 Found 187 hits with Last Name = 'foster' and Initial = 'l'
Found 187 hits with Last Name = 'foster' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50354849
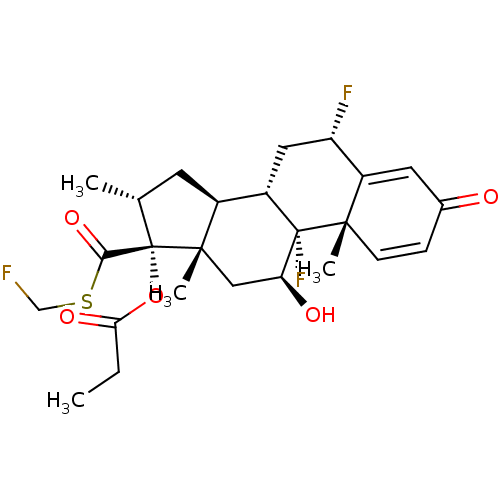
(CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCF |r,c:18,t:14| Show InChI InChI=1S/C25H31F3O5S/c1-5-20(31)33-25(21(32)34-12-26)13(2)8-15-16-10-18(27)17-9-14(29)6-7-22(17,3)24(16,28)19(30)11-23(15,25)4/h6-7,9,13,15-16,18-19,30H,5,8,10-12H2,1-4H3/t13-,15+,16+,18+,19+,22+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 568-74 (2005)
Article DOI: 10.1124/jpet.105.085217
BindingDB Entry DOI: 10.7270/Q2CC0Z7J |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM86696
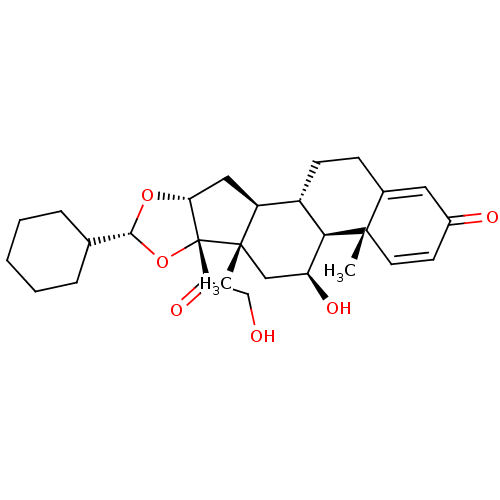
(des-CIC | desisobutyryl-ciclesonide)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=CC(=O)C=C[C@]34C)[C@@H]1C[C@H]1O[C@H](O[C@@]21C(=O)CO)C1CCCCC1 |c:13,t:9| Show InChI InChI=1S/C28H38O6/c1-26-11-10-18(30)12-17(26)8-9-19-20-13-23-28(22(32)15-29,27(20,2)14-21(31)24(19)26)34-25(33-23)16-6-4-3-5-7-16/h10-12,16,19-21,23-25,29,31H,3-9,13-15H2,1-2H3/t19-,20-,21-,23+,24+,25+,26-,27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 568-74 (2005)
Article DOI: 10.1124/jpet.105.085217
BindingDB Entry DOI: 10.7270/Q2CC0Z7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 568-74 (2005)
Article DOI: 10.1124/jpet.105.085217
BindingDB Entry DOI: 10.7270/Q2CC0Z7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359779
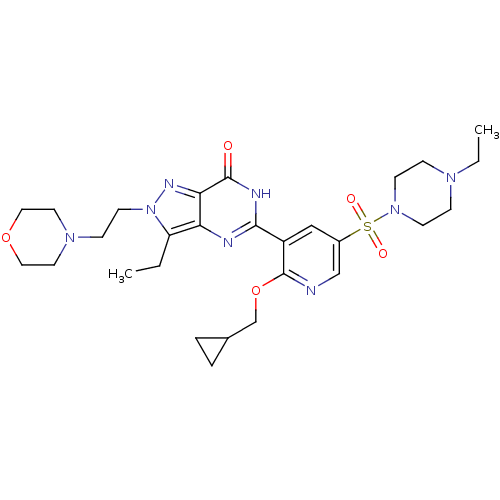
(CHEMBL1928271)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC2CC2)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C28H40N8O5S/c1-3-23-24-25(32-36(23)12-9-34-13-15-40-16-14-34)27(37)31-26(30-24)22-17-21(18-29-28(22)41-19-20-5-6-20)42(38,39)35-10-7-33(4-2)8-11-35/h17-18,20H,3-16,19H2,1-2H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359773
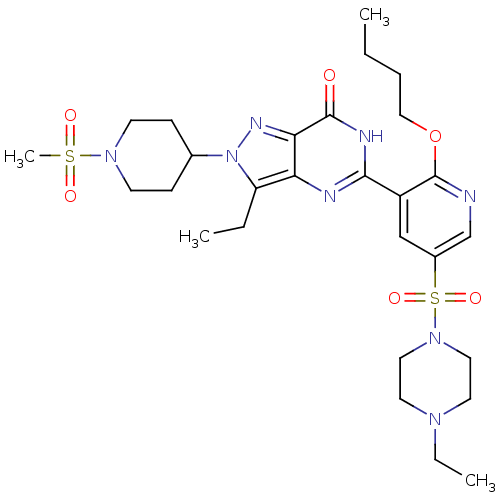
(CHEMBL1928265)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(CC1)S(C)(=O)=O)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O6S2/c1-5-8-17-42-28-22(18-21(19-29-28)44(40,41)35-15-13-33(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-34(12-10-20)43(4,38)39/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50197517
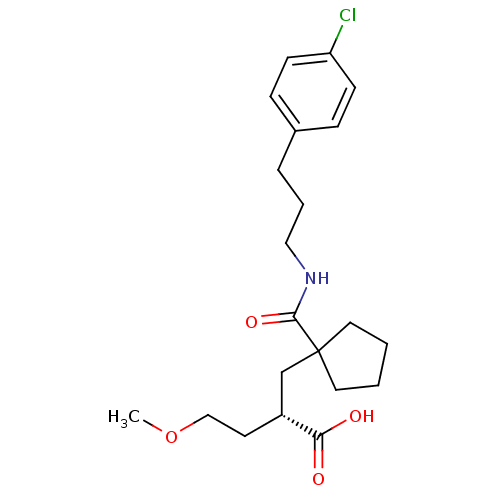
((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359795
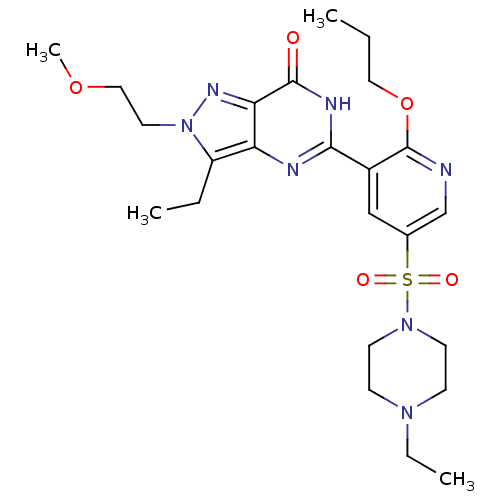
(CHEMBL1928258)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C24H35N7O5S/c1-5-13-36-24-18(15-17(16-25-24)37(33,34)30-10-8-29(7-3)9-11-30)22-26-20-19(6-2)31(12-14-35-4)28-21(20)23(32)27-22/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359767
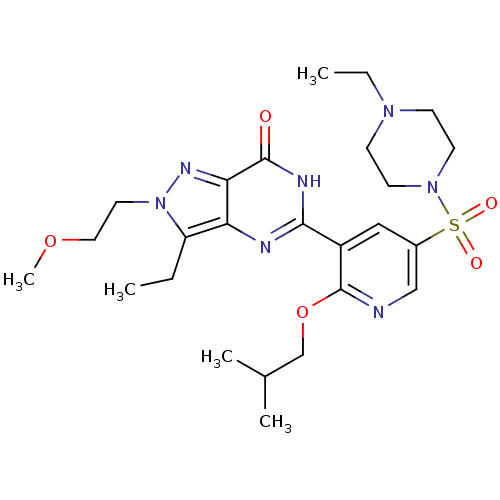
(CHEMBL1928259)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)12-13-36-5)24(33)28-23(27-21)19-14-18(15-26-25(19)37-16-17(3)4)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359774

(CHEMBL1928266)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCN(C)CC1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C28H42N8O4S/c1-5-8-17-40-28-22(18-21(19-29-28)41(38,39)35-15-13-34(7-3)14-16-35)26-30-24-23(6-2)36(32-25(24)27(37)31-26)20-9-11-33(4)12-10-20/h18-20H,5-17H2,1-4H3,(H,30,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359768
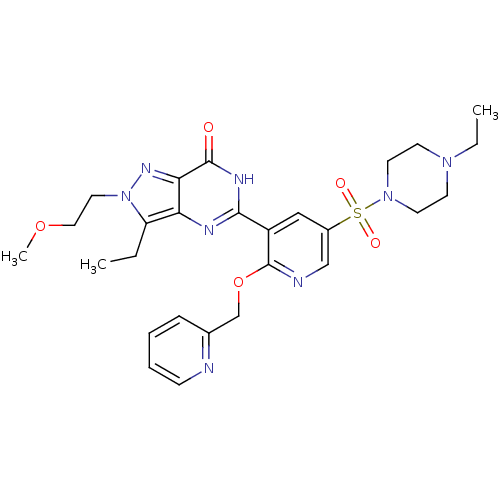
(CHEMBL1928260)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCc2ccccn2)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H34N8O5S/c1-4-22-23-24(32-35(22)14-15-39-3)26(36)31-25(30-23)21-16-20(41(37,38)34-12-10-33(5-2)11-13-34)17-29-27(21)40-18-19-8-6-7-9-28-19/h6-9,16-17H,4-5,10-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359777
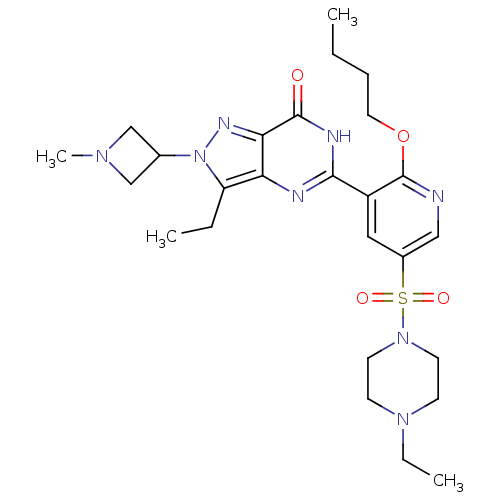
(CHEMBL1928269)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CN(C)C1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O4S/c1-5-8-13-38-26-20(14-19(15-27-26)39(36,37)33-11-9-32(7-3)10-12-33)24-28-22-21(6-2)34(18-16-31(4)17-18)30-23(22)25(35)29-24/h14-15,18H,5-13,16-17H2,1-4H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359778
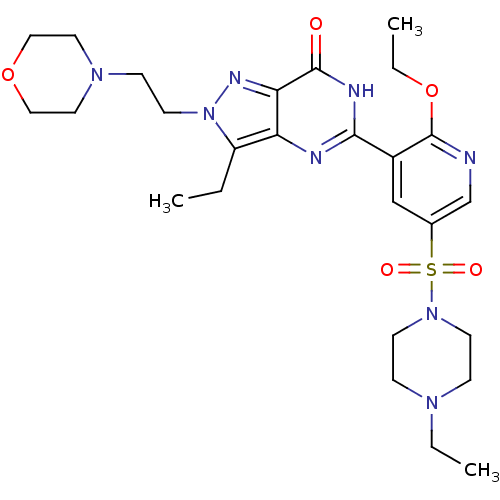
(CHEMBL1928270)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H38N8O5S/c1-4-21-22-23(30-34(21)12-9-32-13-15-38-16-14-32)25(35)29-24(28-22)20-17-19(18-27-26(20)39-6-3)40(36,37)33-10-7-31(5-2)8-11-33/h17-18H,4-16H2,1-3H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359790

(CHEMBL1928253)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC3CC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)16-17-6-7-17)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-3)38(34,35)31-10-8-30(5-2)9-11-31/h14-15,17H,4-13,16H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359771
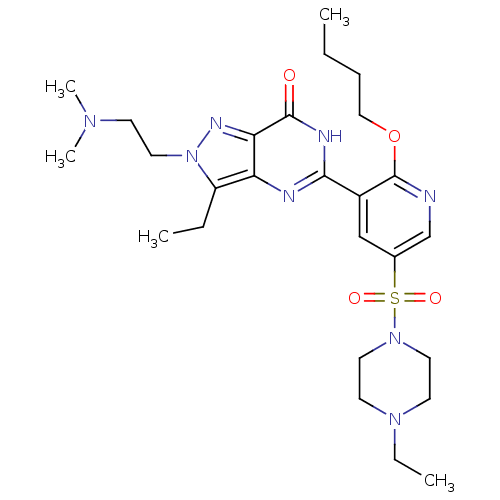
(CHEMBL1928263)Show SMILES CCCCOc1ncc(cc1-c1nc2c(CC)n(CCN(C)C)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C26H40N8O4S/c1-6-9-16-38-26-20(17-19(18-27-26)39(36,37)33-13-11-32(8-3)12-14-33)24-28-22-21(7-2)34(15-10-31(4)5)30-23(22)25(35)29-24/h17-18H,6-16H2,1-5H3,(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359789
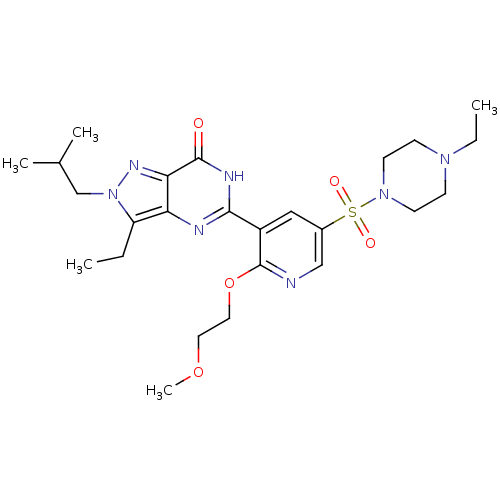
(CHEMBL1928252)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CC(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C25H37N7O5S/c1-6-20-21-22(29-32(20)16-17(3)4)24(33)28-23(27-21)19-14-18(15-26-25(19)37-13-12-36-5)38(34,35)31-10-8-30(7-2)9-11-31/h14-15,17H,6-13,16H2,1-5H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50246483
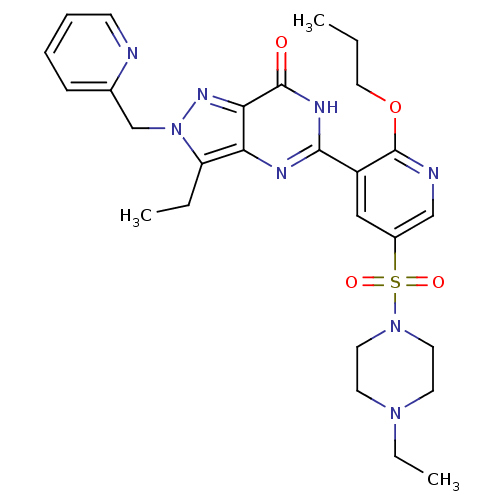
(3-ethyl-5-(5-(4-ethylpiperazin-1-ylsulfonyl)-2-pro...)Show SMILES CCCOc1ncc(cc1-c1nc2c(CC)n(Cc3ccccn3)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C27H34N8O4S/c1-4-15-39-27-21(16-20(17-29-27)40(37,38)34-13-11-33(6-3)12-14-34)25-30-23-22(5-2)35(32-24(23)26(36)31-25)18-19-9-7-8-10-28-19/h7-10,16-17H,4-6,11-15,18H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359770
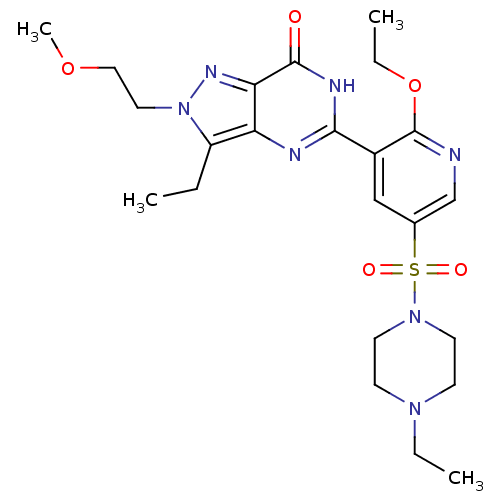
(CHEMBL1928262)Show SMILES CCOc1ncc(cc1-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H33N7O5S/c1-5-18-19-20(27-30(18)12-13-34-4)22(31)26-21(25-19)17-14-16(15-24-23(17)35-7-3)36(32,33)29-10-8-28(6-2)9-11-29/h14-15H,5-13H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359780
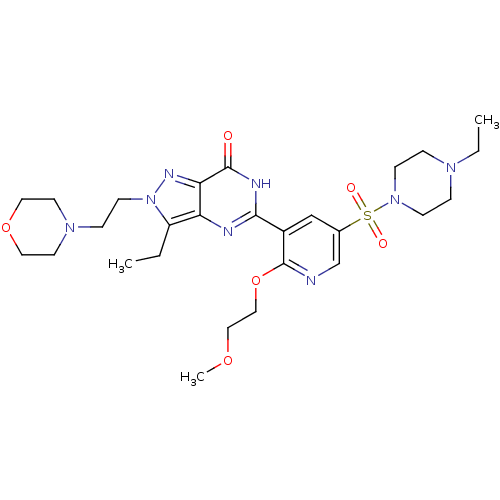
(CHEMBL1928272)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCN3CCOCC3)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H40N8O6S/c1-4-22-23-24(31-35(22)11-8-33-12-14-40-15-13-33)26(36)30-25(29-23)21-18-20(19-28-27(21)41-17-16-39-3)42(37,38)34-9-6-32(5-2)7-10-34/h18-19H,4-17H2,1-3H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359787
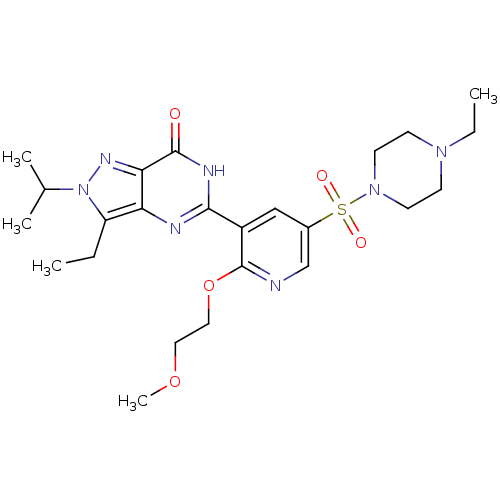
(CHEMBL1928250)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C(C)C Show InChI InChI=1S/C24H35N7O5S/c1-6-19-20-21(28-31(19)16(3)4)23(32)27-22(26-20)18-14-17(15-25-24(18)36-13-12-35-5)37(33,34)30-10-8-29(7-2)9-11-30/h14-16H,6-13H2,1-5H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359769

(CHEMBL1928261)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(CCOC)nc2c(=O)[nH]1 Show InChI InChI=1S/C24H35N7O6S/c1-5-19-20-21(28-31(19)11-12-35-3)23(32)27-22(26-20)18-15-17(16-25-24(18)37-14-13-36-4)38(33,34)30-9-7-29(6-2)8-10-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359791

(CHEMBL1928254)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C26H37N7O6S/c1-4-21-22-23(30-33(21)18-6-12-38-13-7-18)25(34)29-24(28-22)20-16-19(17-27-26(20)39-15-14-37-3)40(35,36)32-10-8-31(5-2)9-11-32/h16-18H,4-15H2,1-3H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359788
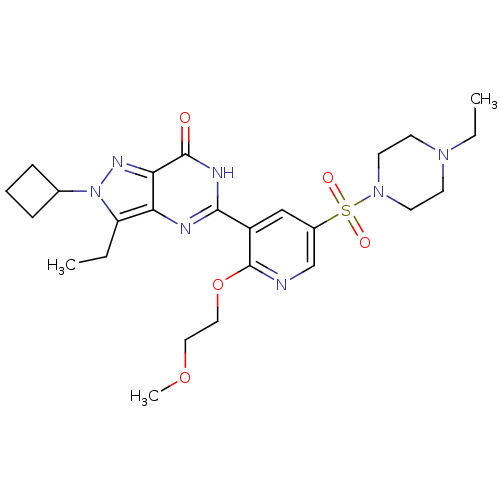
(CHEMBL1928251)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c(CC)n(nc2c(=O)[nH]1)C1CCC1 Show InChI InChI=1S/C25H35N7O5S/c1-4-20-21-22(29-32(20)17-7-6-8-17)24(33)28-23(27-21)19-15-18(16-26-25(19)37-14-13-36-3)38(34,35)31-11-9-30(5-2)10-12-31/h15-17H,4-14H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359786

(CHEMBL1928249)Show SMILES CCCn1nc2c(nc([nH]c2=O)-c2cc(cnc2OCCOC)S(=O)(=O)N2CCN(CC)CC2)c1CC Show InChI InChI=1S/C24H35N7O5S/c1-5-8-31-19(6-2)20-21(28-31)23(32)27-22(26-20)18-15-17(16-25-24(18)36-14-13-35-4)37(33,34)30-11-9-29(7-3)10-12-30/h15-16H,5-14H2,1-4H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359772

(CHEMBL1928264)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCC(C)C)c(c1)-c1nc2c(CC)n(CCCN(C)C)nc2c(=O)[nH]1 Show InChI InChI=1S/C27H42N8O4S/c1-7-22-23-24(31-35(22)11-9-10-32(5)6)26(36)30-25(29-23)21-16-20(17-28-27(21)39-18-19(3)4)40(37,38)34-14-12-33(8-2)13-15-34/h16-17,19H,7-15,18H2,1-6H3,(H,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359782

(CHEMBL1928274)Show SMILES CCCOc1ncc(cc1-c1nc2c3CCCCn3nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H31N7O4S/c1-3-13-34-23-17(14-16(15-24-23)35(32,33)29-11-9-28(4-2)10-12-29)21-25-19-18-7-5-6-8-30(18)27-20(19)22(31)26-21/h14-15H,3-13H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359785
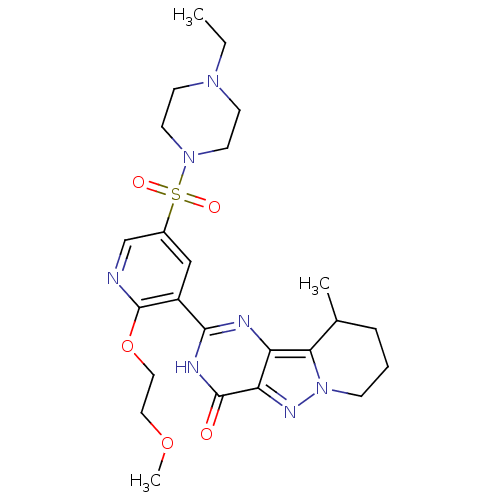
(CHEMBL1928277)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c3C(C)CCCn3nc2c(=O)[nH]1 Show InChI InChI=1S/C24H33N7O5S/c1-4-29-8-10-30(11-9-29)37(33,34)17-14-18(24(25-15-17)36-13-12-35-3)22-26-19-20(23(32)27-22)28-31-7-5-6-16(2)21(19)31/h14-16H,4-13H2,1-3H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359793

(CHEMBL1928256)Show SMILES CCc1n(CC(C)C)nc2c1nc([nH]c2=O)-c1cc(cnc1OCCOC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C24H35N7O5S/c1-6-19-20-21(28-31(19)15-16(2)3)23(32)27-22(26-20)18-13-17(14-25-24(18)36-12-11-35-5)37(33,34)30-9-7-29(4)8-10-30/h13-14,16H,6-12,15H2,1-5H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359784
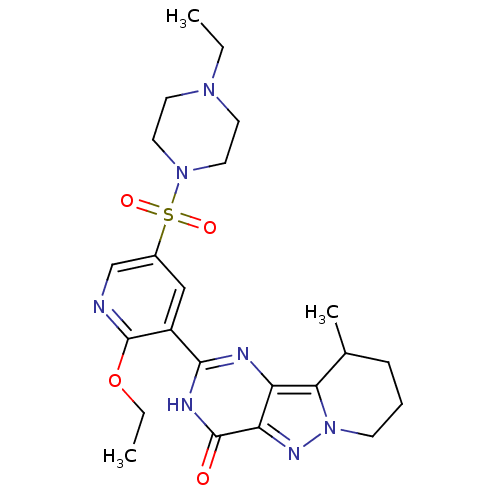
(CHEMBL1928276)Show SMILES CCOc1ncc(cc1-c1nc2c3C(C)CCCn3nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H31N7O4S/c1-4-28-9-11-29(12-10-28)35(32,33)16-13-17(23(24-14-16)34-5-2)21-25-18-19(22(31)26-21)27-30-8-6-7-15(3)20(18)30/h13-15H,4-12H2,1-3H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197513
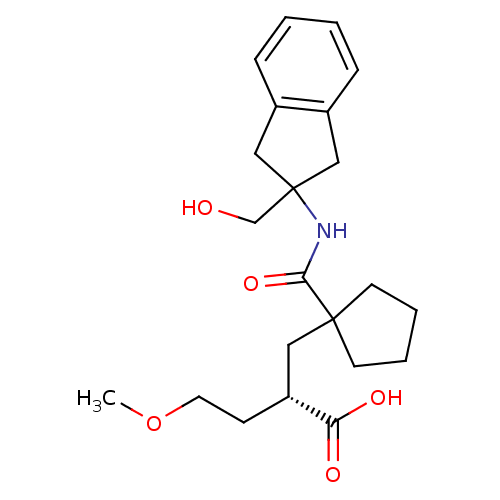
((S)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-28-11-8-18(19(25)26)12-21(9-4-5-10-21)20(27)23-22(15-24)13-16-6-2-3-7-17(16)14-22/h2-3,6-7,18,24H,4-5,8-15H2,1H3,(H,23,27)(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359792
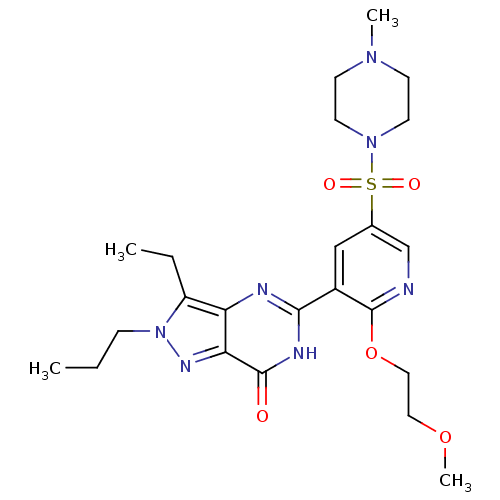
(CHEMBL1928255)Show SMILES CCCn1nc2c(nc([nH]c2=O)-c2cc(cnc2OCCOC)S(=O)(=O)N2CCN(C)CC2)c1CC Show InChI InChI=1S/C23H33N7O5S/c1-5-7-30-18(6-2)19-20(27-30)22(31)26-21(25-19)17-14-16(15-24-23(17)35-13-12-34-4)36(32,33)29-10-8-28(3)9-11-29/h14-15H,5-13H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197519

((S)-2-{1-[3-(4-fluoro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C21H30FNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50219254

(CHEMBL347592)Show SMILES C[C@@]1(CO[C@@H](OC1)c1nc(c([nH]1)-c1ccnc(NCc2ccccc2)n1)-c1ccc(F)cc1)C(=O)N1CCOCC1 |wU:4.7,1.0,wD:1.37,(10.24,-.19,;9.47,-1.52,;8.56,-.28,;7.03,-.44,;6.42,-1.85,;7.31,-3.09,;8.84,-2.94,;4.88,-2.01,;4.11,-3.34,;2.61,-3.03,;2.45,-1.5,;3.85,-.87,;1.1,-.73,;1.1,.81,;-.23,1.58,;-1.56,.81,;-1.56,-.73,;-2.89,-1.5,;-4.22,-.73,;-4.22,.81,;-5.55,1.58,;-5.55,3.1,;-4.22,3.89,;-2.89,3.1,;-2.89,1.56,;-.23,-1.5,;1.47,-4.07,;,-3.59,;-1.14,-4.62,;-.82,-6.12,;-1.96,-7.16,;.65,-6.59,;1.8,-5.56,;10.8,-2.29,;10.8,-3.83,;12.13,-1.52,;13.46,-2.31,;14.79,-1.54,;14.8,,;13.47,.77,;12.13,.02,)| Show InChI InChI=1S/C30H31FN6O4/c1-30(28(38)37-13-15-39-16-14-37)18-40-27(41-19-30)26-35-24(21-7-9-22(31)10-8-21)25(36-26)23-11-12-32-29(34-23)33-17-20-5-3-2-4-6-20/h2-12,27H,13-19H2,1H3,(H,35,36)(H,32,33,34)/t27-,30- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibition of murine Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197507

((S)-4-methoxy-2-{1-[(1R,2S)-2-(4-methoxy-phenyl)-c...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)N[C@@H]1C[C@H]1c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-27-12-9-16(20(24)25)14-22(10-3-4-11-22)21(26)23-19-13-18(19)15-5-7-17(28-2)8-6-15/h5-8,16,18-19H,3-4,9-14H2,1-2H3,(H,23,26)(H,24,25)/t16-,18+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50219252
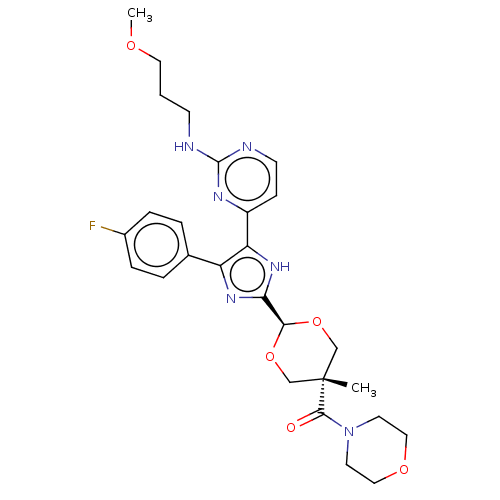
(CHEMBL159932)Show SMILES COCCCNc1nccc(n1)-c1[nH]c(nc1-c1ccc(F)cc1)[C@H]1OC[C@@](C)(CO1)C(=O)N1CCOCC1 |wU:24.26,27.30,wD:27.34,(-7.57,.44,;-6.24,-.34,;-4.9,.42,;-3.57,-.35,;-2.24,.42,;-.9,-.35,;.43,.42,;.43,1.96,;1.76,2.74,;3.12,1.96,;3.12,.42,;1.76,-.35,;4.45,-.35,;5.85,.28,;6.91,-.87,;6.13,-2.2,;4.61,-1.88,;3.47,-2.92,;3.8,-4.42,;2.65,-5.45,;1.18,-4.98,;.03,-6.02,;.85,-3.47,;2,-2.44,;8.43,-.7,;9.06,.72,;10.58,.88,;11.49,-.37,;12.26,.96,;10.86,-1.78,;9.34,-1.94,;12.82,-1.15,;12.82,-2.69,;14.15,-.37,;14.15,1.17,;15.51,1.93,;16.84,1.16,;16.82,-.38,;15.49,-1.15,)| Show InChI InChI=1S/C27H33FN6O5/c1-27(25(35)34-11-14-37-15-12-34)16-38-24(39-17-27)23-32-21(18-4-6-19(28)7-5-18)22(33-23)20-8-10-30-26(31-20)29-9-3-13-36-2/h4-8,10,24H,3,9,11-17H2,1-2H3,(H,32,33)(H,29,30,31)/t24-,27- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibition of murine Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359776

(CHEMBL1928268)Show SMILES CCOc1ncc(cc1C(=O)Nc1c(CC)n(nc1C(N)=O)C1CN(C)C1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C24H36N8O5S/c1-5-19-20(21(22(25)33)28-32(19)16-14-29(4)15-16)27-23(34)18-12-17(13-26-24(18)37-7-3)38(35,36)31-10-8-30(6-2)9-11-31/h12-13,16H,5-11,14-15H2,1-4H3,(H2,25,33)(H,27,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359783
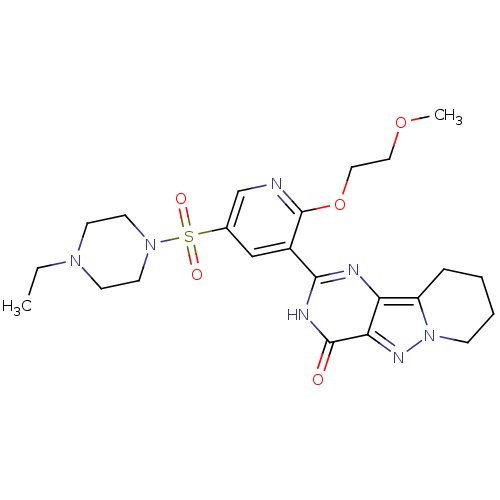
(CHEMBL1928275)Show SMILES CCN1CCN(CC1)S(=O)(=O)c1cnc(OCCOC)c(c1)-c1nc2c3CCCCn3nc2c(=O)[nH]1 Show InChI InChI=1S/C23H31N7O5S/c1-3-28-8-10-29(11-9-28)36(32,33)16-14-17(23(24-15-16)35-13-12-34-2)21-25-19-18-6-4-5-7-30(18)27-20(19)22(31)26-21/h14-15H,3-13H2,1-2H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50219253

(CHEMBL161197)Show SMILES C[C@@]1(CO[C@@H](OC1)c1nc(c([nH]1)-c1ccnc(NC2CC2)n1)-c1ccc(F)cc1)C(=O)N1CCOCC1 |wU:4.7,1.0,wD:1.33,(10.32,.96,;9.56,-.37,;8.65,.88,;7.12,.72,;6.51,-.7,;7.4,-1.94,;8.93,-1.78,;4.98,-.86,;4.21,-2.19,;2.69,-1.87,;2.53,-.35,;3.94,.28,;1.2,.42,;1.2,1.96,;-.14,2.73,;-1.47,1.96,;-1.47,.42,;-2.81,-.35,;-4.14,.42,;-5.64,.02,;-4.73,1.97,;-.14,-.35,;1.55,-2.92,;1.87,-4.41,;.75,-5.43,;-.72,-4.97,;-1.87,-6,;-1.05,-3.46,;.09,-2.43,;10.89,-1.14,;10.89,-2.68,;12.22,-.37,;13.55,-1.15,;14.88,-.38,;14.89,1.16,;13.56,1.93,;12.21,1.17,)| Show InChI InChI=1S/C26H29FN6O4/c1-26(24(34)33-10-12-35-13-11-33)14-36-23(37-15-26)22-31-20(16-2-4-17(27)5-3-16)21(32-22)19-8-9-28-25(30-19)29-18-6-7-18/h2-5,8-9,18,23H,6-7,10-15H2,1H3,(H,31,32)(H,28,29,30)/t23-,26- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibition of murine Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197513

((S)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-28-11-8-18(19(25)26)12-21(9-4-5-10-21)20(27)23-22(15-24)13-16-6-2-3-7-17(16)14-22/h2-3,6-7,18,24H,4-5,8-15H2,1H3,(H,23,27)(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50190746
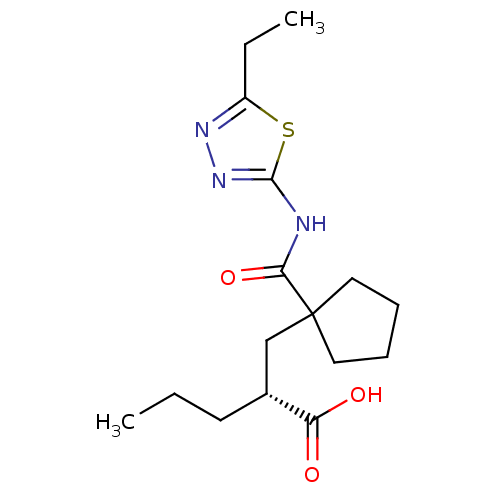
((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190771
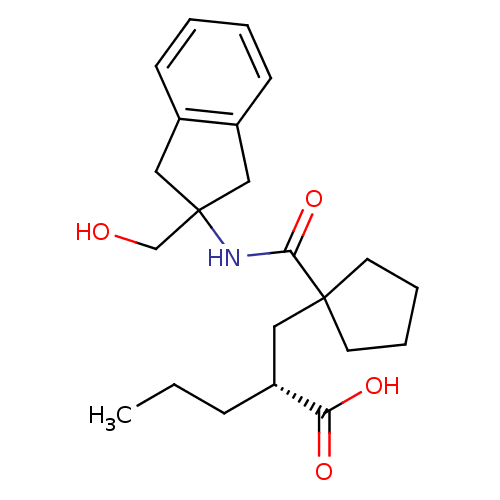
((R)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-7-18(19(25)26)12-21(10-5-6-11-21)20(27)23-22(15-24)13-16-8-3-4-9-17(16)14-22/h3-4,8-9,18,24H,2,5-7,10-15H2,1H3,(H,23,27)(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50359781
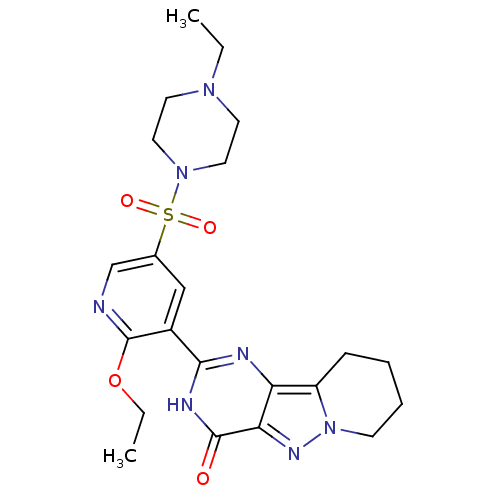
(CHEMBL1928273)Show SMILES CCOc1ncc(cc1-c1nc2c3CCCCn3nc2c(=O)[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C22H29N7O4S/c1-3-27-9-11-28(12-10-27)34(31,32)15-13-16(22(23-14-15)33-4-2)20-24-18-17-7-5-6-8-29(17)26-19(18)21(30)25-20/h13-14H,3-12H2,1-2H3,(H,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE5 isolated from corpus cavernosum after 30 to 60 mins by scintillation counting method |
Bioorg Med Chem 20: 498-509 (2011)
Article DOI: 10.1016/j.bmc.2011.10.022
BindingDB Entry DOI: 10.7270/Q2M9094T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197519

((S)-2-{1-[3-(4-fluoro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C21H30FNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197507

((S)-4-methoxy-2-{1-[(1R,2S)-2-(4-methoxy-phenyl)-c...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)N[C@@H]1C[C@H]1c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-27-12-9-16(20(24)25)14-22(10-3-4-11-22)21(26)23-19-13-18(19)15-5-7-17(28-2)8-6-15/h5-8,16,18-19H,3-4,9-14H2,1-2H3,(H,23,26)(H,24,25)/t16-,18+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data