 Found 130 hits with Last Name = 'euan macintyre' and Initial = 'd'
Found 130 hits with Last Name = 'euan macintyre' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at cannabinoid CB1 receptor (unknown origin) |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259222
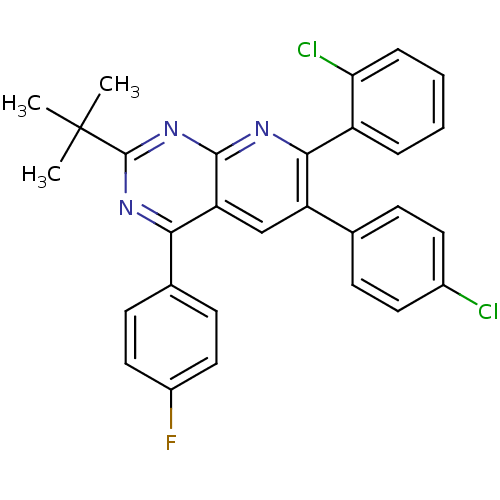
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CC(C)(C)c1nc(-c2ccc(F)cc2)c2cc(-c3ccc(Cl)cc3)c(nc2n1)-c1ccccc1Cl Show InChI InChI=1S/C29H22Cl2FN3/c1-29(2,3)28-34-25(18-10-14-20(32)15-11-18)23-16-22(17-8-12-19(30)13-9-17)26(33-27(23)35-28)21-6-4-5-7-24(21)31/h4-16H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259391
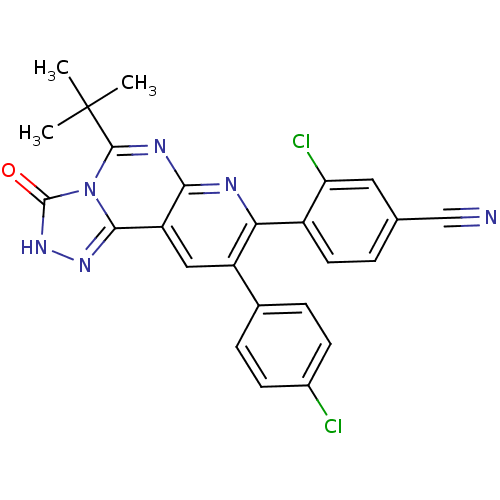
(4-(5-tert-butyl-9-(4-chlorophenyl)-3-oxo-2,3-dihyd...)Show SMILES CC(C)(C)c1nc2nc(-c3ccc(cc3Cl)C#N)c(cc2c2n[nH]c(=O)n12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H18Cl2N6O/c1-25(2,3)23-30-21-18(22-31-32-24(34)33(22)23)11-17(14-5-7-15(26)8-6-14)20(29-21)16-9-4-13(12-28)10-19(16)27/h4-11H,1-3H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259337
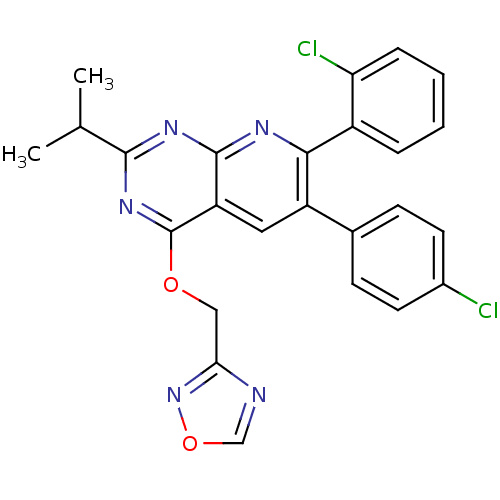
(3-((7-(2-chlorophenyl)-6-(4-chlorophenyl)-2-isopro...)Show SMILES CC(C)c1nc(OCc2ncon2)c2cc(-c3ccc(Cl)cc3)c(nc2n1)-c1ccccc1Cl Show InChI InChI=1S/C25H19Cl2N5O2/c1-14(2)23-30-24-19(25(31-23)33-12-21-28-13-34-32-21)11-18(15-7-9-16(26)10-8-15)22(29-24)17-5-3-4-6-20(17)27/h3-11,13-14H,12H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259492
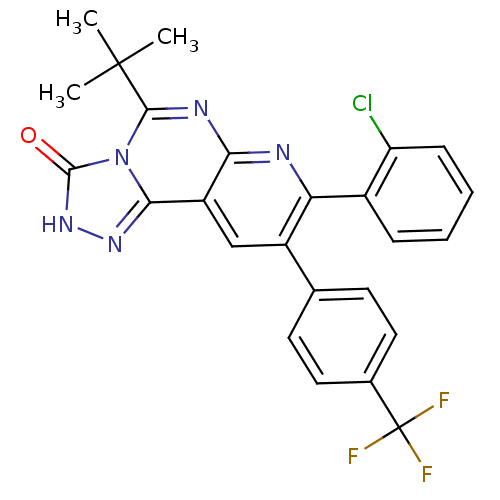
(5-tert-butyl-8-(2-chlorophenyl)-9-(4-(trifluoromet...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3Cl)c(cc2c2n[nH]c(=O)n12)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C25H19ClF3N5O/c1-24(2,3)22-31-20-17(21-32-33-23(35)34(21)22)12-16(13-8-10-14(11-9-13)25(27,28)29)19(30-20)15-6-4-5-7-18(15)26/h4-12H,1-3H3,(H,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235501
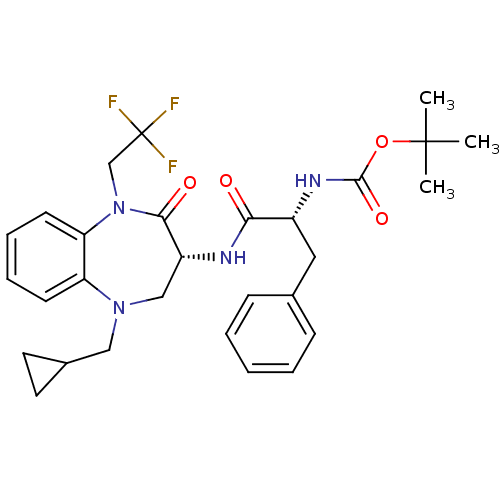
(CHEMBL254707 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CC(C)(C)OC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H]1CN(CC2CC2)c2ccccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C29H35F3N4O4/c1-28(2,3)40-27(39)34-21(15-19-9-5-4-6-10-19)25(37)33-22-17-35(16-20-13-14-20)23-11-7-8-12-24(23)36(26(22)38)18-29(30,31)32/h4-12,20-22H,13-18H2,1-3H3,(H,33,37)(H,34,39)/t21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells at a membrane potential of -70 mV by whole cell voltage clamp technique |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259339
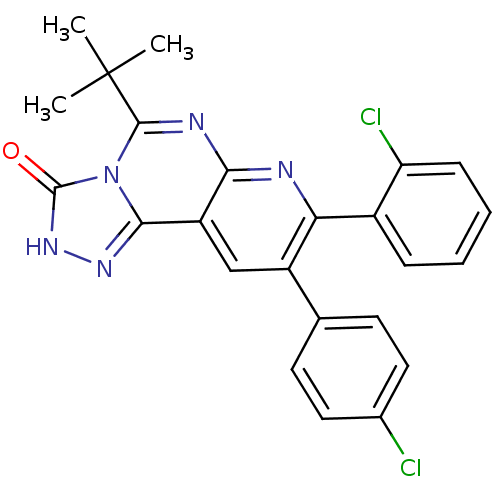
(5-tert-butyl-8-(2-chlorophenyl)-9-(4-chlorophenyl)...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3Cl)c(cc2c2n[nH]c(=O)n12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19Cl2N5O/c1-24(2,3)22-28-20-17(21-29-30-23(32)31(21)22)12-16(13-8-10-14(25)11-9-13)19(27-20)15-6-4-5-7-18(15)26/h4-12H,1-3H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259223
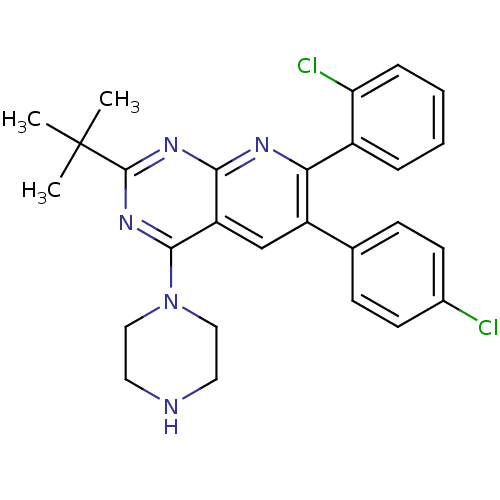
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CC(C)(C)c1nc(N2CCNCC2)c2cc(-c3ccc(Cl)cc3)c(nc2n1)-c1ccccc1Cl Show InChI InChI=1S/C27H27Cl2N5/c1-27(2,3)26-32-24-21(25(33-26)34-14-12-30-13-15-34)16-20(17-8-10-18(28)11-9-17)23(31-24)19-6-4-5-7-22(19)29/h4-11,16,30H,12-15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259224
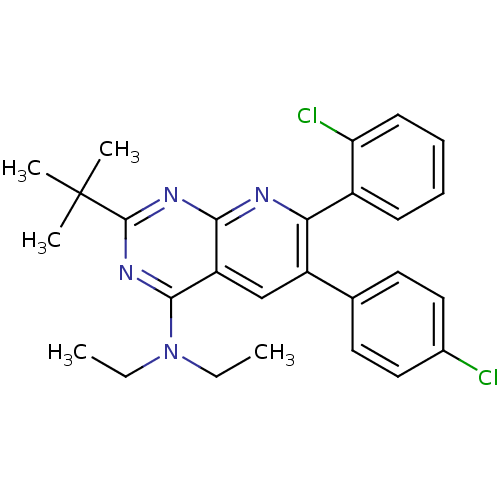
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CCN(CC)c1nc(nc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C27H28Cl2N4/c1-6-33(7-2)25-21-16-20(17-12-14-18(28)15-13-17)23(19-10-8-9-11-22(19)29)30-24(21)31-26(32-25)27(3,4)5/h8-16H,6-7H2,1-5H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259225
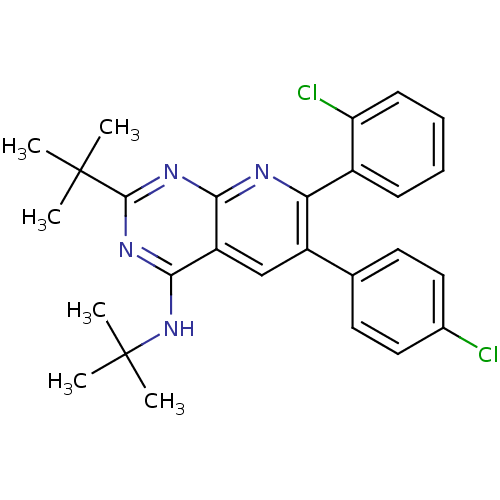
(CHEMBL513648 | N,2-di-tert-butyl-7-(2-chlorophenyl...)Show SMILES CC(C)(C)Nc1nc(nc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C27H28Cl2N4/c1-26(2,3)25-31-23-20(24(32-25)33-27(4,5)6)15-19(16-11-13-17(28)14-12-16)22(30-23)18-9-7-8-10-21(18)29/h7-15H,1-6H3,(H,30,31,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259491
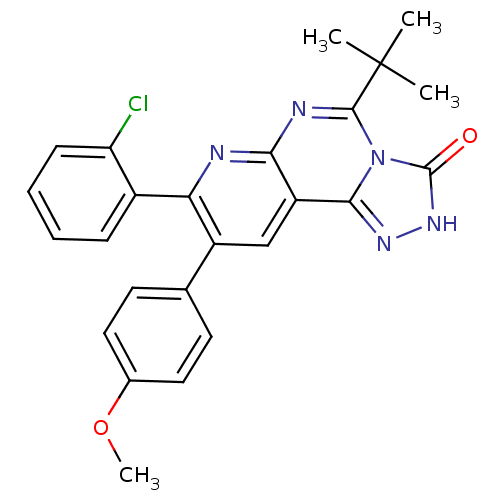
(5-tert-butyl-8-(2-chlorophenyl)-9-(4-methoxyphenyl...)Show SMILES COc1ccc(cc1)-c1cc2c3n[nH]c(=O)n3c(nc2nc1-c1ccccc1Cl)C(C)(C)C Show InChI InChI=1S/C25H22ClN5O2/c1-25(2,3)23-28-21-18(22-29-30-24(32)31(22)23)13-17(14-9-11-15(33-4)12-10-14)20(27-21)16-7-5-6-8-19(16)26/h5-13H,1-4H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259392
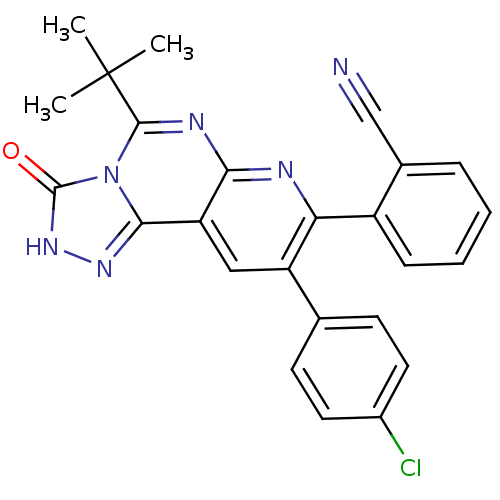
(2-(5-tert-butyl-9-(4-chlorophenyl)-3-oxo-2,3-dihyd...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3C#N)c(cc2c2n[nH]c(=O)n12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H19ClN6O/c1-25(2,3)23-29-21-19(22-30-31-24(33)32(22)23)12-18(14-8-10-16(26)11-9-14)20(28-21)17-7-5-4-6-15(17)13-27/h4-12H,1-3H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50204174
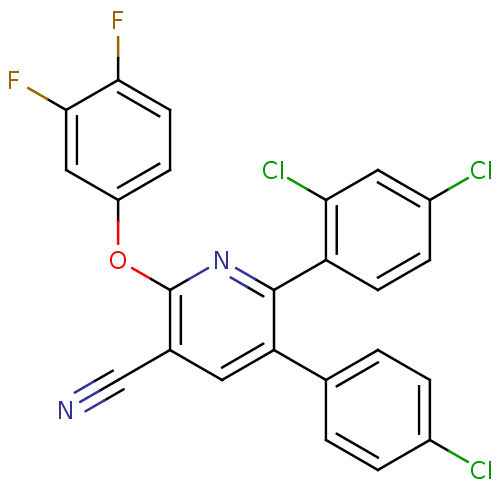
(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)-2-(3,4-d...)Show SMILES Fc1ccc(Oc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C24H11Cl3F2N2O/c25-15-3-1-13(2-4-15)19-9-14(12-30)24(32-17-6-8-21(28)22(29)11-17)31-23(19)18-7-5-16(26)10-20(18)27/h1-11H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at cannabinoid CB1 receptor (unknown origin) |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259269
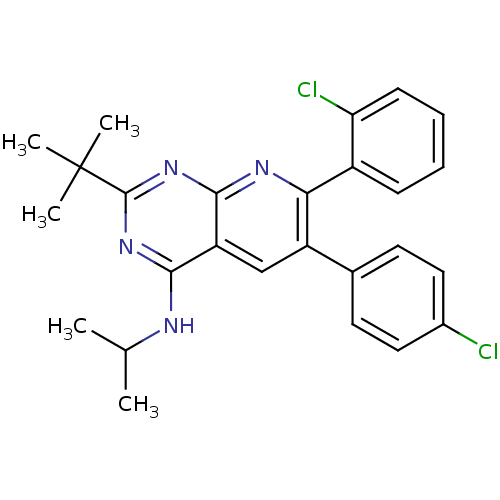
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CC(C)Nc1nc(nc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C26H26Cl2N4/c1-15(2)29-23-20-14-19(16-10-12-17(27)13-11-16)22(18-8-6-7-9-21(18)28)30-24(20)32-25(31-23)26(3,4)5/h6-15H,1-5H3,(H,29,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259390
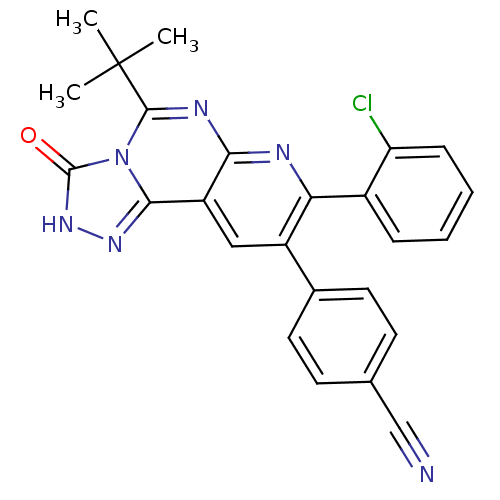
(4-(5-tert-butyl-8-(2-chlorophenyl)-3-oxo-2,3-dihyd...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3Cl)c(cc2c2n[nH]c(=O)n12)-c1ccc(cc1)C#N Show InChI InChI=1S/C25H19ClN6O/c1-25(2,3)23-29-21-18(22-30-31-24(33)32(22)23)12-17(15-10-8-14(13-27)9-11-15)20(28-21)16-6-4-5-7-19(16)26/h4-12H,1-3H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259140
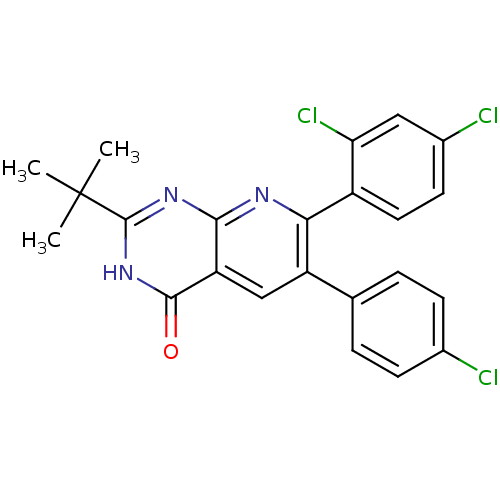
(2-tert-butyl-6-(4-chlorophenyl)-7-(2,4-dichlorophe...)Show SMILES CC(C)(C)c1nc2nc(-c3ccc(Cl)cc3Cl)c(cc2c(=O)[nH]1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H18Cl3N3O/c1-23(2,3)22-28-20-17(21(30)29-22)11-16(12-4-6-13(24)7-5-12)19(27-20)15-9-8-14(25)10-18(15)26/h4-11H,1-3H3,(H,27,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259139
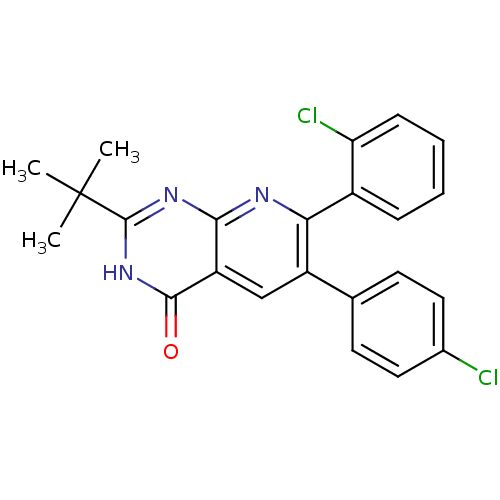
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3Cl)c(cc2c(=O)[nH]1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H19Cl2N3O/c1-23(2,3)22-27-20-17(21(29)28-22)12-16(13-8-10-14(24)11-9-13)19(26-20)15-6-4-5-7-18(15)25/h4-12H,1-3H3,(H,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176435
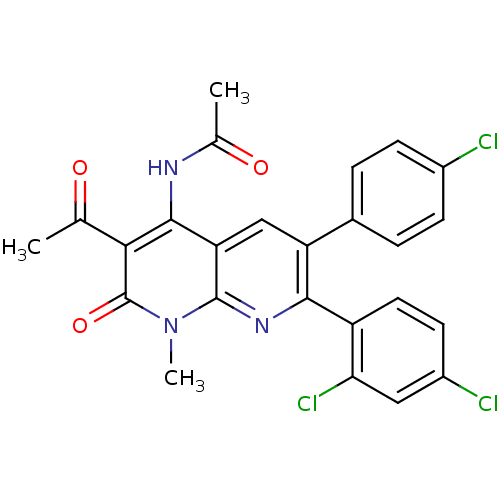
(CHEMBL204232 | N-(3-acetyl-6-(4-chlorophenyl)-7-(2...)Show SMILES CC(=O)Nc1c(C(C)=O)c(=O)n(C)c2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H18Cl3N3O3/c1-12(32)21-23(29-13(2)33)19-11-18(14-4-6-15(26)7-5-14)22(30-24(19)31(3)25(21)34)17-9-8-16(27)10-20(17)28/h4-11H,1-3H3,(H,29,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at cannabinoid CB1 receptor (unknown origin) |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259271
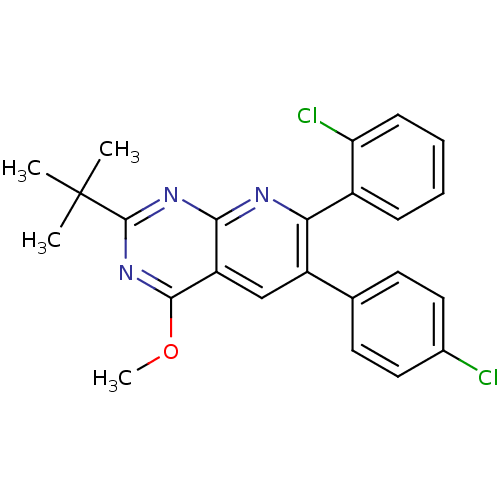
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES COc1nc(nc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C24H21Cl2N3O/c1-24(2,3)23-28-21-18(22(29-23)30-4)13-17(14-9-11-15(25)12-10-14)20(27-21)16-7-5-6-8-19(16)26/h5-13H,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259574
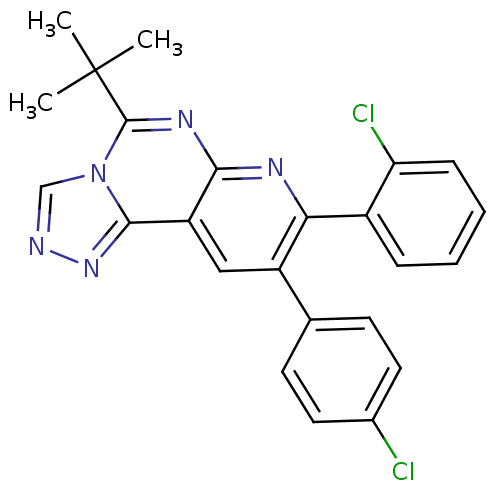
(5-tert-butyl-8-(2-chlorophenyl)-9-(4-chlorophenyl)...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3Cl)c(cc2c2nncn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19Cl2N5/c1-24(2,3)23-29-21-18(22-30-27-13-31(22)23)12-17(14-8-10-15(25)11-9-14)20(28-21)16-6-4-5-7-19(16)26/h4-13H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259272
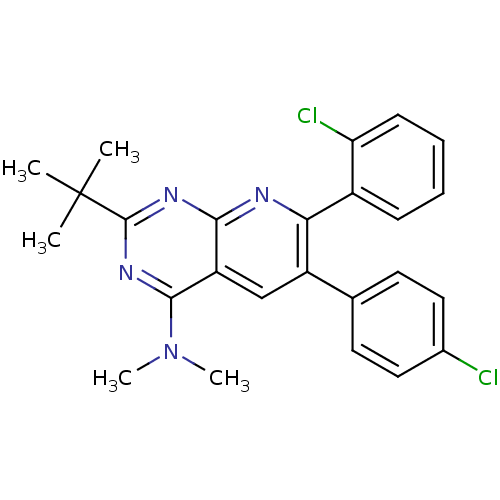
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CN(C)c1nc(nc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C25H24Cl2N4/c1-25(2,3)24-29-22-19(23(30-24)31(4)5)14-18(15-10-12-16(26)13-11-15)21(28-22)17-8-6-7-9-20(17)27/h6-14H,1-5H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259336
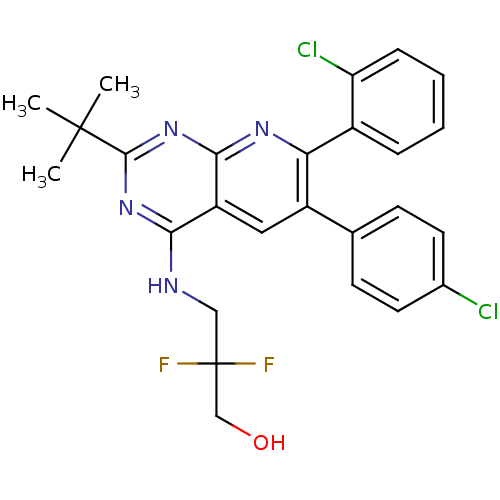
(3-(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophen...)Show SMILES CC(C)(C)c1nc(NCC(F)(F)CO)c2cc(-c3ccc(Cl)cc3)c(nc2n1)-c1ccccc1Cl Show InChI InChI=1S/C26H24Cl2F2N4O/c1-25(2,3)24-33-22(31-13-26(29,30)14-35)19-12-18(15-8-10-16(27)11-9-15)21(32-23(19)34-24)17-6-4-5-7-20(17)28/h4-12,35H,13-14H2,1-3H3,(H,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259493
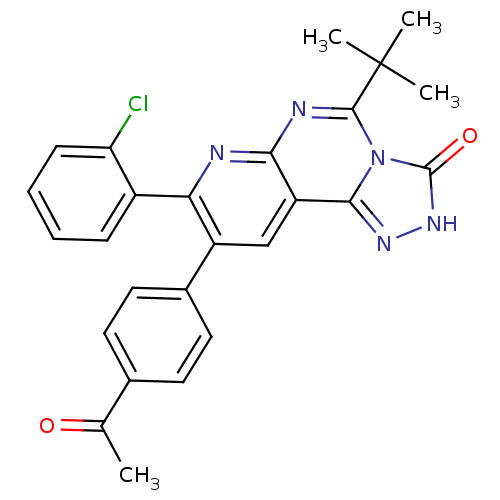
(9-(4-acetylphenyl)-5-tert-butyl-8-(2-chlorophenyl)...)Show SMILES CC(=O)c1ccc(cc1)-c1cc2c3n[nH]c(=O)n3c(nc2nc1-c1ccccc1Cl)C(C)(C)C Show InChI InChI=1S/C26H22ClN5O2/c1-14(33)15-9-11-16(12-10-15)18-13-19-22(28-21(18)17-7-5-6-8-20(17)27)29-24(26(2,3)4)32-23(19)30-31-25(32)34/h5-13H,1-4H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259182
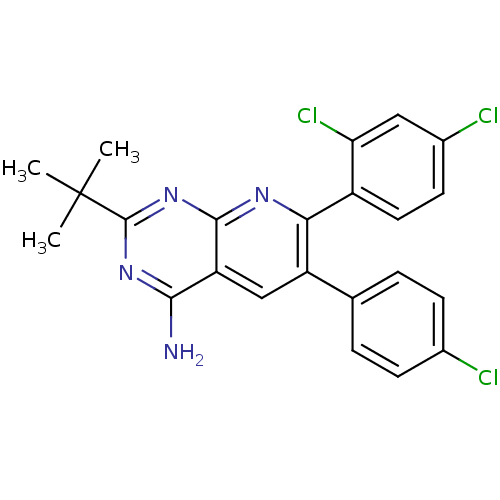
(2-tert-butyl-6-(4-chlorophenyl)-7-(2,4-dichlorophe...)Show SMILES CC(C)(C)c1nc(N)c2cc(-c3ccc(Cl)cc3)c(nc2n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H19Cl3N4/c1-23(2,3)22-29-20(27)17-11-16(12-4-6-13(24)7-5-12)19(28-21(17)30-22)15-9-8-14(25)10-18(15)26/h4-11H,1-3H3,(H2,27,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164777
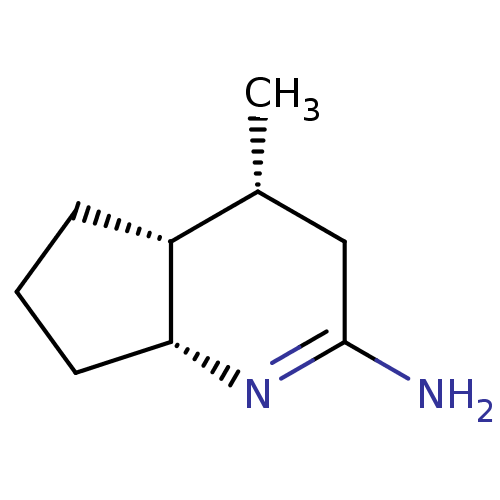
((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259184
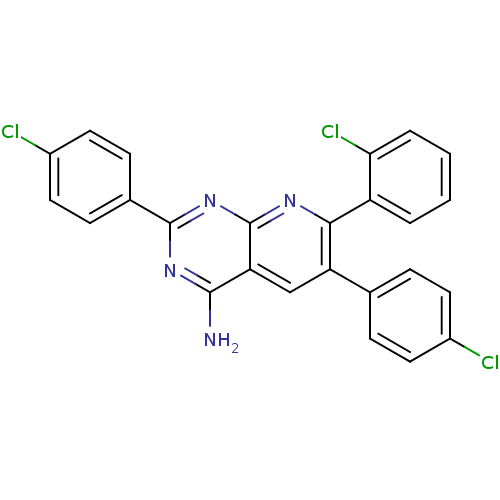
(7-(2-chlorophenyl)-2,6-bis(4-chlorophenyl)pyrido[2...)Show SMILES Nc1nc(nc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H15Cl3N4/c26-16-9-5-14(6-10-16)19-13-20-23(29)31-24(15-7-11-17(27)12-8-15)32-25(20)30-22(19)18-3-1-2-4-21(18)28/h1-13H,(H2,29,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062133
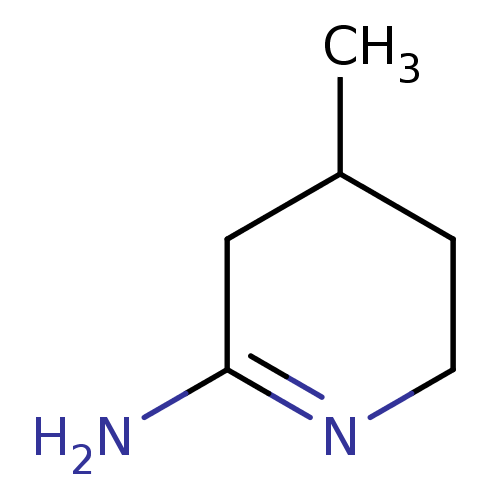
(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235499
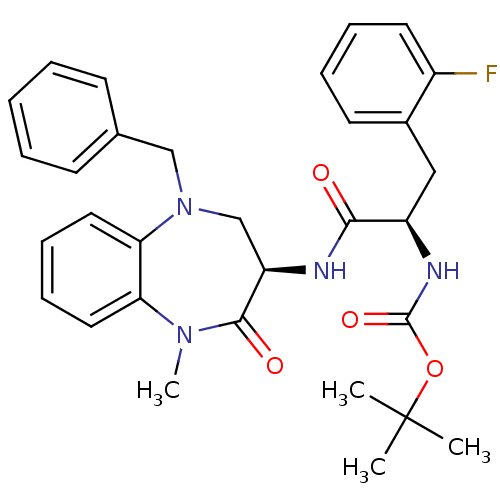
(CHEMBL438869 | tert-butyl (R)-1-((R)-5-benzyl-1-me...)Show SMILES CN1c2ccccc2N(Cc2ccccc2)C[C@@H](NC(=O)[C@@H](Cc2ccccc2F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C31H35FN4O4/c1-31(2,3)40-30(39)34-24(18-22-14-8-9-15-23(22)32)28(37)33-25-20-36(19-21-12-6-5-7-13-21)27-17-11-10-16-26(27)35(4)29(25)38/h5-17,24-25H,18-20H2,1-4H3,(H,33,37)(H,34,39)/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259494
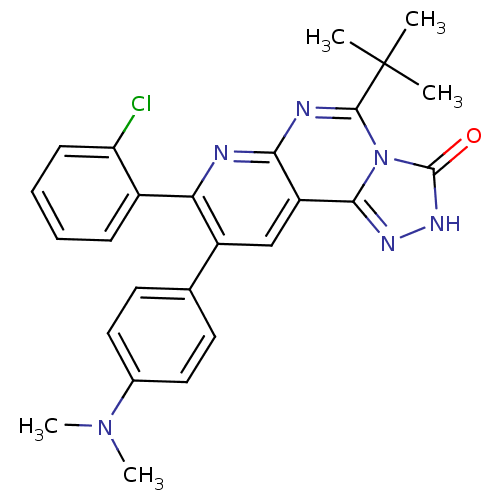
(5-tert-butyl-8-(2-chlorophenyl)-9-(4-(dimethylamin...)Show SMILES CN(C)c1ccc(cc1)-c1cc2c3n[nH]c(=O)n3c(nc2nc1-c1ccccc1Cl)C(C)(C)C Show InChI InChI=1S/C26H25ClN6O/c1-26(2,3)24-29-22-19(23-30-31-25(34)33(23)24)14-18(15-10-12-16(13-11-15)32(4)5)21(28-22)17-8-6-7-9-20(17)27/h6-14H,1-5H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235501

(CHEMBL254707 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CC(C)(C)OC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H]1CN(CC2CC2)c2ccccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C29H35F3N4O4/c1-28(2,3)40-27(39)34-21(15-19-9-5-4-6-10-19)25(37)33-22-17-35(16-20-13-14-20)23-11-7-8-12-24(23)36(26(22)38)18-29(30,31)32/h4-12,20-22H,13-18H2,1-3H3,(H,33,37)(H,34,39)/t21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259389
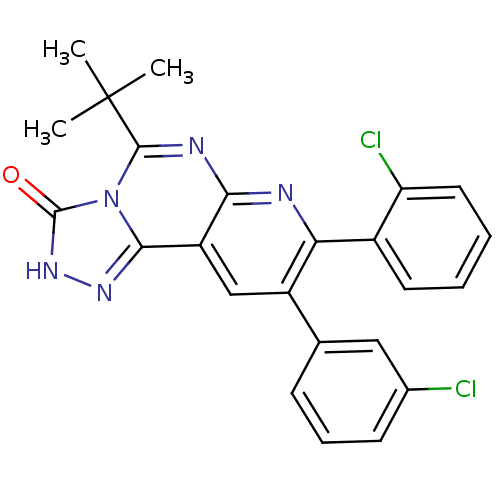
(5-tert-butyl-8-(2-chlorophenyl)-9-(3-chlorophenyl)...)Show SMILES CC(C)(C)c1nc2nc(-c3ccccc3Cl)c(cc2c2n[nH]c(=O)n12)-c1cccc(Cl)c1 Show InChI InChI=1S/C24H19Cl2N5O/c1-24(2,3)22-28-20-17(21-29-30-23(32)31(21)22)12-16(13-7-6-8-14(25)11-13)19(27-20)15-9-4-5-10-18(15)26/h4-12H,1-3H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50216671
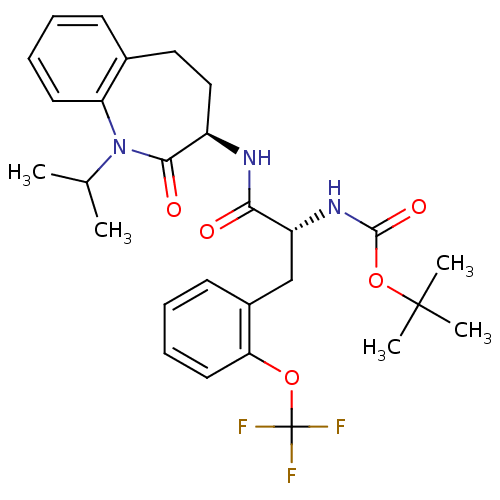
(CHEMBL247828 | tert-butyl (R)-1-((R)-1-isopropyl-2...)Show SMILES CC(C)N1c2ccccc2CC[C@@H](NC(=O)[C@@H](Cc2ccccc2OC(F)(F)F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C28H34F3N3O5/c1-17(2)34-22-12-8-6-10-18(22)14-15-20(25(34)36)32-24(35)21(33-26(37)39-27(3,4)5)16-19-11-7-9-13-23(19)38-28(29,30)31/h6-13,17,20-21H,14-16H2,1-5H3,(H,32,35)(H,33,37)/t20-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164784
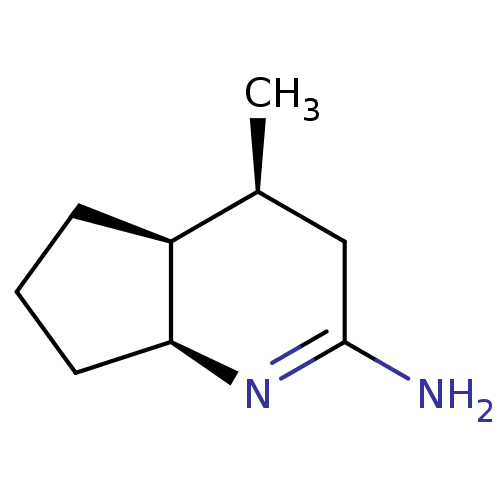
((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164779
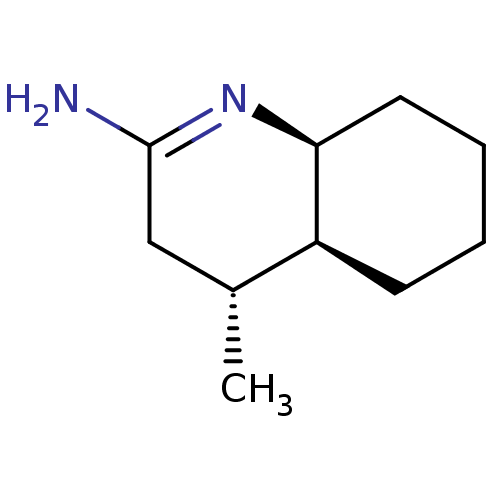
((4R,4aS,5S)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259181
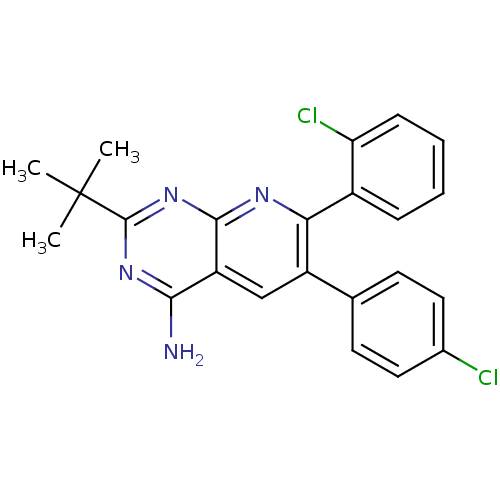
(2-tert-butyl-7-(2-chlorophenyl)-6-(4-chlorophenyl)...)Show SMILES CC(C)(C)c1nc(N)c2cc(-c3ccc(Cl)cc3)c(nc2n1)-c1ccccc1Cl Show InChI InChI=1S/C23H20Cl2N4/c1-23(2,3)22-28-20(26)17-12-16(13-8-10-14(24)11-9-13)19(27-21(17)29-22)15-6-4-5-7-18(15)25/h4-12H,1-3H3,(H2,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235517
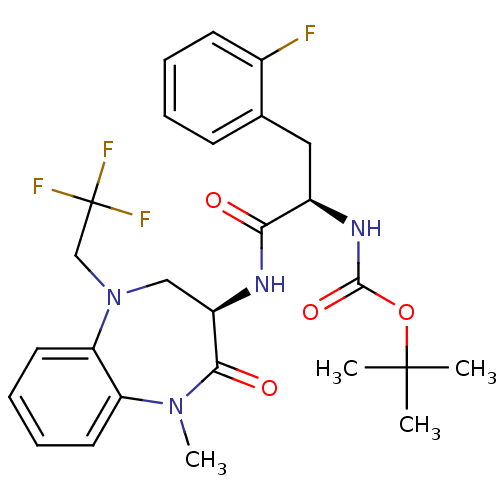
(CHEMBL254699 | tert-butyl (R)-3-(2-fluorophenyl)-1...)Show SMILES CN1c2ccccc2N(CC(F)(F)F)C[C@@H](NC(=O)[C@@H](Cc2ccccc2F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C26H30F4N4O4/c1-25(2,3)38-24(37)32-18(13-16-9-5-6-10-17(16)27)22(35)31-19-14-34(15-26(28,29)30)21-12-8-7-11-20(21)33(4)23(19)36/h5-12,18-19H,13-15H2,1-4H3,(H,31,35)(H,32,37)/t18-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235512
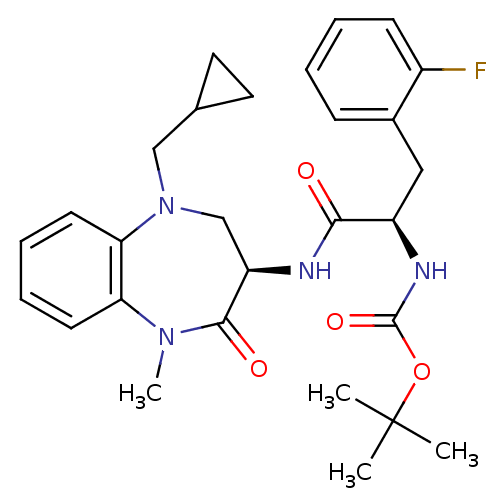
(CHEMBL401343 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CN1c2ccccc2N(CC2CC2)C[C@@H](NC(=O)[C@@H](Cc2ccccc2F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C28H35FN4O4/c1-28(2,3)37-27(36)31-21(15-19-9-5-6-10-20(19)29)25(34)30-22-17-33(16-18-13-14-18)24-12-8-7-11-23(24)32(4)26(22)35/h5-12,18,21-22H,13-17H2,1-4H3,(H,30,34)(H,31,36)/t21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235513
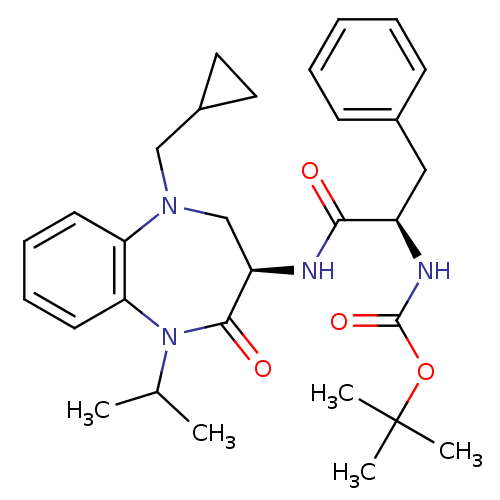
(CHEMBL399886 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CC(C)N1c2ccccc2N(CC2CC2)C[C@@H](NC(=O)[C@@H](Cc2ccccc2)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C30H40N4O4/c1-20(2)34-26-14-10-9-13-25(26)33(18-22-15-16-22)19-24(28(34)36)31-27(35)23(17-21-11-7-6-8-12-21)32-29(37)38-30(3,4)5/h6-14,20,22-24H,15-19H2,1-5H3,(H,31,35)(H,32,37)/t23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259183
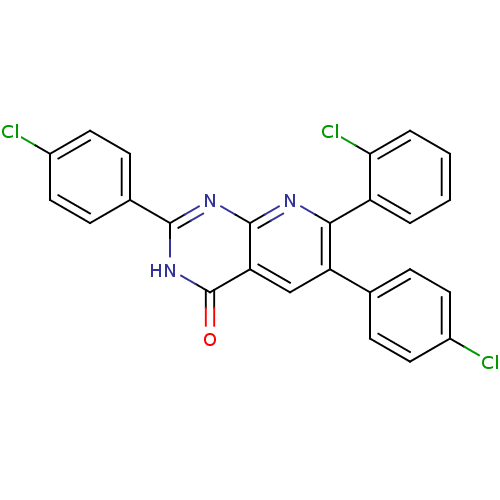
(7-(2-chlorophenyl)-2,6-bis(4-chlorophenyl)pyrido[2...)Show SMILES Clc1ccc(cc1)-c1cc2c(nc([nH]c2=O)-c2ccc(Cl)cc2)nc1-c1ccccc1Cl Show InChI InChI=1S/C25H14Cl3N3O/c26-16-9-5-14(6-10-16)19-13-20-24(29-22(19)18-3-1-2-4-21(18)28)30-23(31-25(20)32)15-7-11-17(27)12-8-15/h1-13H,(H,29,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235511
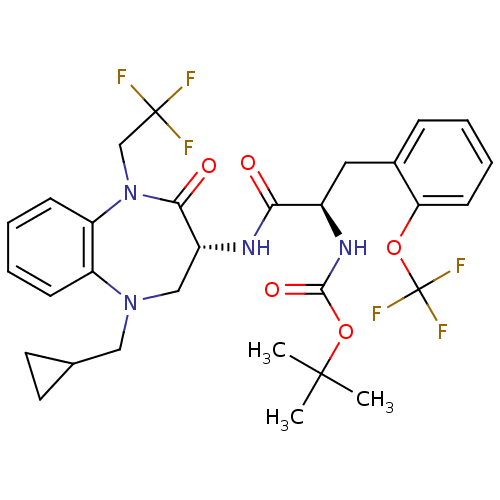
(CHEMBL253819 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CC(C)(C)OC(=O)N[C@H](Cc1ccccc1OC(F)(F)F)C(=O)N[C@@H]1CN(CC2CC2)c2ccccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C30H34F6N4O5/c1-28(2,3)45-27(43)38-20(14-19-8-4-7-11-24(19)44-30(34,35)36)25(41)37-21-16-39(15-18-12-13-18)22-9-5-6-10-23(22)40(26(21)42)17-29(31,32)33/h4-11,18,20-21H,12-17H2,1-3H3,(H,37,41)(H,38,43)/t20-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235510
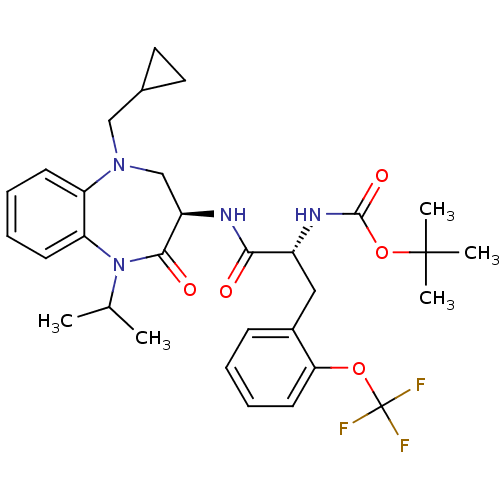
(CHEMBL254928 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CC(C)N1c2ccccc2N(CC2CC2)C[C@@H](NC(=O)[C@@H](Cc2ccccc2OC(F)(F)F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C31H39F3N4O5/c1-19(2)38-25-12-8-7-11-24(25)37(17-20-14-15-20)18-23(28(38)40)35-27(39)22(36-29(41)43-30(3,4)5)16-21-10-6-9-13-26(21)42-31(32,33)34/h6-13,19-20,22-23H,14-18H2,1-5H3,(H,35,39)(H,36,41)/t22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235498

(CHEMBL253877 | tert-butyl (R)-1-((R)-5-(cyclobutyl...)Show SMILES CN1c2ccccc2N(CC2CCC2)C[C@@H](NC(=O)[C@@H](Cc2ccccc2F)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C29H37FN4O4/c1-29(2,3)38-28(37)32-22(16-20-12-5-6-13-21(20)30)26(35)31-23-18-34(17-19-10-9-11-19)25-15-8-7-14-24(25)33(4)27(23)36/h5-8,12-15,19,22-23H,9-11,16-18H2,1-4H3,(H,31,35)(H,32,37)/t22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50259338
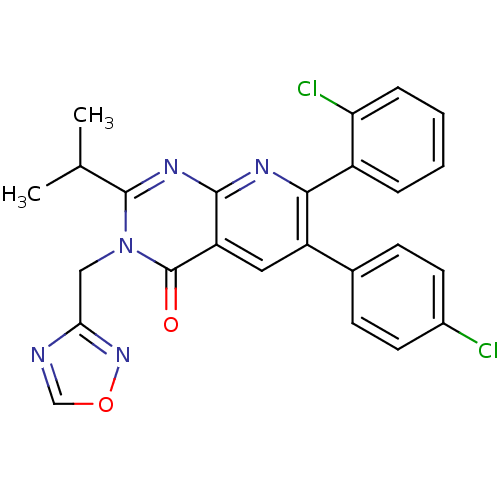
(3-((1,2,4-oxadiazol-3-yl)methyl)-7-(2-chlorophenyl...)Show SMILES CC(C)c1nc2nc(-c3ccccc3Cl)c(cc2c(=O)n1Cc1ncon1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H19Cl2N5O2/c1-14(2)24-30-23-19(25(33)32(24)12-21-28-13-34-31-21)11-18(15-7-9-16(26)10-8-15)22(29-23)17-5-3-4-6-20(17)27/h3-11,13-14H,12H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1R expressed in CHO cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50235506
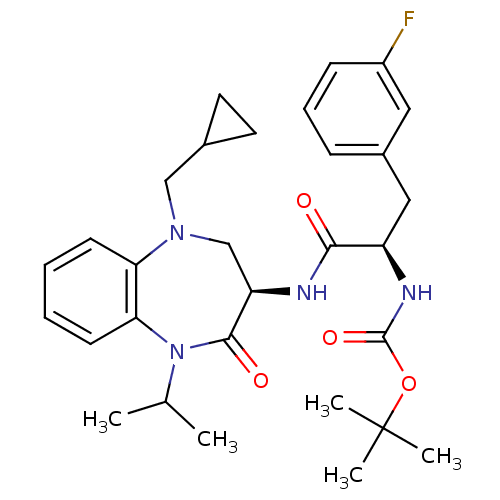
(CHEMBL252783 | tert-butyl (R)-1-((R)-5-(cyclopropy...)Show SMILES CC(C)N1c2ccccc2N(CC2CC2)C[C@@H](NC(=O)[C@@H](Cc2cccc(F)c2)NC(=O)OC(C)(C)C)C1=O Show InChI InChI=1S/C30H39FN4O4/c1-19(2)35-26-12-7-6-11-25(26)34(17-20-13-14-20)18-24(28(35)37)32-27(36)23(33-29(38)39-30(3,4)5)16-21-9-8-10-22(31)15-21/h6-12,15,19-20,23-24H,13-14,16-18H2,1-5H3,(H,32,36)(H,33,38)/t23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 sodium channel expressed in HEK293 cells by FRET assay |
Bioorg Med Chem Lett 18: 1963-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.123
BindingDB Entry DOI: 10.7270/Q24T6J4T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data