 Found 807 hits with Last Name = 'ellis' and Initial = 'p'
Found 807 hits with Last Name = 'ellis' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50459408
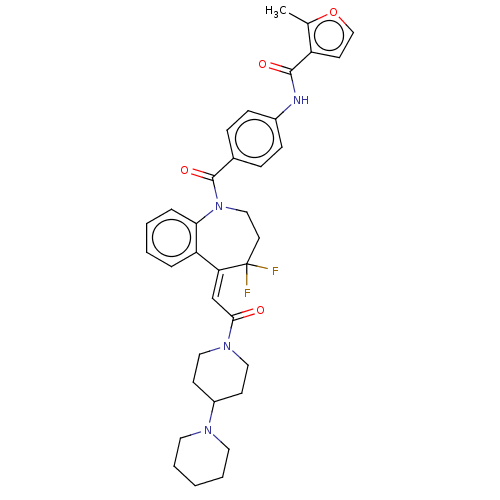
(CHEMBL3307200)Show SMILES Cc1occc1C(=O)Nc1ccc(cc1)C(=O)N1CCC(F)(F)\C(=C/C(=O)N2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C35H38F2N4O4/c1-24-28(15-22-45-24)33(43)38-26-11-9-25(10-12-26)34(44)41-21-16-35(36,37)30(29-7-3-4-8-31(29)41)23-32(42)40-19-13-27(14-20-40)39-17-5-2-6-18-39/h3-4,7-12,15,22-23,27H,2,5-6,13-14,16-21H2,1H3,(H,38,43)/b30-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387945
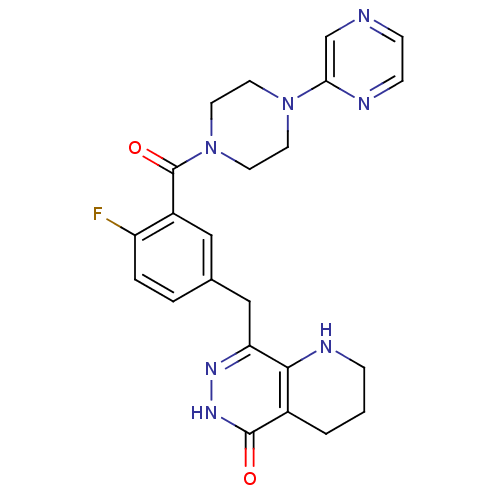
(CHEMBL2058927 | US9283222, 511)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1cnccn1 Show InChI InChI=1S/C23H24FN7O2/c24-18-4-3-15(13-19-21-16(2-1-5-27-21)22(32)29-28-19)12-17(18)23(33)31-10-8-30(9-11-31)20-14-25-6-7-26-20/h3-4,6-7,12,14,27H,1-2,5,8-11,13H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387946

(CHEMBL2058928 | US9283222, 536)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C23H24FN7O2/c24-18-5-4-15(14-19-20-16(3-1-6-25-20)21(32)29-28-19)13-17(18)22(33)30-9-11-31(12-10-30)23-26-7-2-8-27-23/h2,4-5,7-8,13,25H,1,3,6,9-12,14H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095
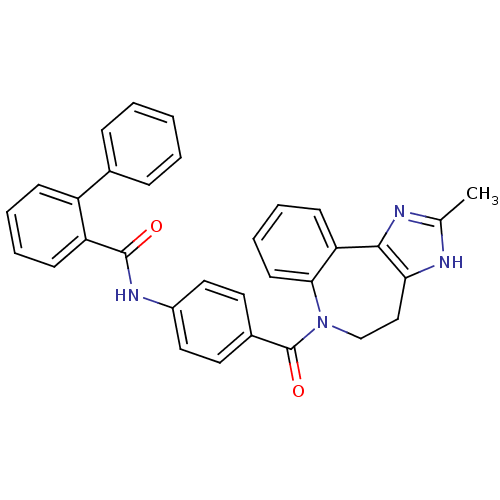
(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from V1A receptor in Wistar rat liver membranes incubated for 60 mins by microplate scintillation counting method |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V2 receptor expressed in CHO cell membranes by radioligand binding assay |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387914
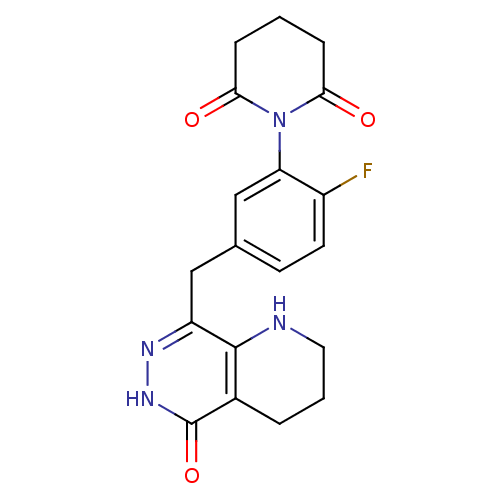
(CHEMBL2058682 | US9283222, 507)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1N1C(=O)CCCC1=O Show InChI InChI=1S/C19H19FN4O3/c20-13-7-6-11(10-15(13)24-16(25)4-1-5-17(24)26)9-14-18-12(3-2-8-21-18)19(27)23-22-14/h6-7,10,21H,1-5,8-9H2,(H,23,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387941
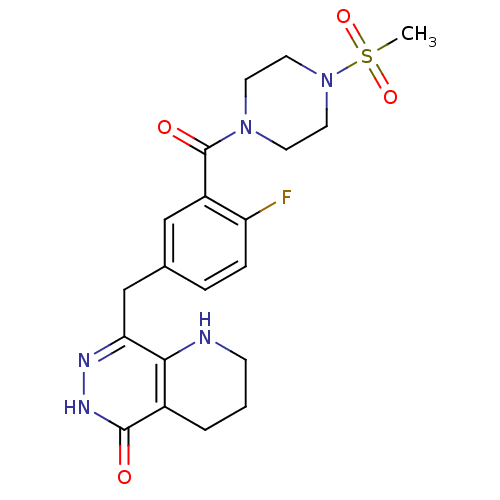
(CHEMBL2058922 | US9283222, 556)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3CCCNc23)ccc1F Show InChI InChI=1S/C20H24FN5O4S/c1-31(29,30)26-9-7-25(8-10-26)20(28)15-11-13(4-5-16(15)21)12-17-18-14(3-2-6-22-18)19(27)24-23-17/h4-5,11,22H,2-3,6-10,12H2,1H3,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(MOUSE) | BDBM86428
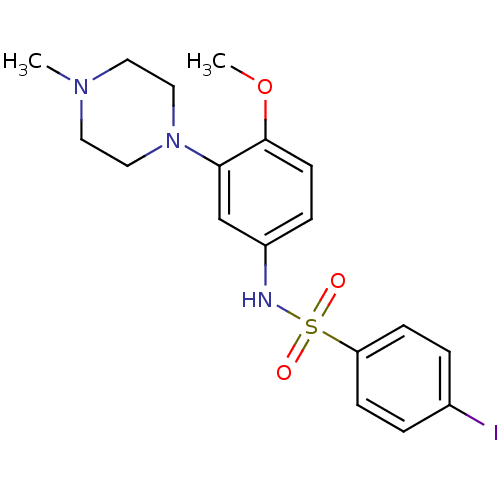
(SB-258585)Show SMILES COc1ccc(NS(=O)(=O)c2ccc(I)cc2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C18H22IN3O3S/c1-21-9-11-22(12-10-21)17-13-15(5-8-18(17)25-2)20-26(23,24)16-6-3-14(19)4-7-16/h3-8,13,20H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse wild type 5HT6 receptor expressed in COS7 cells assessed as inhibition of seratonin-induced cAMP accumulation by HTRF as... |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300018
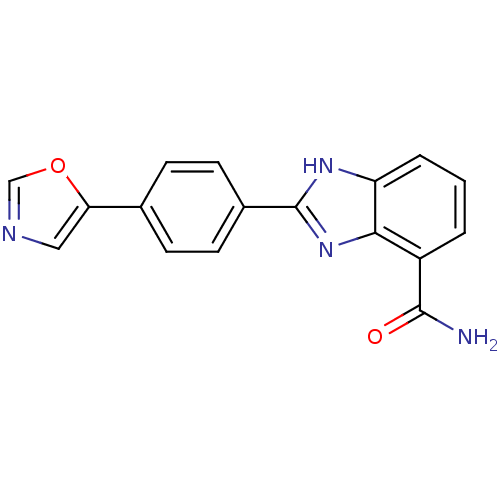
(2-(4-(Oxazol-5-yl)phenyl)-1H-benzo[d]imidazole-4-c...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1cnco1 Show InChI InChI=1S/C17H12N4O2/c18-16(22)12-2-1-3-13-15(12)21-17(20-13)11-6-4-10(5-7-11)14-8-19-9-23-14/h1-9H,(H2,18,22)(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387944
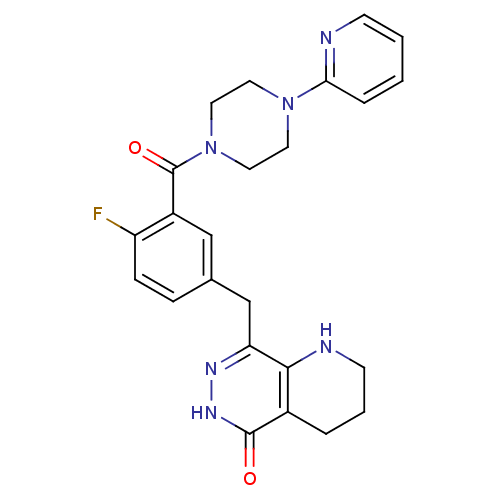
(CHEMBL2058926 | US9283222, 545)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1ccccn1 Show InChI InChI=1S/C24H25FN6O2/c25-19-7-6-16(15-20-22-17(4-3-9-27-22)23(32)29-28-20)14-18(19)24(33)31-12-10-30(11-13-31)21-5-1-2-8-26-21/h1-2,5-8,14,27H,3-4,9-13,15H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387938

(CHEMBL2058919)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)C(=O)C(F)(F)F Show InChI InChI=1S/C21H21F4N5O3/c22-15-4-3-12(11-16-17-13(2-1-5-26-17)18(31)28-27-16)10-14(15)19(32)29-6-8-30(9-7-29)20(33)21(23,24)25/h3-4,10,26H,1-2,5-9,11H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387935
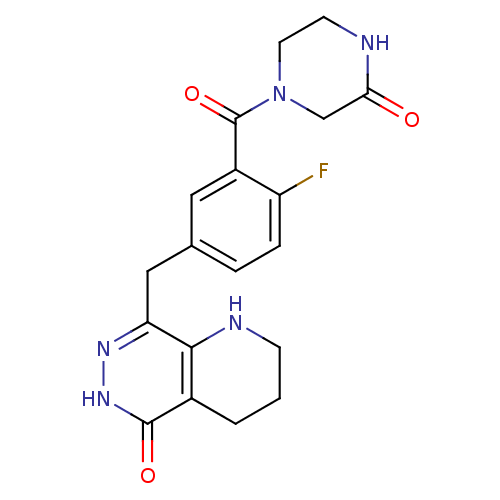
(CHEMBL2058916 | US9283222, 607)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCNC(=O)C1 Show InChI InChI=1S/C19H20FN5O3/c20-14-4-3-11(8-13(14)19(28)25-7-6-21-16(26)10-25)9-15-17-12(2-1-5-22-17)18(27)24-23-15/h3-4,8,22H,1-2,5-7,9-10H2,(H,21,26)(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387948
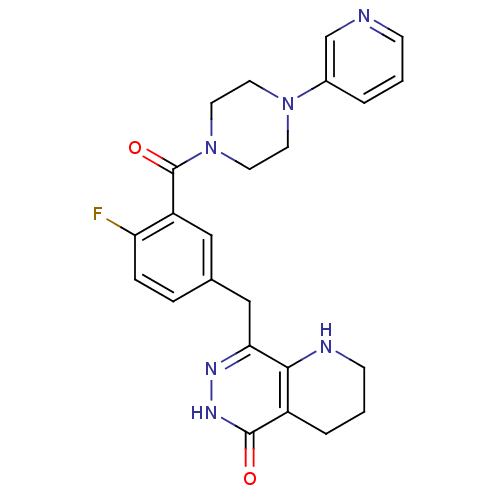
(CHEMBL2058925 | US9283222, 695)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1cccnc1 Show InChI InChI=1S/C24H25FN6O2/c25-20-6-5-16(14-21-22-18(4-2-8-27-22)23(32)29-28-21)13-19(20)24(33)31-11-9-30(10-12-31)17-3-1-7-26-15-17/h1,3,5-7,13,15,27H,2,4,8-12,14H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387939
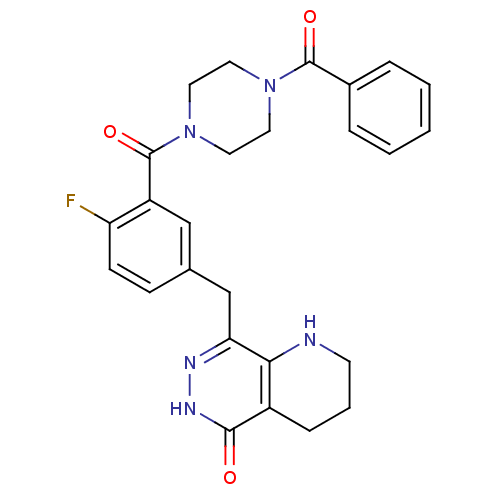
(CHEMBL2058920 | US9283222, 551)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C26H26FN5O3/c27-21-9-8-17(16-22-23-19(7-4-10-28-23)24(33)30-29-22)15-20(21)26(35)32-13-11-31(12-14-32)25(34)18-5-2-1-3-6-18/h1-3,5-6,8-9,15,28H,4,7,10-14,16H2,(H,30,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300015

(2-(4-(Pyridin-3-yl)phenyl)-1H-benzo[d]imidazole-4-...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C19H14N4O/c20-18(24)15-4-1-5-16-17(15)23-19(22-16)13-8-6-12(7-9-13)14-3-2-10-21-11-14/h1-11H,(H2,20,24)(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300008

(2-(4-(1,3,4-Oxadiazol-2-yl)phenyl)-1H-benzo[d]imid...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1nnco1 Show InChI InChI=1S/C16H11N5O2/c17-14(22)11-2-1-3-12-13(11)20-15(19-12)9-4-6-10(7-5-9)16-21-18-8-23-16/h1-8H,(H2,17,22)(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387940

(CHEMBL2058921 | US9283222, 512)Show SMILES CN(C)C(=O)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3CCCNc23)ccc1F Show InChI InChI=1S/C22H27FN6O3/c1-27(2)22(32)29-10-8-28(9-11-29)21(31)16-12-14(5-6-17(16)23)13-18-19-15(4-3-7-24-19)20(30)26-25-18/h5-6,12,24H,3-4,7-11,13H2,1-2H3,(H,26,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387942
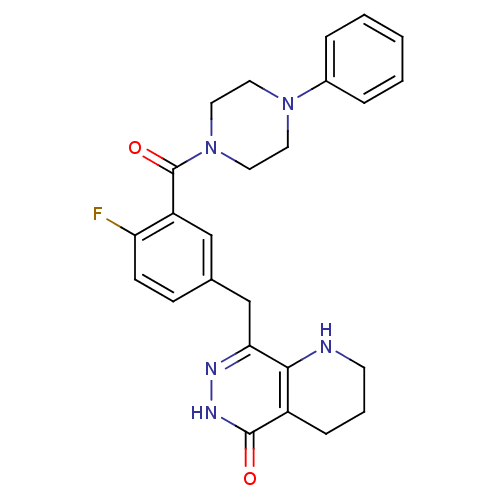
(CHEMBL2058923 | US9283222, 546)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C25H26FN5O2/c26-21-9-8-17(16-22-23-19(7-4-10-27-23)24(32)29-28-22)15-20(21)25(33)31-13-11-30(12-14-31)18-5-2-1-3-6-18/h1-3,5-6,8-9,15,27H,4,7,10-14,16H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300019

(2-(4-(1H-Imidazol-5-yl)phenyl)-1H-benzo[d]imidazol...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1c[nH]cn1 Show InChI InChI=1S/C17H13N5O/c18-16(23)12-2-1-3-13-15(12)22-17(21-13)11-6-4-10(5-7-11)14-8-19-9-20-14/h1-9H,(H2,18,23)(H,19,20)(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387943
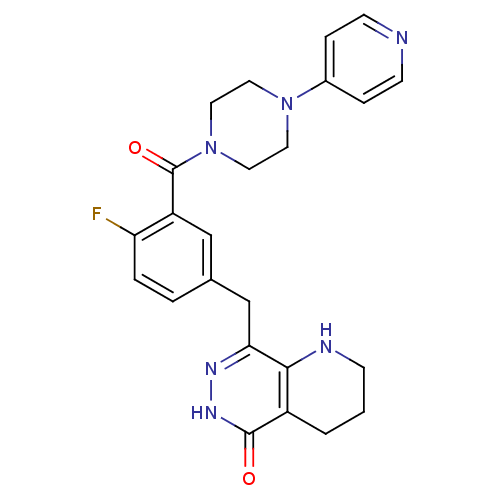
(CHEMBL2058924 | US9283222, 510)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C24H25FN6O2/c25-20-4-3-16(15-21-22-18(2-1-7-27-22)23(32)29-28-21)14-19(20)24(33)31-12-10-30(11-13-31)17-5-8-26-9-6-17/h3-6,8-9,14,27H,1-2,7,10-13,15H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387936
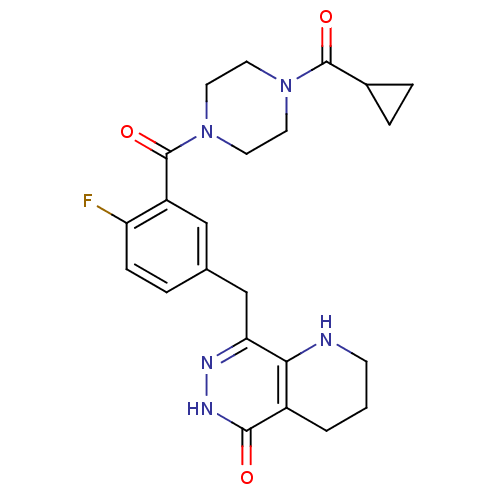
(CHEMBL2058917 | US9283222, 506)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C23H26FN5O3/c24-18-6-3-14(13-19-20-16(2-1-7-25-20)21(30)27-26-19)12-17(18)23(32)29-10-8-28(9-11-29)22(31)15-4-5-15/h3,6,12,15,25H,1-2,4-5,7-11,13H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387925
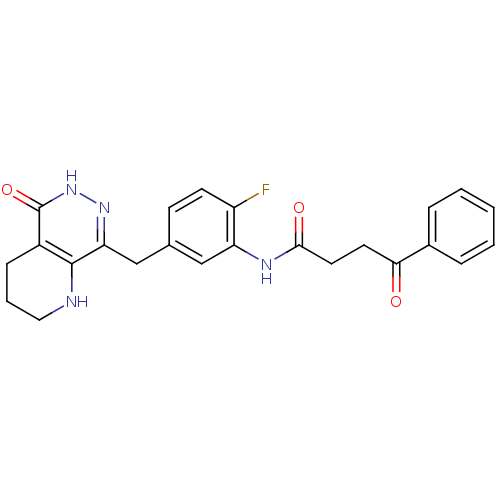
(CHEMBL2058688 | US9283222, 479)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1NC(=O)CCC(=O)c1ccccc1 Show InChI InChI=1S/C24H23FN4O3/c25-18-9-8-15(14-20-23-17(7-4-12-26-23)24(32)29-28-20)13-19(18)27-22(31)11-10-21(30)16-5-2-1-3-6-16/h1-3,5-6,8-9,13,26H,4,7,10-12,14H2,(H,27,31)(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387937
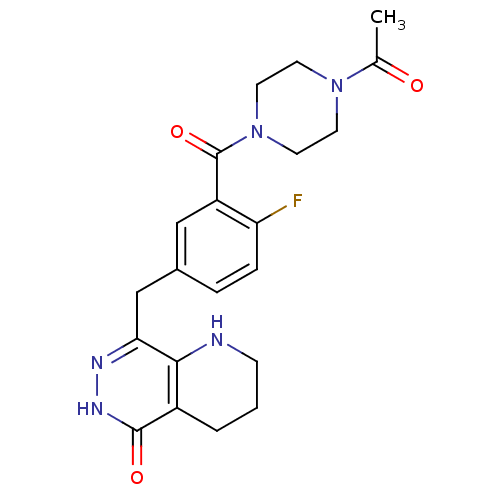
(CHEMBL2058918 | US9283222, 559)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3CCCNc23)ccc1F Show InChI InChI=1S/C21H24FN5O3/c1-13(28)26-7-9-27(10-8-26)21(30)16-11-14(4-5-17(16)22)12-18-19-15(3-2-6-23-19)20(29)25-24-18/h4-5,11,23H,2-3,6-10,12H2,1H3,(H,25,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387924

(CHEMBL2058687 | US9283222, 474)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1NC(=O)CCCC(=O)Nc1ccccc1 Show InChI InChI=1S/C25H26FN5O3/c26-19-12-11-16(15-21-24-18(8-5-13-27-24)25(34)31-30-21)14-20(19)29-23(33)10-4-9-22(32)28-17-6-2-1-3-7-17/h1-3,6-7,11-12,14,27H,4-5,8-10,13,15H2,(H,28,32)(H,29,33)(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387916

(CHEMBL2058681)Show SMILES Oc1ccc(O)n1-c1cc(Cc2n[nH]c(=O)c3CCCNc23)ccc1F |(12.24,-8.93,;13.39,-9.95,;14.9,-9.63,;15.67,-10.96,;14.64,-12.11,;14.96,-13.61,;13.25,-11.48,;11.91,-12.25,;10.58,-11.49,;9.26,-12.26,;7.93,-11.49,;7.93,-9.95,;9.26,-9.18,;9.25,-7.64,;7.92,-6.88,;7.92,-5.34,;6.6,-7.65,;5.27,-6.87,;3.94,-7.65,;3.94,-9.19,;5.27,-9.95,;6.6,-9.18,;9.25,-13.8,;10.58,-14.57,;11.92,-13.8,;13.25,-14.57,)| Show InChI InChI=1S/C18H17FN4O3/c19-12-4-3-10(9-14(12)23-15(24)5-6-16(23)25)8-13-17-11(2-1-7-20-17)18(26)22-21-13/h3-6,9,20,24-25H,1-2,7-8H2,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300009
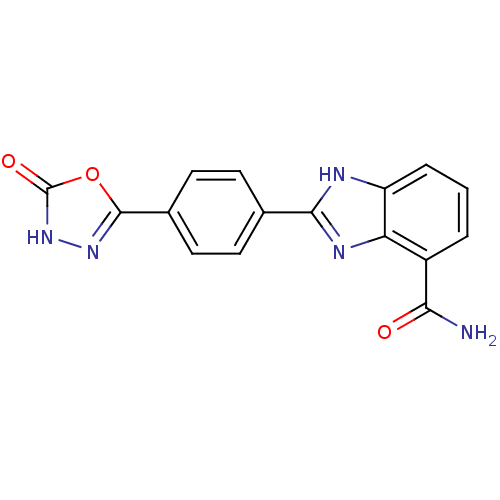
(2-(4-(5-Oxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)pheny...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1n[nH]c(=O)o1 Show InChI InChI=1S/C16H11N5O3/c17-13(22)10-2-1-3-11-12(10)19-14(18-11)8-4-6-9(7-5-8)15-20-21-16(23)24-15/h1-7H,(H2,17,22)(H,18,19)(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387931

(CHEMBL2058912 | US9283222, 708)Show SMILES NC1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3CCCNc23)ccc1F Show InChI InChI=1S/C20H24FN5O2/c21-16-4-3-12(10-15(16)20(28)26-8-5-13(22)6-9-26)11-17-18-14(2-1-7-23-18)19(27)25-24-17/h3-4,10,13,23H,1-2,5-9,11,22H2,(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300021
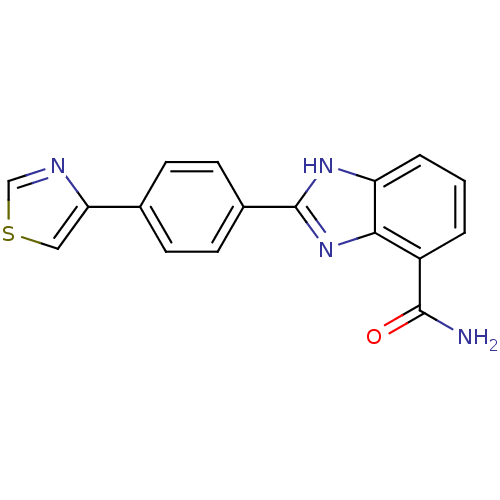
(2-(4-(Thiazol-4-yl)phenyl)-1H-benzo[d]imidazole-4-...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1cscn1 Show InChI InChI=1S/C17H12N4OS/c18-16(22)12-2-1-3-13-15(12)21-17(20-13)11-6-4-10(5-7-11)14-8-23-9-19-14/h1-9H,(H2,18,22)(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387930
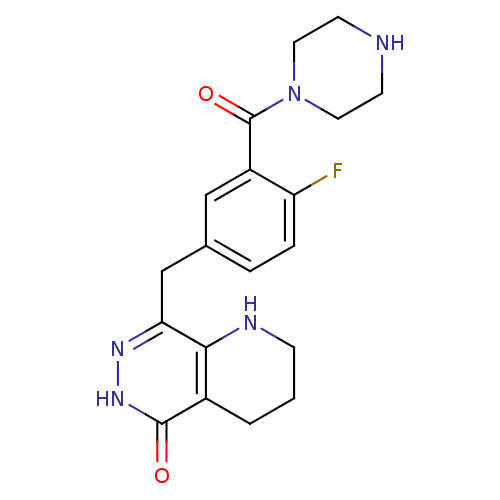
(CHEMBL2058911 | US9283222, 617)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCNCC1 Show InChI InChI=1S/C19H22FN5O2/c20-15-4-3-12(10-14(15)19(27)25-8-6-21-7-9-25)11-16-17-13(2-1-5-22-17)18(26)24-23-16/h3-4,10,21-22H,1-2,5-9,11H2,(H,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300016
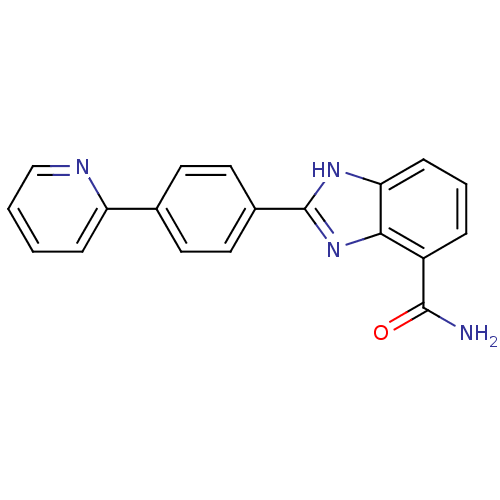
(2-(4-(Pyridin-2-yl)phenyl)-1H-benzo[d]imidazole-4-...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C19H14N4O/c20-18(24)14-4-3-6-16-17(14)23-19(22-16)13-9-7-12(8-10-13)15-5-1-2-11-21-15/h1-11H,(H2,20,24)(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300017
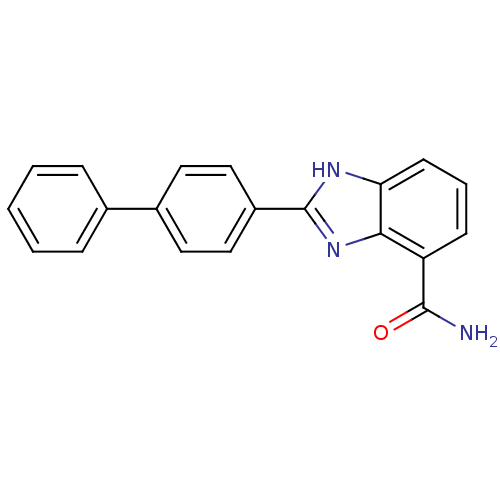
(2-(biphenyl-4-yl)-1H-benzo[d]imidazole-4-carboxami...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O/c21-19(24)16-7-4-8-17-18(16)23-20(22-17)15-11-9-14(10-12-15)13-5-2-1-3-6-13/h1-12H,(H2,21,24)(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300023
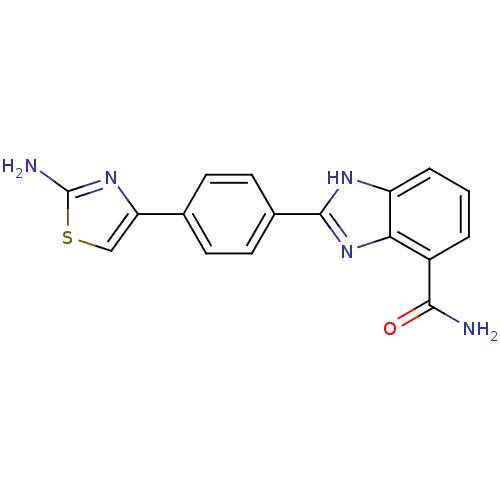
(2-(4-(2-Aminothiazol-4-yl)phenyl)-1H-benzo[d]imida...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1csc(N)n1 Show InChI InChI=1S/C17H13N5OS/c18-15(23)11-2-1-3-12-14(11)22-16(20-12)10-6-4-9(5-7-10)13-8-24-17(19)21-13/h1-8H,(H2,18,23)(H2,19,21)(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300020
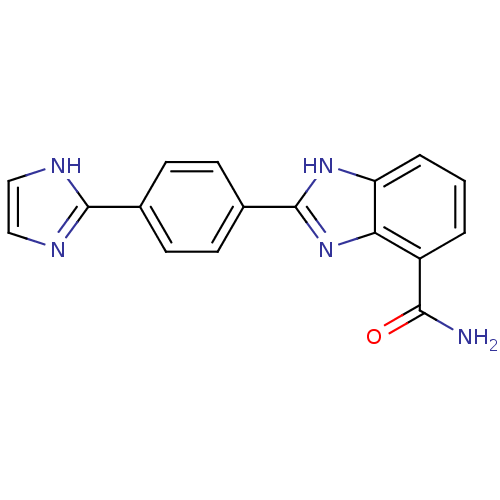
(2-(4-(1H-Imidazol-2-yl)phenyl)-1H-benzo[d]imidazol...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1ncc[nH]1 Show InChI InChI=1S/C17H13N5O/c18-15(23)12-2-1-3-13-14(12)22-17(21-13)11-6-4-10(5-7-11)16-19-8-9-20-16/h1-9H,(H2,18,23)(H,19,20)(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50459401

(CHEMBL4210854)Show InChI InChI=1S/C23H21NO/c25-23(24-17-7-6-11-20-10-4-5-12-22(20)24)21-15-13-19(14-16-21)18-8-2-1-3-9-18/h1-5,8-10,12-16H,6-7,11,17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes by radioligand binding assay |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300022
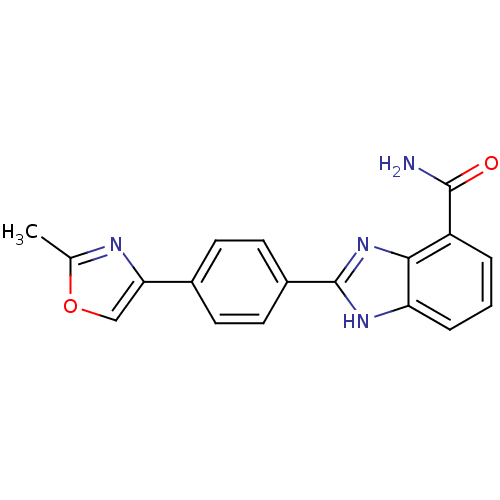
(2-(4-(2-Methyloxazol-4-yl)phenyl)-1H-benzo[d]imida...)Show SMILES Cc1nc(co1)-c1ccc(cc1)-c1nc2c(cccc2[nH]1)C(N)=O Show InChI InChI=1S/C18H14N4O2/c1-10-20-15(9-24-10)11-5-7-12(8-6-11)18-21-14-4-2-3-13(17(19)23)16(14)22-18/h2-9H,1H3,(H2,19,23)(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387934
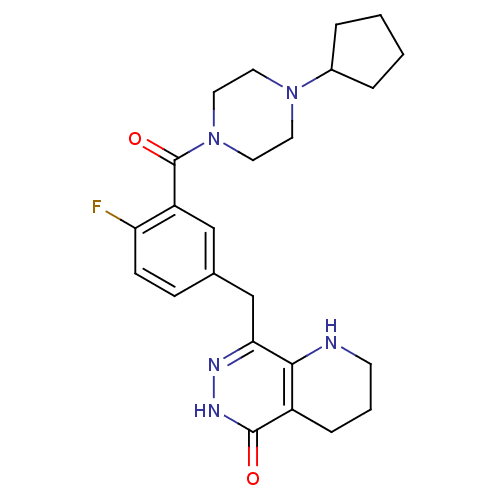
(CHEMBL2058915 | US9283222, 564)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3CCCNc23)cc1C(=O)N1CCN(CC1)C1CCCC1 Show InChI InChI=1S/C24H30FN5O2/c25-20-8-7-16(15-21-22-18(6-3-9-26-22)23(31)28-27-21)14-19(20)24(32)30-12-10-29(11-13-30)17-4-1-2-5-17/h7-8,14,17,26H,1-6,9-13,15H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes by radioligand binding assay |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300005
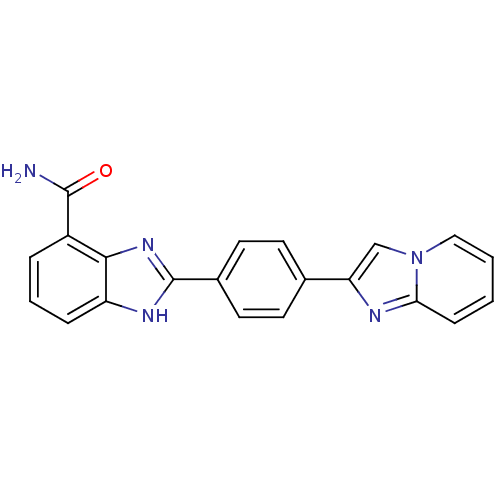
(2-(4-(Imidazo[1,2-a]pyridin-2-yl)phenyl)-1H-benzo[...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1cn2ccccc2n1 Show InChI InChI=1S/C21H15N5O/c22-20(27)15-4-3-5-16-19(15)25-21(24-16)14-9-7-13(8-10-14)17-12-26-11-2-1-6-18(26)23-17/h1-12H,(H2,22,27)(H,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387918
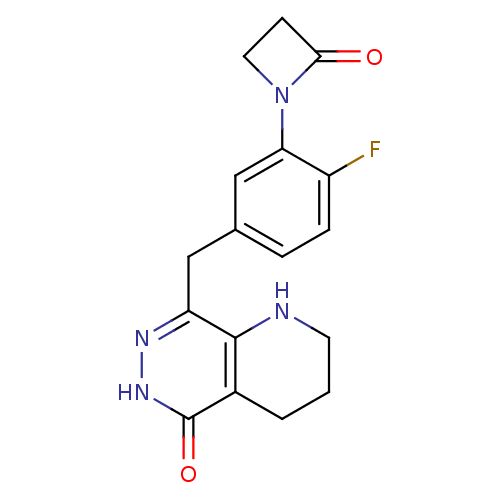
(CHEMBL2058680 | US9283222, 459)Show InChI InChI=1S/C17H17FN4O2/c18-12-4-3-10(9-14(12)22-7-5-15(22)23)8-13-16-11(2-1-6-19-16)17(24)21-20-13/h3-4,9,19H,1-2,5-8H2,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from V2 receptor in Wistar rat kidney membranes incubated for 60 mins by microplate scintillation counting method |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300014
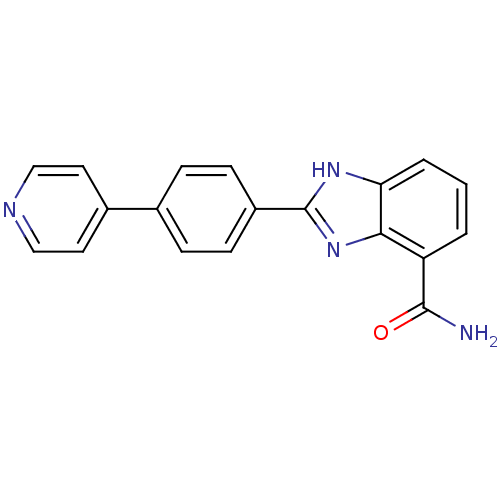
(2-(4-(Pyridin-4-yl)phenyl)-1H-benzimidazole-4-carb...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C19H14N4O/c20-18(24)15-2-1-3-16-17(15)23-19(22-16)14-6-4-12(5-7-14)13-8-10-21-11-9-13/h1-11H,(H2,20,24)(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387929

(CHEMBL2058693 | US9283222, 563)Show SMILES CN1CCN(CCNC(=O)c2cc(Cc3n[nH]c(=O)c4CCCNc34)ccc2F)CC1 Show InChI InChI=1S/C22H29FN6O2/c1-28-9-11-29(12-10-28)8-7-25-21(30)17-13-15(4-5-18(17)23)14-19-20-16(3-2-6-24-20)22(31)27-26-19/h4-5,13,24H,2-3,6-12,14H2,1H3,(H,25,30)(H,27,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300011
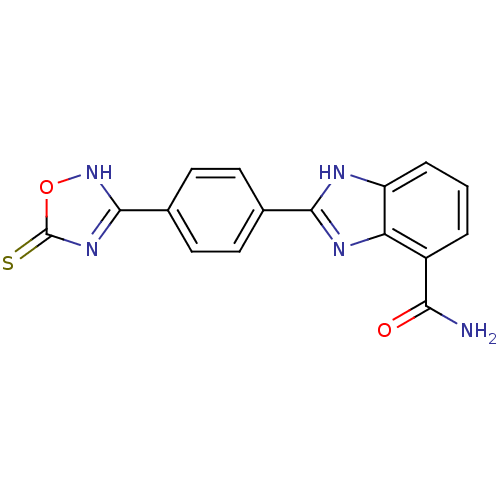
(2-(4-(5-Thioxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)ph...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1nc(=S)o[nH]1 Show InChI InChI=1S/C16H11N5O2S/c17-13(22)10-2-1-3-11-12(10)19-14(18-11)8-4-6-9(7-5-8)15-20-16(24)23-21-15/h1-7H,(H2,17,22)(H,18,19)(H,20,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50387923

(CHEMBL2058686 | US9283222, 477)Show SMILES CC(=O)CCCC(=O)Nc1cc(Cc2n[nH]c(=O)c3CCCNc23)ccc1F Show InChI InChI=1S/C20H23FN4O3/c1-12(26)4-2-6-18(27)23-16-10-13(7-8-15(16)21)11-17-19-14(5-3-9-22-19)20(28)25-24-17/h7-8,10,22H,2-6,9,11H2,1H3,(H,23,27)(H,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation counting |
Bioorg Med Chem 20: 4635-45 (2012)
Article DOI: 10.1016/j.bmc.2012.06.021
BindingDB Entry DOI: 10.7270/Q2PN96P7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
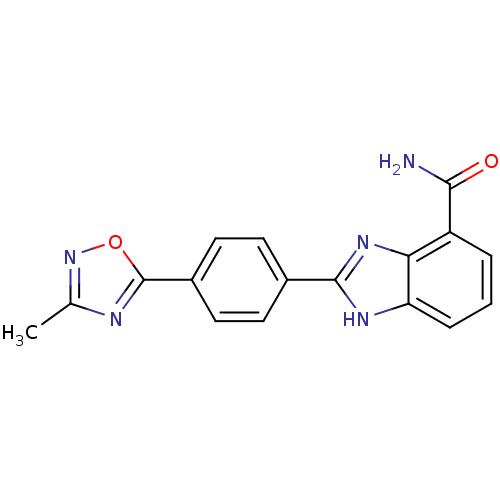
(Homo sapiens (Human)) | BDBM50300007
(2-(4-(3-Methyl-1,2,4-oxadiazol-5-yl)phenyl)-1H-ben...)Show SMILES Cc1noc(n1)-c1ccc(cc1)-c1nc2c(cccc2[nH]1)C(N)=O Show InChI InChI=1S/C17H13N5O2/c1-9-19-17(24-22-9)11-7-5-10(6-8-11)16-20-13-4-2-3-12(15(18)23)14(13)21-16/h2-8H,1H3,(H2,18,23)(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50300013

(2-(4-(1-Methyl-1H-pyrazol-3-yl)phenyl)-1H-benzo[d]...)Show SMILES Cn1ccc(n1)-c1ccc(cc1)-c1nc2c(cccc2[nH]1)C(N)=O Show InChI InChI=1S/C18H15N5O/c1-23-10-9-14(22-23)11-5-7-12(8-6-11)18-20-15-4-2-3-13(17(19)24)16(15)21-18/h2-10H,1H3,(H2,19,24)(H,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50304001

(CHEMBL584690 | [4-(4-Methylpiperazin-1-yl)-1H-benz...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(nc12)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H22N4O/c1-26-12-14-27(15-13-26)20-11-5-10-19-21(20)25-23(24-19)22(28)18-9-4-7-16-6-2-3-8-17(16)18/h2-11H,12-15H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 receptor expressed in COS7 cells assessed as inhibition of seratonin-induced cAMP accumulation by HTRF assay |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(MOUSE) | BDBM50304001

(CHEMBL584690 | [4-(4-Methylpiperazin-1-yl)-1H-benz...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(nc12)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H22N4O/c1-26-12-14-27(15-13-26)20-11-5-10-19-21(20)25-23(24-19)22(28)18-9-4-7-16-6-2-3-8-17(16)18/h2-11H,12-15H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse wild type 5HT6 receptor expressed in COS7 cells assessed as inhibition of seratonin-induced cAMP accumulation by HTRF as... |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27135

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
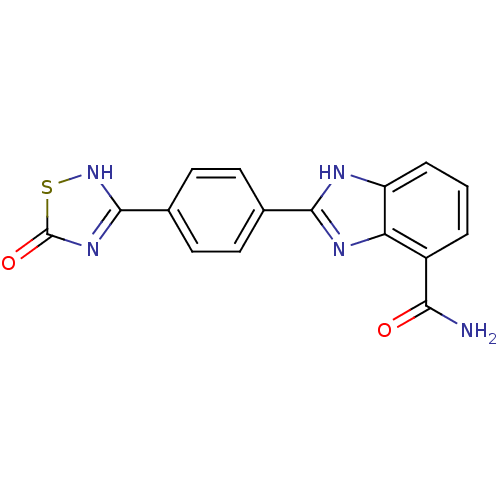
(Homo sapiens (Human)) | BDBM50300010
(2-(4-(5-Oxo-4,5-dihydro-1,2,4-thiadiazol-3-yl)phen...)Show SMILES NC(=O)c1cccc2[nH]c(nc12)-c1ccc(cc1)-c1nc(=O)s[nH]1 Show InChI InChI=1S/C16H11N5O2S/c17-13(22)10-2-1-3-11-12(10)19-14(18-11)8-4-6-9(7-5-8)15-20-16(23)24-21-15/h1-7H,(H2,17,22)(H,18,19)(H,20,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PARP1 by scintillation counting |
J Med Chem 52: 6803-13 (2009)
Article DOI: 10.1021/jm900697r
BindingDB Entry DOI: 10.7270/Q2DN4548 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data