 Found 609 hits with Last Name = 'evindar' and Initial = 'g'
Found 609 hits with Last Name = 'evindar' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099331
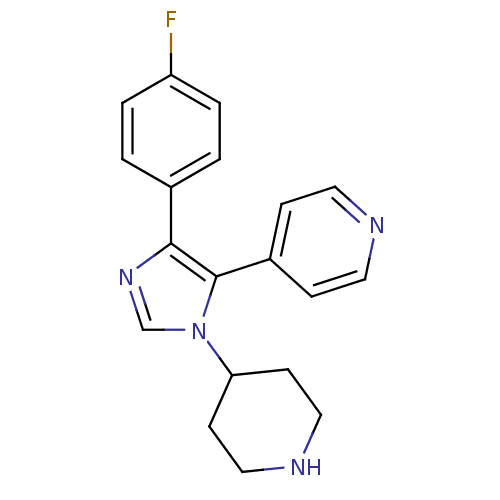
(4-(4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...)Show InChI InChI=1S/C19H19FN4/c20-16-3-1-14(2-4-16)18-19(15-5-9-21-10-6-15)24(13-23-18)17-7-11-22-12-8-17/h1-6,9-10,13,17,22H,7-8,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha kinase |
ACS Med Chem Lett 2: 758-763 (2011)
Article DOI: 10.1021/ml2001455
BindingDB Entry DOI: 10.7270/Q26D5V19 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM15244
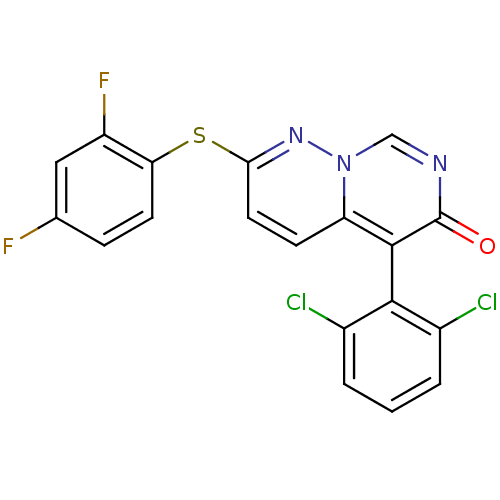
(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p38beta using KRELVEPLTPSGEAPNQALLR as substrate for 10 mins by lactate dehydrogenase-coupled spectrophotometric assay |
ACS Med Chem Lett 2: 758-763 (2011)
Article DOI: 10.1021/ml2001455
BindingDB Entry DOI: 10.7270/Q26D5V19 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277149
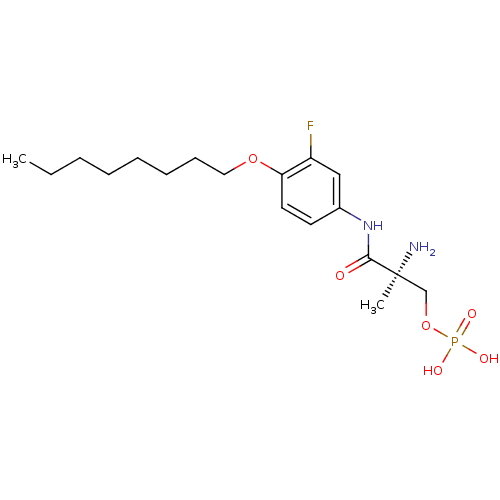
((S)-2-amino-3-(3-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc1F |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-16-10-9-14(12-15(16)19)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249294

((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H30N3O5P/c1-23(24,17-31-32(27,28)29)22-25-16-21(26-22)19-11-13-20(14-12-19)30-15-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-14,16H,3,6-7,10,15,17,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249114
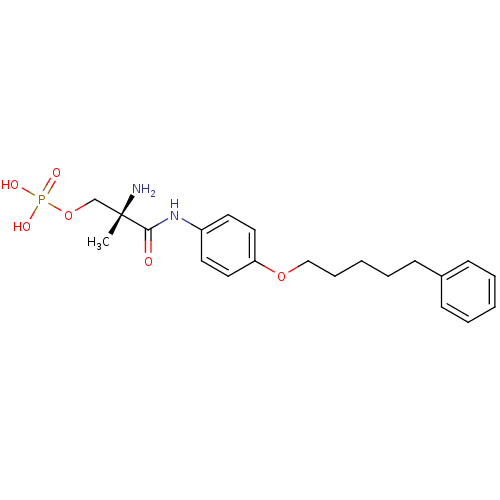
((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315562
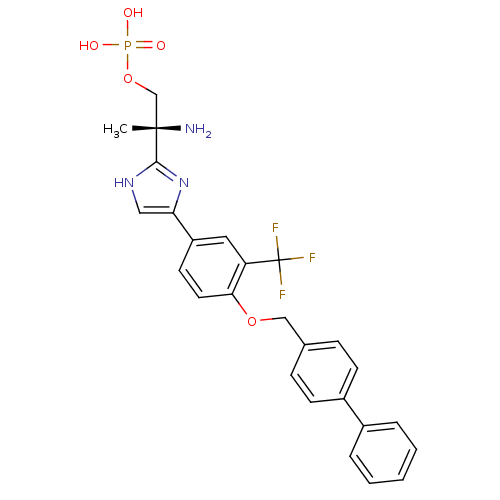
((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H25F3N3O5P/c1-25(30,16-37-38(33,34)35)24-31-14-22(32-24)20-11-12-23(21(13-20)26(27,28)29)36-15-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-14H,15-16,30H2,1H3,(H,31,32)(H2,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315559
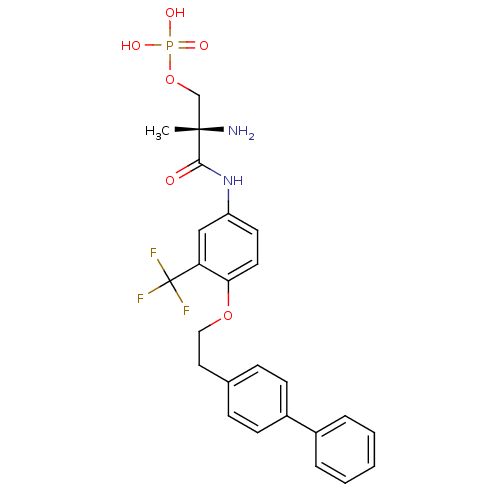
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C25H26F3N2O6P/c1-24(29,16-36-37(32,33)34)23(31)30-20-11-12-22(21(15-20)25(26,27)28)35-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,29H2,1H3,(H,30,31)(H2,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277186
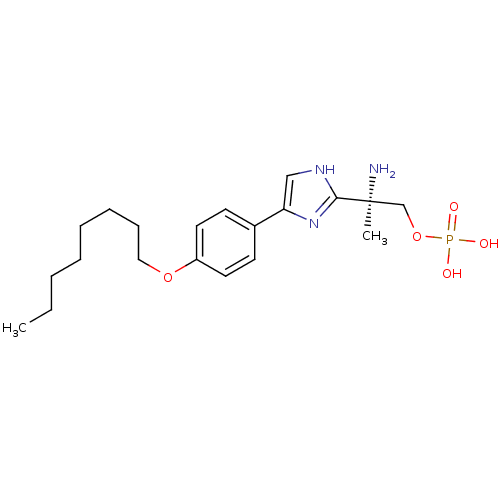
((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277185
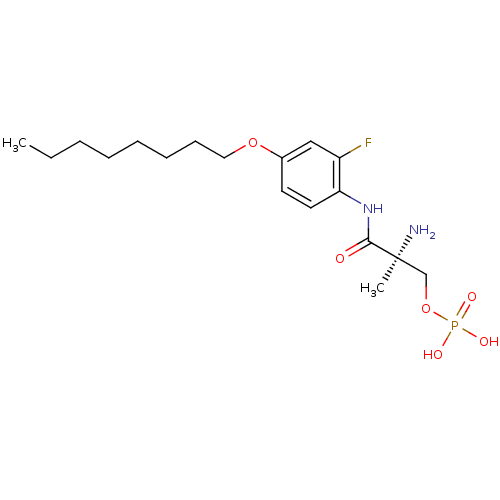
((S)-2-amino-3-(2-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)c(F)c1 |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-14-9-10-16(15(19)12-14)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277187
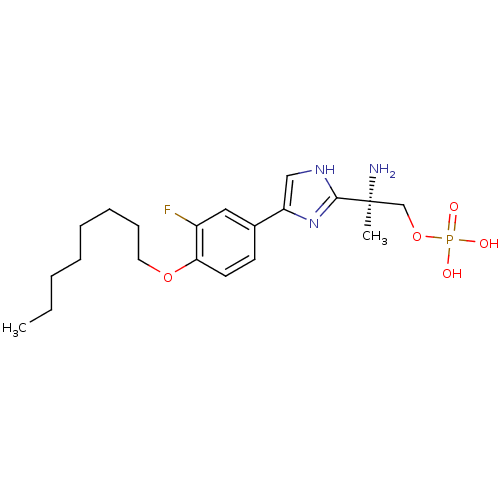
((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50249114

((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277148
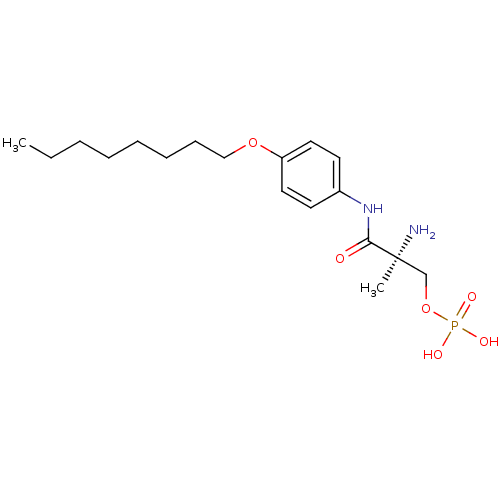
((S)-2-amino-2-methyl-3-(4-(octyloxy)phenylamino)-3...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C18H31N2O6P/c1-3-4-5-6-7-8-13-25-16-11-9-15(10-12-16)20-17(21)18(2,19)14-26-27(22,23)24/h9-12H,3-8,13-14,19H2,1-2H3,(H,20,21)(H2,22,23,24)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249259
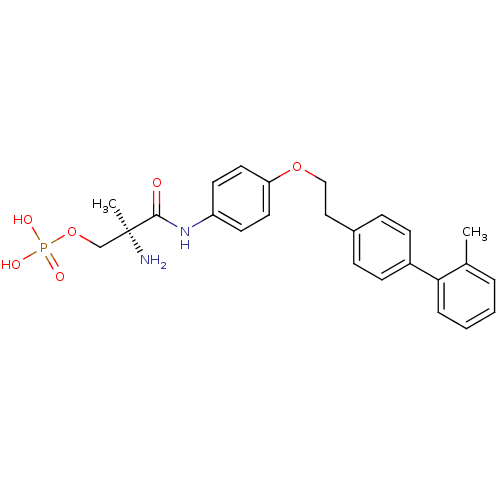
((S)-2-amino-2-methyl-3-(4-(2-(2'-methylbiphenyl-4-...)Show SMILES Cc1ccccc1-c1ccc(CCOc2ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc2)cc1 |r| Show InChI InChI=1S/C25H29N2O6P/c1-18-5-3-4-6-23(18)20-9-7-19(8-10-20)15-16-32-22-13-11-21(12-14-22)27-24(28)25(2,26)17-33-34(29,30)31/h3-14H,15-17,26H2,1-2H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315556
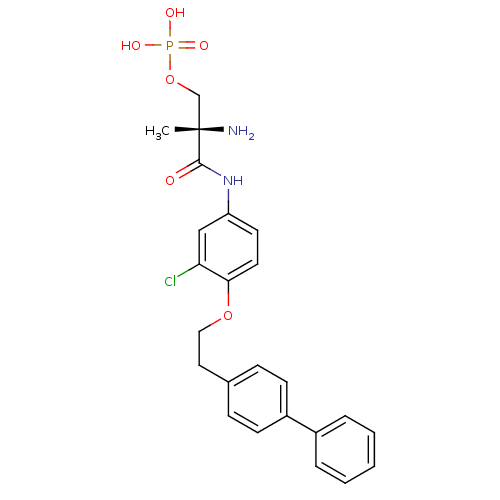
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-chlor...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C24H26ClN2O6P/c1-24(26,16-33-34(29,30)31)23(28)27-20-11-12-22(21(25)15-20)32-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,26H2,1H3,(H,27,28)(H2,29,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249260
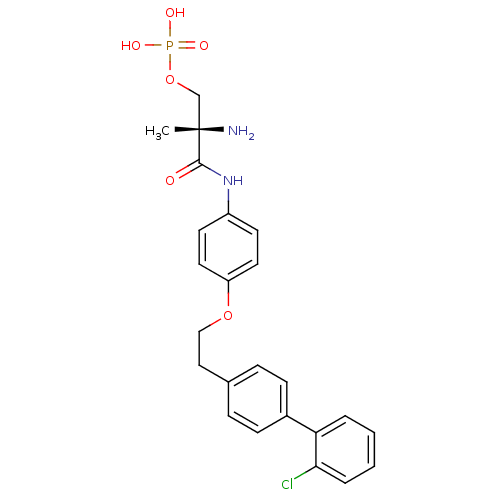
((S)-2-amino-3-(4-(2-(2'-chlorobiphenyl-4-yl)ethoxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2Cl)cc1 |r| Show InChI InChI=1S/C24H26ClN2O6P/c1-24(26,16-33-34(29,30)31)23(28)27-19-10-12-20(13-11-19)32-15-14-17-6-8-18(9-7-17)21-4-2-3-5-22(21)25/h2-13H,14-16,26H2,1H3,(H,27,28)(H2,29,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249263
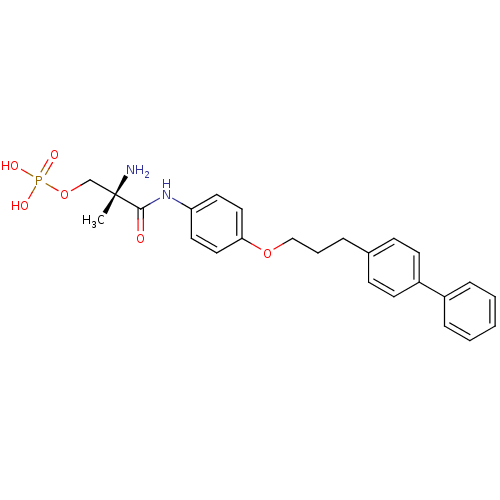
((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propoxy)phenyla...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H29N2O6P/c1-25(26,18-33-34(29,30)31)24(28)27-22-13-15-23(16-14-22)32-17-5-6-19-9-11-21(12-10-19)20-7-3-2-4-8-20/h2-4,7-16H,5-6,17-18,26H2,1H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249293
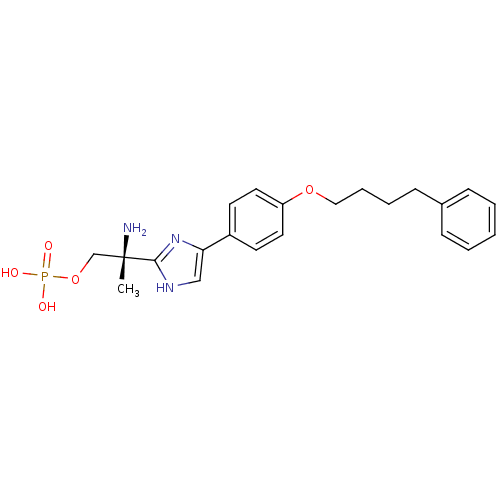
((R)-2-amino-2-(4-(4-(4-phenylbutoxy)phenyl)-1H-imi...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H28N3O5P/c1-22(23,16-30-31(26,27)28)21-24-15-20(25-21)18-10-12-19(13-11-18)29-14-6-5-9-17-7-3-2-4-8-17/h2-4,7-8,10-13,15H,5-6,9,14,16,23H2,1H3,(H,24,25)(H2,26,27,28)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249266
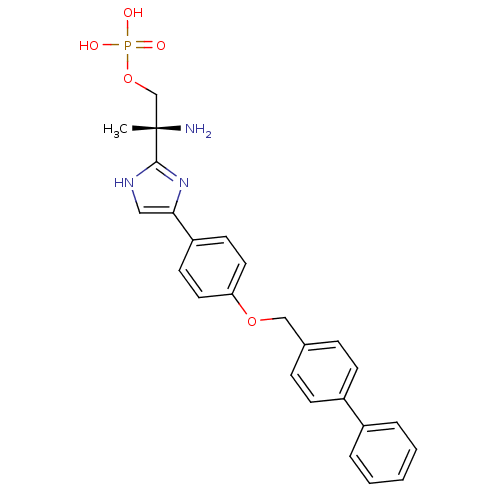
((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H26N3O5P/c1-25(26,17-33-34(29,30)31)24-27-15-23(28-24)21-11-13-22(14-12-21)32-16-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-15H,16-17,26H2,1H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249266

((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H26N3O5P/c1-25(26,17-33-34(29,30)31)24-27-15-23(28-24)21-11-13-22(14-12-21)32-16-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-15H,16-17,26H2,1H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249240
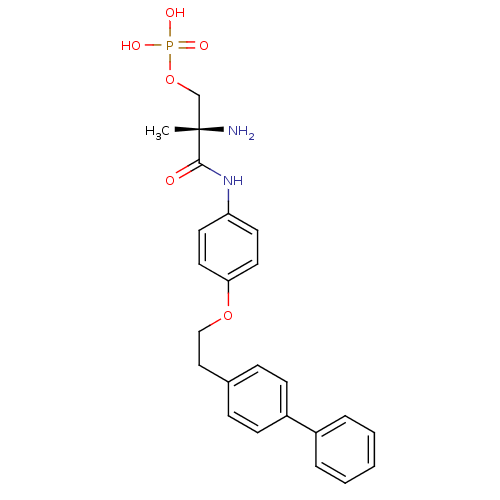
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C24H27N2O6P/c1-24(25,17-32-33(28,29)30)23(27)26-21-11-13-22(14-12-21)31-16-15-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-14H,15-17,25H2,1H3,(H,26,27)(H2,28,29,30)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249240

((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C24H27N2O6P/c1-24(25,17-32-33(28,29)30)23(27)26-21-11-13-22(14-12-21)31-16-15-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-14H,15-17,25H2,1H3,(H,26,27)(H2,28,29,30)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50431093
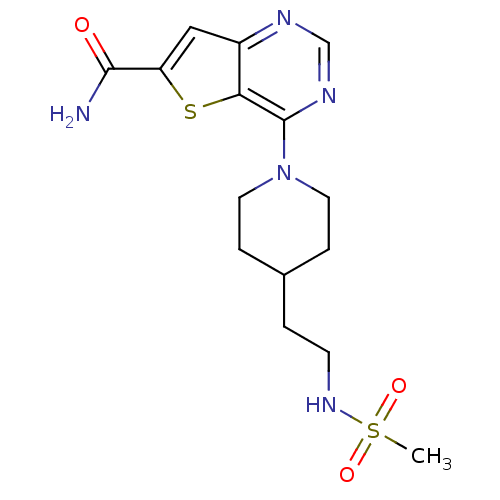
(CHEMBL2338810)Show SMILES CS(=O)(=O)NCCC1CCN(CC1)c1ncnc2cc(sc12)C(N)=O Show InChI InChI=1S/C15H21N5O3S2/c1-25(22,23)19-5-2-10-3-6-20(7-4-10)15-13-11(17-9-18-15)8-12(24-13)14(16)21/h8-10,19H,2-7H2,1H3,(H2,16,21) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... |
J Med Chem 56: 3666-79 (2013)
Article DOI: 10.1021/jm400204k
BindingDB Entry DOI: 10.7270/Q2D50P9C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50277186

((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50277187

((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249153

((S)-2-amino-3-(4-(4-cyclohexylbutoxy)phenylamino)-...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCC2CCCCC2)cc1 |r| Show InChI InChI=1S/C20H33N2O6P/c1-20(21,15-28-29(24,25)26)19(23)22-17-10-12-18(13-11-17)27-14-6-5-9-16-7-3-2-4-8-16/h10-13,16H,2-9,14-15,21H2,1H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50249294

((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H30N3O5P/c1-23(24,17-31-32(27,28)29)22-25-16-21(26-22)19-11-13-20(14-12-19)30-15-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-14,16H,3,6-7,10,15,17,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50431121
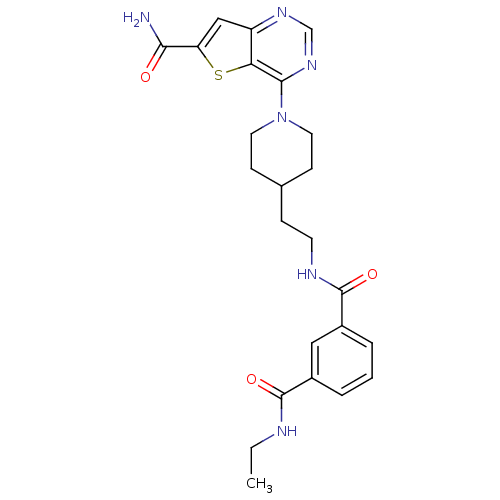
(CHEMBL2332037)Show SMILES CCNC(=O)c1cccc(c1)C(=O)NCCC1CCN(CC1)c1ncnc2cc(sc12)C(N)=O Show InChI InChI=1S/C24H28N6O3S/c1-2-26-23(32)16-4-3-5-17(12-16)24(33)27-9-6-15-7-10-30(11-8-15)22-20-18(28-14-29-22)13-19(34-20)21(25)31/h3-5,12-15H,2,6-11H2,1H3,(H2,25,31)(H,26,32)(H,27,33) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... |
J Med Chem 56: 3666-79 (2013)
Article DOI: 10.1021/jm400204k
BindingDB Entry DOI: 10.7270/Q2D50P9C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315557
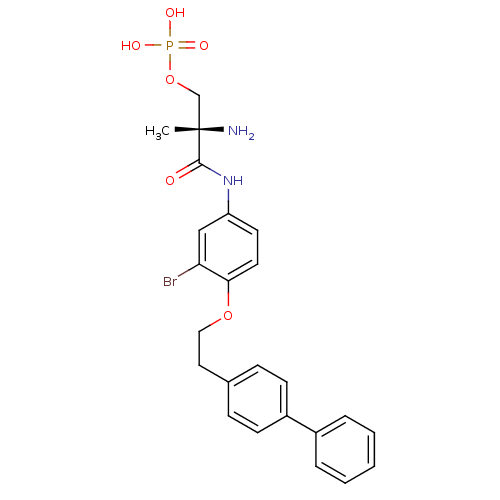
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-bromo...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(Br)c1 |r| Show InChI InChI=1S/C24H26BrN2O6P/c1-24(26,16-33-34(29,30)31)23(28)27-20-11-12-22(21(25)15-20)32-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,26H2,1H3,(H,27,28)(H2,29,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50277186

((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50277185

((S)-2-amino-3-(2-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)c(F)c1 |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-14-9-10-16(15(19)12-14)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50431118
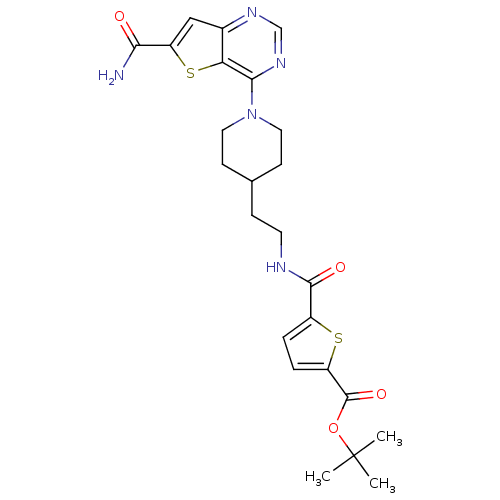
(CHEMBL2332041)Show SMILES CC(C)(C)OC(=O)c1ccc(s1)C(=O)NCCC1CCN(CC1)c1ncnc2cc(sc12)C(N)=O Show InChI InChI=1S/C24H29N5O4S2/c1-24(2,3)33-23(32)17-5-4-16(34-17)22(31)26-9-6-14-7-10-29(11-8-14)21-19-15(27-13-28-21)12-18(35-19)20(25)30/h4-5,12-14H,6-11H2,1-3H3,(H2,25,30)(H,26,31) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... |
J Med Chem 56: 3666-79 (2013)
Article DOI: 10.1021/jm400204k
BindingDB Entry DOI: 10.7270/Q2D50P9C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50277187

((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448871

(CHEMBL3125101)Show SMILES CCc1cc(C(=O)N[C@@H]2C[C@H](N(C2)C(=O)c2coc3ccccc23)C(=O)NCc2ccc3COB(O)c3c2)n(CC)n1 |r| Show InChI InChI=1S/C30H32BN5O6/c1-3-20-12-26(36(4-2)34-20)29(38)33-21-13-25(28(37)32-14-18-9-10-19-16-42-31(40)24(19)11-18)35(15-21)30(39)23-17-41-27-8-6-5-7-22(23)27/h5-12,17,21,25,40H,3-4,13-16H2,1-2H3,(H,32,37)(H,33,38)/t21-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... |
J Med Chem 57: 1276-88 (2014)
Article DOI: 10.1021/jm401326j
BindingDB Entry DOI: 10.7270/Q2NP25XB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091691
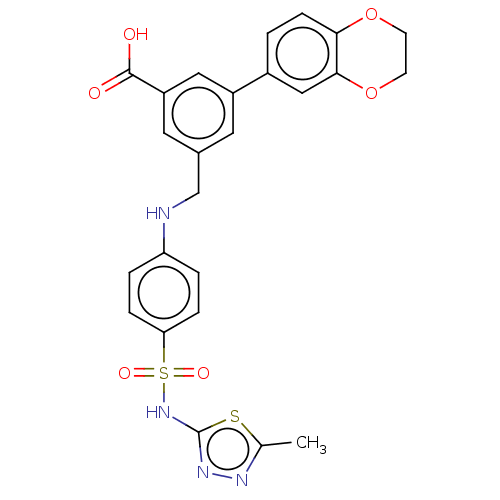
(CHEMBL3582356)Show SMILES Cc1nnc(NS(=O)(=O)c2ccc(NCc3cc(cc(c3)-c3ccc4OCCOc4c3)C(O)=O)cc2)s1 Show InChI InChI=1S/C25H22N4O6S2/c1-15-27-28-25(36-15)29-37(32,33)21-5-3-20(4-6-21)26-14-16-10-18(12-19(11-16)24(30)31)17-2-7-22-23(13-17)35-9-8-34-22/h2-7,10-13,26H,8-9,14H2,1H3,(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448867

(CHEMBL3125235)Show SMILES CCc1cc(C(=O)N[C@@H]2C[C@H](N(C2)C(=O)c2coc3ccccc23)C(=O)NCc2ccccn2)n(CC)n1 |r| Show InChI InChI=1S/C28H30N6O4/c1-3-18-13-24(34(4-2)32-18)27(36)31-20-14-23(26(35)30-15-19-9-7-8-12-29-19)33(16-20)28(37)22-17-38-25-11-6-5-10-21(22)25/h5-13,17,20,23H,3-4,14-16H2,1-2H3,(H,30,35)(H,31,36)/t20-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... |
J Med Chem 57: 1276-88 (2014)
Article DOI: 10.1021/jm401326j
BindingDB Entry DOI: 10.7270/Q2NP25XB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249113
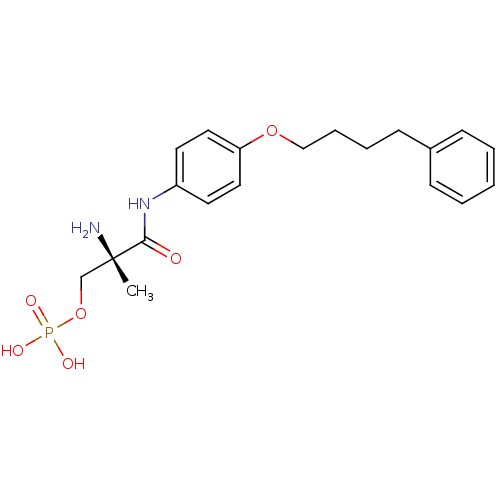
((S)-2-amino-2-methyl-3-oxo-3-(4-(4-phenylbutoxy)ph...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C20H27N2O6P/c1-20(21,15-28-29(24,25)26)19(23)22-17-10-12-18(13-11-17)27-14-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,10-13H,5-6,9,14-15,21H2,1H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514606
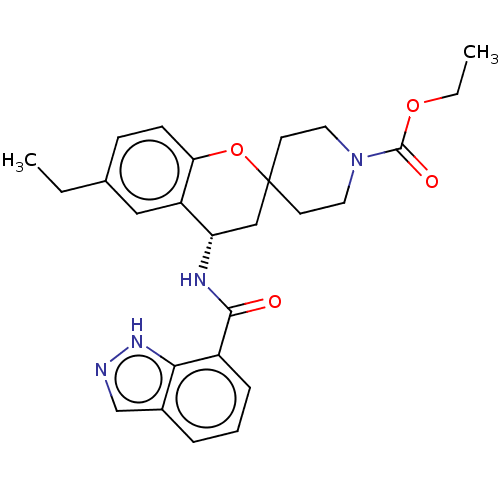
(CHEMBL4517539)Show SMILES CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(CC)ccc1O2 |r| Show InChI InChI=1S/C26H30N4O4/c1-3-17-8-9-22-20(14-17)21(28-24(31)19-7-5-6-18-16-27-29-23(18)19)15-26(34-22)10-12-30(13-11-26)25(32)33-4-2/h5-9,14,16,21H,3-4,10-13,15H2,1-2H3,(H,27,29)(H,28,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay |
J Med Chem 63: 3552-3562 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01799
BindingDB Entry DOI: 10.7270/Q2M33045 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249154
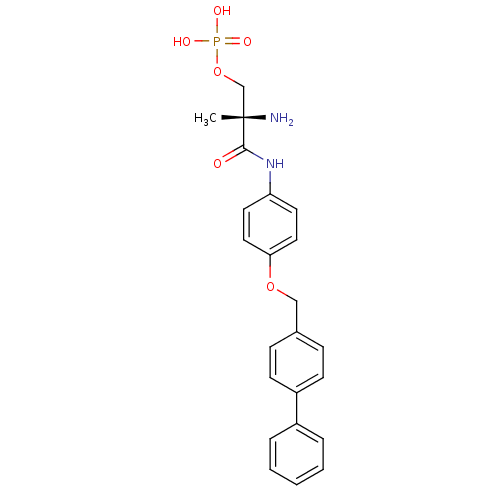
((S)-2-amino-3-(4-(biphenyl-4-ylmethoxy)phenylamino...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C23H25N2O6P/c1-23(24,16-31-32(27,28)29)22(26)25-20-11-13-21(14-12-20)30-15-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-14H,15-16,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091689
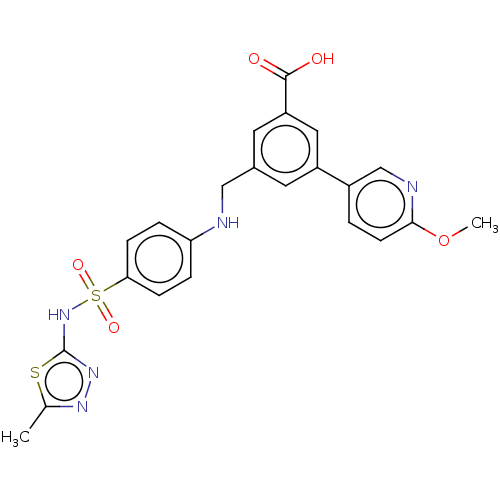
(CHEMBL3582354)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(O)=O Show InChI InChI=1S/C23H21N5O5S2/c1-14-26-27-23(34-14)28-35(31,32)20-6-4-19(5-7-20)24-12-15-9-17(11-18(10-15)22(29)30)16-3-8-21(33-2)25-13-16/h3-11,13,24H,12H2,1-2H3,(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50431097
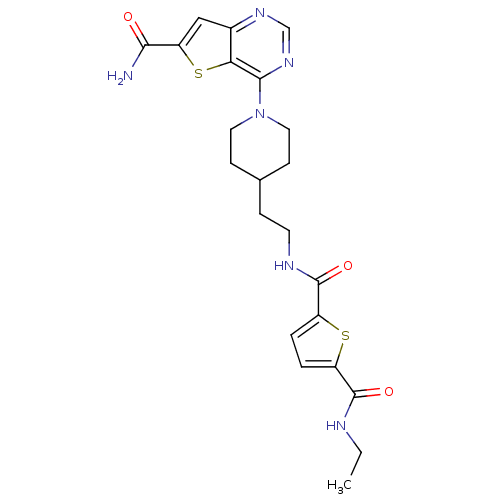
(CHEMBL2332039)Show SMILES CCNC(=O)c1ccc(s1)C(=O)NCCC1CCN(CC1)c1ncnc2cc(sc12)C(N)=O Show InChI InChI=1S/C22H26N6O3S2/c1-2-24-21(30)15-3-4-16(32-15)22(31)25-8-5-13-6-9-28(10-7-13)20-18-14(26-12-27-20)11-17(33-18)19(23)29/h3-4,11-13H,2,5-10H2,1H3,(H2,23,29)(H,24,30)(H,25,31) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... |
J Med Chem 56: 3666-79 (2013)
Article DOI: 10.1021/jm400204k
BindingDB Entry DOI: 10.7270/Q2D50P9C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50249114

((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448875

(CHEMBL3125266)Show SMILES CCc1cc(C(=O)N[C@@H]2C[C@H](N(C2)C(=O)c2coc3ccccc23)C(=O)NCC(=O)OC)n(CC)n1 |r| Show InChI InChI=1S/C25H29N5O6/c1-4-15-10-20(30(5-2)28-15)24(33)27-16-11-19(23(32)26-12-22(31)35-3)29(13-16)25(34)18-14-36-21-9-7-6-8-17(18)21/h6-10,14,16,19H,4-5,11-13H2,1-3H3,(H,26,32)(H,27,33)/t16-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... |
J Med Chem 57: 1276-88 (2014)
Article DOI: 10.1021/jm401326j
BindingDB Entry DOI: 10.7270/Q2NP25XB |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448868

(CHEMBL3125104)Show SMILES CCc1cc(C(=O)N[C@@H]2C[C@H](N(C2)C(=O)c2coc3ccccc23)C(=O)NCCO)n(CC)n1 |r| Show InChI InChI=1S/C24H29N5O5/c1-3-15-11-20(29(4-2)27-15)23(32)26-16-12-19(22(31)25-9-10-30)28(13-16)24(33)18-14-34-21-8-6-5-7-17(18)21/h5-8,11,14,16,19,30H,3-4,9-10,12-13H2,1-2H3,(H,25,31)(H,26,32)/t16-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... |
J Med Chem 57: 1276-88 (2014)
Article DOI: 10.1021/jm401326j
BindingDB Entry DOI: 10.7270/Q2NP25XB |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448888

(CHEMBL3125253)Show SMILES CCc1cc(C(=O)N[C@@H]2C[C@H](N(C2)C(=O)c2coc3ccccc23)C(=O)Nc2cnn(CC)n2)n(C)n1 |r| Show InChI InChI=1S/C25H28N8O4/c1-4-15-10-19(31(3)29-15)23(34)27-16-11-20(24(35)28-22-12-26-33(5-2)30-22)32(13-16)25(36)18-14-37-21-9-7-6-8-17(18)21/h6-10,12,14,16,20H,4-5,11,13H2,1-3H3,(H,27,34)(H,28,30,35)/t16-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... |
J Med Chem 57: 1276-88 (2014)
Article DOI: 10.1021/jm401326j
BindingDB Entry DOI: 10.7270/Q2NP25XB |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448880
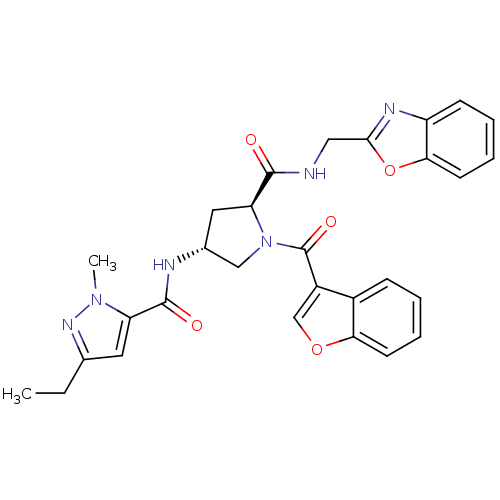
(CHEMBL3125261)Show SMILES CCc1cc(C(=O)N[C@@H]2C[C@H](N(C2)C(=O)c2coc3ccccc23)C(=O)NCc2nc3ccccc3o2)n(C)n1 |r| Show InChI InChI=1S/C29H28N6O5/c1-3-17-12-22(34(2)33-17)28(37)31-18-13-23(27(36)30-14-26-32-21-9-5-7-11-25(21)40-26)35(15-18)29(38)20-16-39-24-10-6-4-8-19(20)24/h4-12,16,18,23H,3,13-15H2,1-2H3,(H,30,36)(H,31,37)/t18-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... |
J Med Chem 57: 1276-88 (2014)
Article DOI: 10.1021/jm401326j
BindingDB Entry DOI: 10.7270/Q2NP25XB |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-3, mitochondrial
(Homo sapiens (Human)) | BDBM50431118

(CHEMBL2332041)Show SMILES CC(C)(C)OC(=O)c1ccc(s1)C(=O)NCCC1CCN(CC1)c1ncnc2cc(sc12)C(N)=O Show InChI InChI=1S/C24H29N5O4S2/c1-24(2,3)33-23(32)17-5-4-16(34-17)22(31)26-9-6-14-7-10-29(11-8-14)21-19-15(27-13-28-21)12-18(35-19)20(25)30/h4-5,12-14H,6-11H2,1-3H3,(H2,25,30)(H,26,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged SIRT3 (102 to 399) expressed in Escherichia coli BL21(DE3) assessed as inhibition of deacetylation of Ac-RHKKAcW-NH2 s... |
J Med Chem 56: 3666-79 (2013)
Article DOI: 10.1021/jm400204k
BindingDB Entry DOI: 10.7270/Q2D50P9C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data