
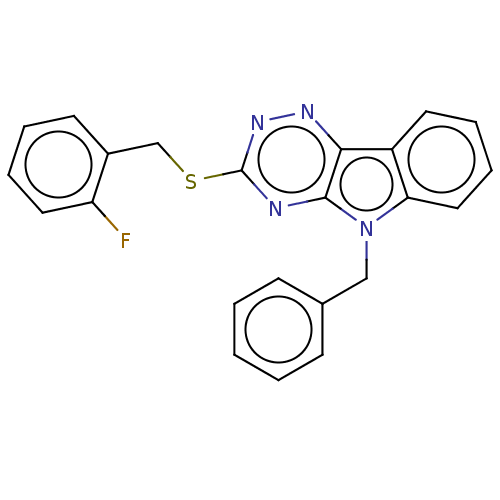
(CHEMBL3353834)Show InChI InChI=1S/C23H17FN4S/c24-19-12-6-4-10-17(19)15-29-23-25-22-21(26-27-23)18-11-5-7-13-20(18)28(22)14-16-8-2-1-3-9-16/h1-13H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50419775

(CHEMBL1950808)Show SMILES OC(=O)[C@@H]1NCCN([C@@H]1C(O)=O)C(=O)c1ccc2c(ccc3ccccc23)c1 |r| Show InChI InChI=1S/C21H18N2O5/c24-19(23-10-9-22-17(20(25)26)18(23)21(27)28)14-7-8-16-13(11-14)6-5-12-3-1-2-4-15(12)16/h1-8,11,17-18,22H,9-10H2,(H,25,26)(H,27,28)/t17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040948
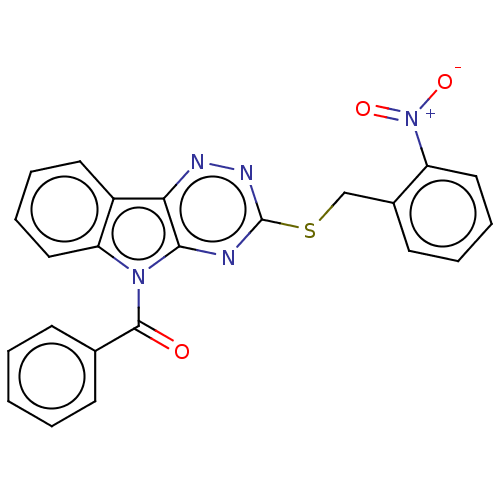
(CHEMBL3354399)Show SMILES [O-][N+](=O)c1ccccc1CSc1nnc2c(n1)n(C(=O)c1ccccc1)c1ccccc21 Show InChI InChI=1S/C23H15N5O3S/c29-22(15-8-2-1-3-9-15)27-19-13-7-5-11-17(19)20-21(27)24-23(26-25-20)32-14-16-10-4-6-12-18(16)28(30)31/h1-13H,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50164549

((2R,3S)-1-(7-Bromo-phenanthrene-2-carbonyl)-pipera...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc2c(ccc3cc(Br)ccc23)c1 Show InChI InChI=1S/C21H17BrN2O5/c22-14-4-6-16-12(10-14)2-1-11-9-13(3-5-15(11)16)19(25)24-8-7-23-17(20(26)27)18(24)21(28)29/h1-6,9-10,17-18,23H,7-8H2,(H,26,27)(H,28,29)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50040935

(CHEMBL3353834)Show InChI InChI=1S/C23H17FN4S/c24-19-12-6-4-10-17(19)15-29-23-25-22-21(26-27-23)18-11-5-7-13-20(18)28(22)14-16-8-2-1-3-9-16/h1-13H,14-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2C
(RAT-Rattus norvegicus (Rat)) | BDBM50164555
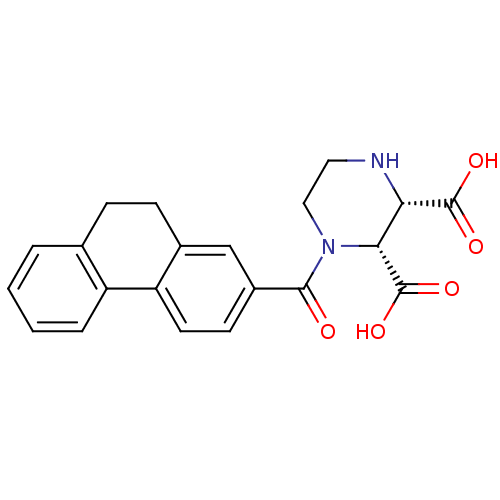
((2R,3S)-1-(9,10-Dihydro-phenanthrene-2-carbonyl)-p...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc-2c(CCc3ccccc-23)c1 Show InChI InChI=1S/C21H20N2O5/c24-19(23-10-9-22-17(20(25)26)18(23)21(27)28)14-7-8-16-13(11-14)6-5-12-3-1-2-4-15(12)16/h1-4,7-8,11,17-18,22H,5-6,9-10H2,(H,25,26)(H,27,28)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2C receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040936

(CHEMBL3353835)Show SMILES [O-][N+](=O)c1ccccc1Cn1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C24H16N6O2S/c25-13-16-7-1-2-9-18(16)15-33-24-26-23-22(27-28-24)19-10-4-6-12-21(19)29(23)14-17-8-3-5-11-20(17)30(31)32/h1-12H,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040947
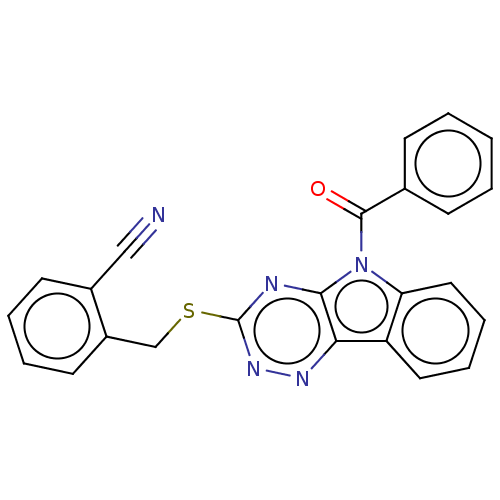
(CHEMBL3354398)Show SMILES O=C(c1ccccc1)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C24H15N5OS/c25-14-17-10-4-5-11-18(17)15-31-24-26-22-21(27-28-24)19-12-6-7-13-20(19)29(22)23(30)16-8-2-1-3-9-16/h1-13H,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2A
(Rattus norvegicus (Rat)-RAT) | BDBM50164549

((2R,3S)-1-(7-Bromo-phenanthrene-2-carbonyl)-pipera...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc2c(ccc3cc(Br)ccc23)c1 Show InChI InChI=1S/C21H17BrN2O5/c22-14-4-6-16-12(10-14)2-1-11-9-13(3-5-15(11)16)19(25)24-8-7-23-17(20(26)27)18(24)21(28)29/h1-6,9-10,17-18,23H,7-8H2,(H,26,27)(H,28,29)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity at rat recombinant GluN1/GluN2A receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040943
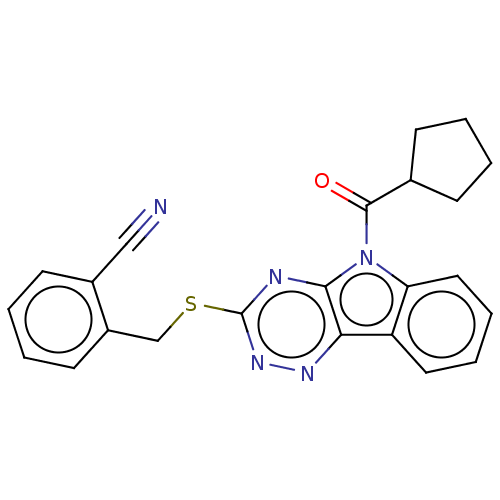
(CHEMBL3353842)Show SMILES O=C(C1CCCC1)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C23H19N5OS/c24-13-16-9-3-4-10-17(16)14-30-23-25-21-20(26-27-23)18-11-5-6-12-19(18)28(21)22(29)15-7-1-2-8-15/h3-6,9-12,15H,1-2,7-8,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040880
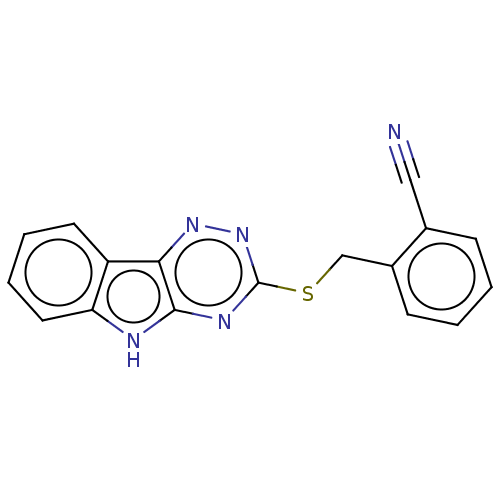
(CHEMBL3353802)Show InChI InChI=1S/C17H11N5S/c18-9-11-5-1-2-6-12(11)10-23-17-20-16-15(21-22-17)13-7-3-4-8-14(13)19-16/h1-8H,10H2,(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040945

(CHEMBL3354396)Show SMILES COC(=O)CCC(=O)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C22H17N5O3S/c1-30-19(29)11-10-18(28)27-17-9-5-4-8-16(17)20-21(27)24-22(26-25-20)31-13-15-7-3-2-6-14(15)12-23/h2-9H,10-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040952
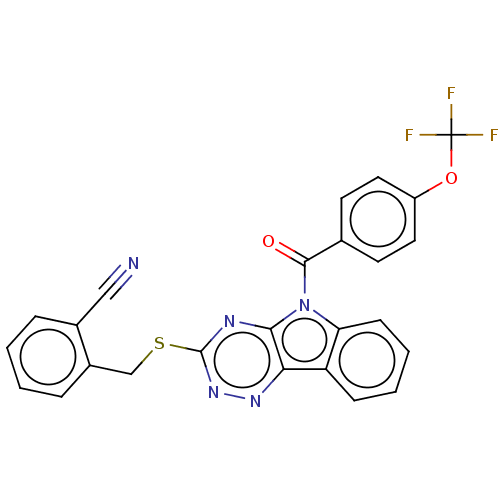
(CHEMBL3354403)Show SMILES FC(F)(F)Oc1ccc(cc1)C(=O)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C25H14F3N5O2S/c26-25(27,28)35-18-11-9-15(10-12-18)23(34)33-20-8-4-3-7-19(20)21-22(33)30-24(32-31-21)36-14-17-6-2-1-5-16(17)13-29/h1-12H,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040956

(CHEMBL3354407)Show SMILES O=C(c1ccno1)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C21H12N6O2S/c22-11-13-5-1-2-6-14(13)12-30-21-24-19-18(25-26-21)15-7-3-4-8-16(15)27(19)20(28)17-9-10-23-29-17/h1-10H,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50040955
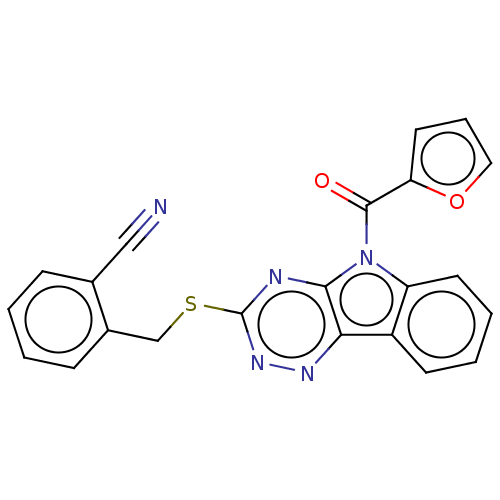
(CHEMBL3354406)Show SMILES O=C(c1ccco1)n1c2ccccc2c2nnc(SCc3ccccc3C#N)nc12 Show InChI InChI=1S/C22H13N5O2S/c23-12-14-6-1-2-7-15(14)13-30-22-24-20-19(25-26-22)16-8-3-4-9-17(16)27(20)21(28)18-10-5-11-29-18/h1-11H,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55212-2 from human CB2R expressed in CHO cells |
Bioorg Med Chem 23: 241-63 (2014)
Article DOI: 10.1016/j.bmc.2014.11.002
BindingDB Entry DOI: 10.7270/Q2930VS3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2C
(RAT-Rattus norvegicus (Rat)) | BDBM50164548
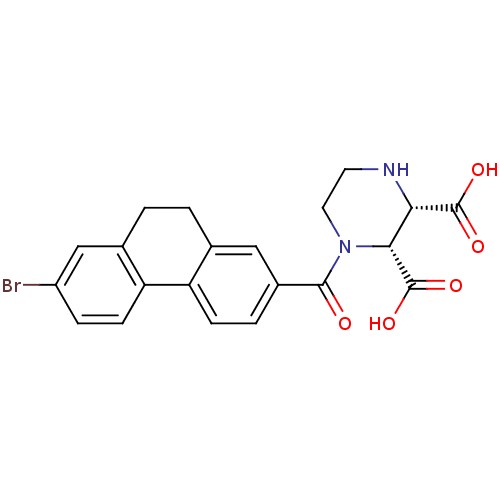
((2R,3S)-1-(7-Bromo-9,10-dihydro-phenanthrene-2-car...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc-2c(CCc3cc(Br)ccc-23)c1 Show InChI InChI=1S/C21H19BrN2O5/c22-14-4-6-16-12(10-14)2-1-11-9-13(3-5-15(11)16)19(25)24-8-7-23-17(20(26)27)18(24)21(28)29/h3-6,9-10,17-18,23H,1-2,7-8H2,(H,26,27)(H,28,29)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2C receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50164555

((2R,3S)-1-(9,10-Dihydro-phenanthrene-2-carbonyl)-p...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc-2c(CCc3ccccc-23)c1 Show InChI InChI=1S/C21H20N2O5/c24-19(23-10-9-22-17(20(25)26)18(23)21(27)28)14-7-8-16-13(11-14)6-5-12-3-1-2-4-15(12)16/h1-4,7-8,11,17-18,22H,5-6,9-10H2,(H,25,26)(H,27,28)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Rattus norvegicus (Rat)-RAT) | BDBM50419786
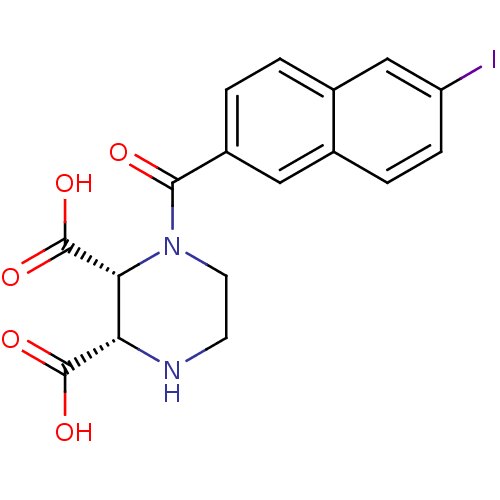
(CHEMBL1950795)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc2cc(I)ccc2c1 |r| Show InChI InChI=1S/C17H15IN2O5/c18-12-4-3-9-7-11(2-1-10(9)8-12)15(21)20-6-5-19-13(16(22)23)14(20)17(24)25/h1-4,7-8,13-14,19H,5-6H2,(H,22,23)(H,24,25)/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2A
(Rattus norvegicus (Rat)-RAT) | BDBM50164555

((2R,3S)-1-(9,10-Dihydro-phenanthrene-2-carbonyl)-p...)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc-2c(CCc3ccccc-23)c1 Show InChI InChI=1S/C21H20N2O5/c24-19(23-10-9-22-17(20(25)26)18(23)21(27)28)14-7-8-16-13(11-14)6-5-12-3-1-2-4-15(12)16/h1-4,7-8,11,17-18,22H,5-6,9-10H2,(H,25,26)(H,27,28)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity at rat recombinant GluN1/GluN2A receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50040671

(1-[5-(3-Aza-bicyclo[3.2.2]non-3-yl)-1-methyl-2-oxo...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)N1CC2CCC(CC2)C1 |c:9,(7.22,-6.97,;7.67,-8.45,;6.69,-9.39,;5.36,-8.63,;4.02,-9.39,;4.02,-10.93,;5.36,-11.69,;6.71,-10.96,;7.63,-11.85,;9.14,-11.6,;9.86,-10.19,;11.4,-10.21,;12.13,-11.53,;11.35,-12.86,;13.67,-11.57,;14.48,-10.26,;13.72,-8.91,;14.5,-7.6,;16.04,-7.62,;16.81,-8.95,;18.33,-8.98,;16.01,-10.29,;9.18,-8.72,;10.1,-7.49,;7.17,-13.34,;5.55,-13.5,;4.73,-14.54,;5.14,-16.05,;6.65,-16.64,;8.09,-15.8,;7.22,-14.74,;6.1,-14.2,;8.22,-14.23,)| Show InChI InChI=1S/C26H31N5O2/c1-17-6-5-7-20(14-17)27-26(33)29-23-25(32)30(2)22-9-4-3-8-21(22)24(28-23)31-15-18-10-11-19(16-31)13-12-18/h3-9,14,18-19,23H,10-13,15-16H2,1-2H3,(H2,27,29,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2C
(RAT-Rattus norvegicus (Rat)) | BDBM50419786

(CHEMBL1950795)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc2cc(I)ccc2c1 |r| Show InChI InChI=1S/C17H15IN2O5/c18-12-4-3-9-7-11(2-1-10(9)8-12)15(21)20-6-5-19-13(16(22)23)14(20)17(24)25/h1-4,7-8,13-14,19H,5-6H2,(H,22,23)(H,24,25)/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2C receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2C
(RAT-Rattus norvegicus (Rat)) | BDBM50364074
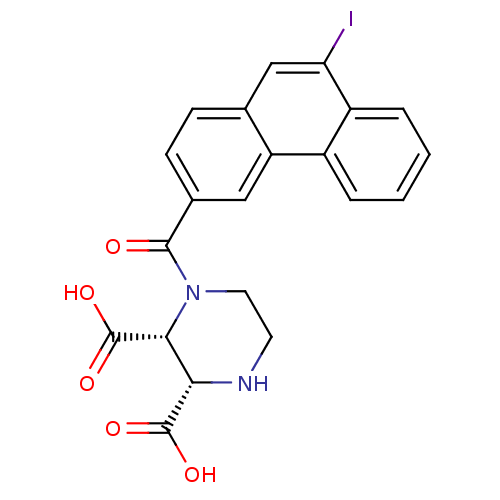
(CHEMBL1950805)Show SMILES OC(=O)[C@H]1NCCN([C@H]1C(O)=O)C(=O)c1ccc2cc(I)c3ccccc3c2c1 |r| Show InChI InChI=1S/C21H17IN2O5/c22-16-10-11-5-6-12(9-15(11)13-3-1-2-4-14(13)16)19(25)24-8-7-23-17(20(26)27)18(24)21(28)29/h1-6,9-10,17-18,23H,7-8H2,(H,26,27)(H,28,29)/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Antagonist activity rat recombinant GluN1/GluN2C receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate-induced response by... |
J Med Chem 55: 327-41 (2012)
Article DOI: 10.1021/jm201230z
BindingDB Entry DOI: 10.7270/Q2TD9XTJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data