 Found 492 hits with Last Name = 'francis' and Initial = 'g'
Found 492 hits with Last Name = 'francis' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364234
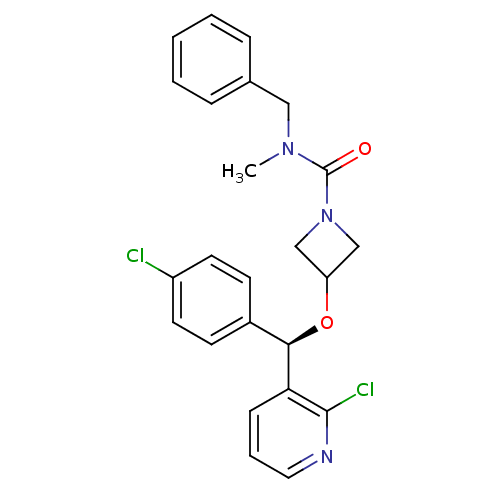
(CHEMBL1952279)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50296485
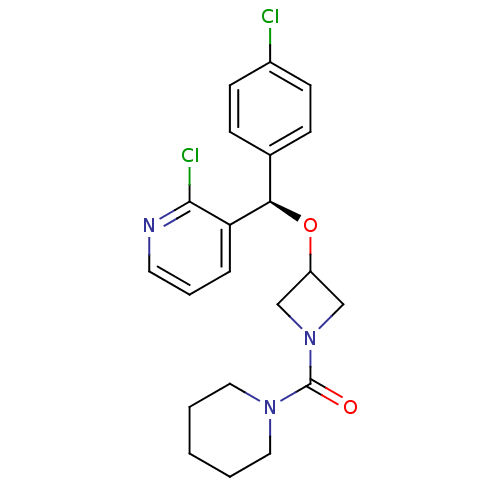
((R)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM16589
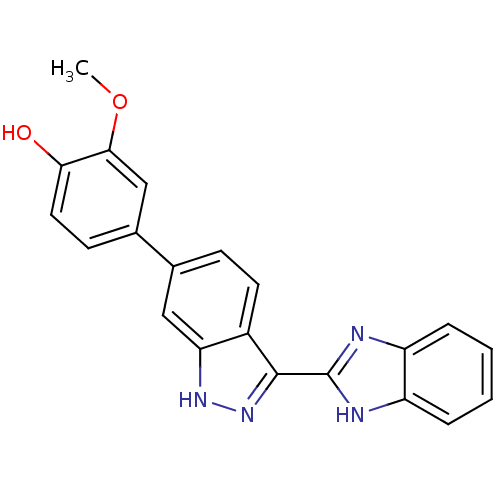
(4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...)Show SMILES COc1cc(ccc1O)-c1ccc2c(n[nH]c2c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C21H16N4O2/c1-27-19-11-13(7-9-18(19)26)12-6-8-14-17(10-12)24-25-20(14)21-22-15-4-2-3-5-16(15)23-21/h2-11,26H,1H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 26 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd
| Assay Description
For Ki determinations a matrix of inhibitor and substrate concentrations were tested. Inhibitor concentrations were tested from four times IC50 with ... |
Bioorg Med Chem 14: 1792-804 (2006)
Article DOI: 10.1016/j.bmc.2005.10.022
BindingDB Entry DOI: 10.7270/Q2154F9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82124
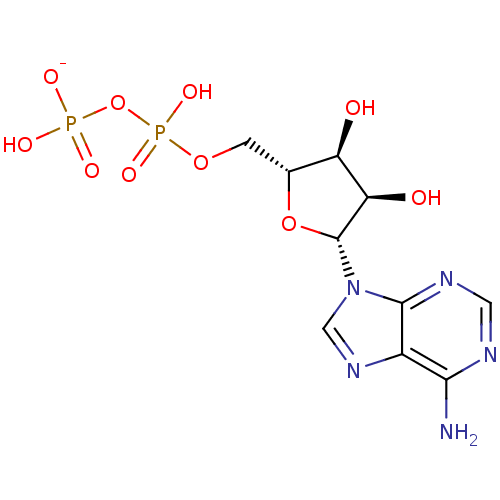
(adenosine-derived inhibitor (Grp78), 1)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/p-1/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32378
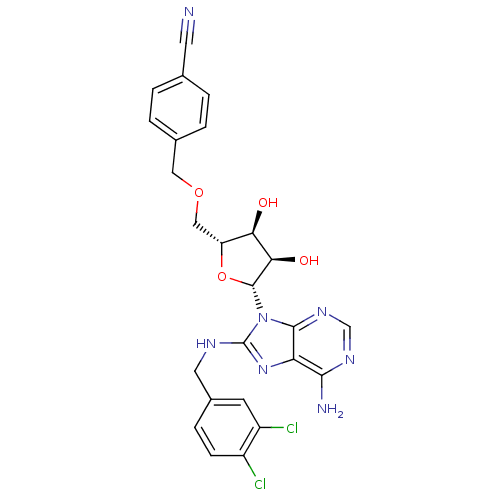
(adenosine-derived inhibitor (Grp78), 13 | adenosin...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](COCc4ccc(cc4)C#N)[C@@H](O)[C@H]3O)c(NCc3ccc(Cl)c(Cl)c3)nc12 |r| Show InChI InChI=1S/C25H23Cl2N7O4/c26-16-6-5-15(7-17(16)27)9-30-25-33-19-22(29)31-12-32-23(19)34(25)24-21(36)20(35)18(38-24)11-37-10-14-3-1-13(8-28)2-4-14/h1-7,12,18,20-21,24,35-36H,9-11H2,(H,30,33)(H2,29,31,32)/t18-,20-,21-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364235
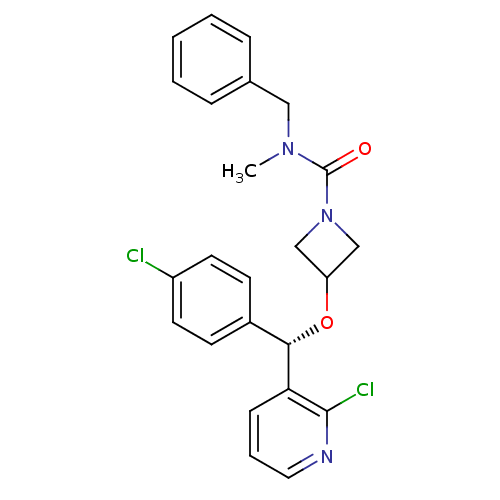
(CHEMBL1952280)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32381
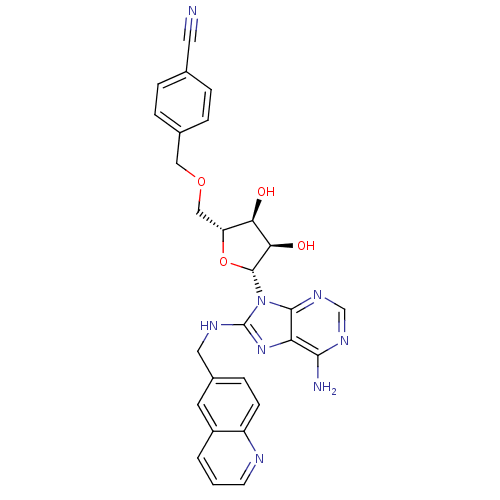
(adenosine-derived inhibitor (Grp78), 14 | adenosin...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](COCc4ccc(cc4)C#N)[C@@H](O)[C@H]3O)c(NCc3ccc4ncccc4c3)nc12 |r| Show InChI InChI=1S/C28H26N8O4/c29-11-16-3-5-17(6-4-16)13-39-14-21-23(37)24(38)27(40-21)36-26-22(25(30)33-15-34-26)35-28(36)32-12-18-7-8-20-19(10-18)2-1-9-31-20/h1-10,15,21,23-24,27,37-38H,12-14H2,(H,32,35)(H2,30,33,34)/t21-,23-,24-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82127
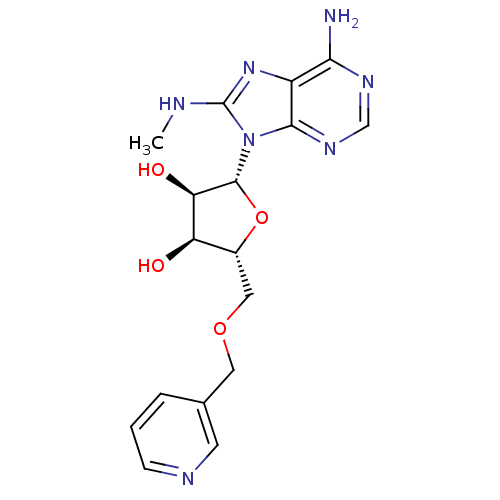
((2R,3R,4S,5R)-2-(6-Amino-8-methylaminopurin-9-yl)-...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCc2cccnc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H21N7O4/c1-19-17-23-11-14(18)21-8-22-15(11)24(17)16-13(26)12(25)10(28-16)7-27-6-9-3-2-4-20-5-9/h2-5,8,10,12-13,16,25-26H,6-7H2,1H3,(H,19,23)(H2,18,21,22)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364234

(CHEMBL1952279)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50296484
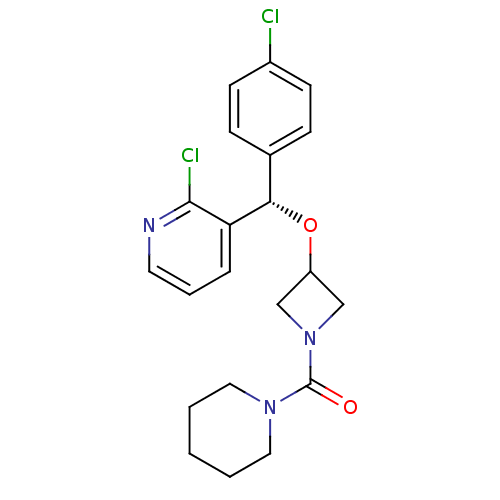
((S)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 19: 4241-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.097
BindingDB Entry DOI: 10.7270/Q28S4PZC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM16591
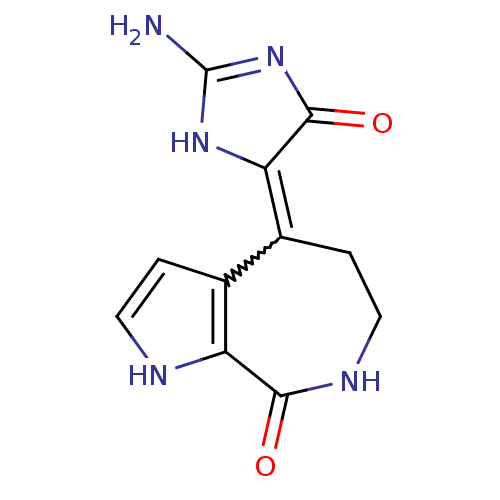
((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...)Show SMILES NC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]ccc12 |w:7.18,t:1| Show InChI InChI=1S/C11H11N5O2/c12-11-15-8(10(18)16-11)6-2-4-14-9(17)7-5(6)1-3-13-7/h1,3,13H,2,4H2,(H,14,17)(H3,12,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 659 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd
| Assay Description
For Ki determinations a matrix of inhibitor and substrate concentrations were tested. Inhibitor concentrations were tested from four times IC50 with ... |
Bioorg Med Chem 14: 1792-804 (2006)
Article DOI: 10.1016/j.bmc.2005.10.022
BindingDB Entry DOI: 10.7270/Q2154F9K |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32377
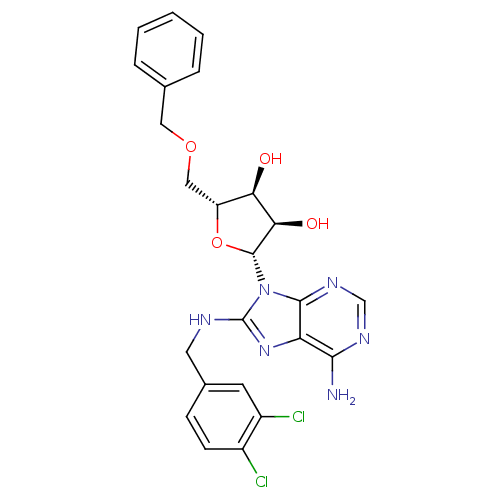
(adenosine-derived inhibitor (Grp78), 12 | adenosin...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](COCc4ccccc4)[C@@H](O)[C@H]3O)c(NCc3ccc(Cl)c(Cl)c3)nc12 |r| Show InChI InChI=1S/C24H24Cl2N6O4/c25-15-7-6-14(8-16(15)26)9-28-24-31-18-21(27)29-12-30-22(18)32(24)23-20(34)19(33)17(36-23)11-35-10-13-4-2-1-3-5-13/h1-8,12,17,19-20,23,33-34H,9-11H2,(H,28,31)(H2,27,29,30)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82125
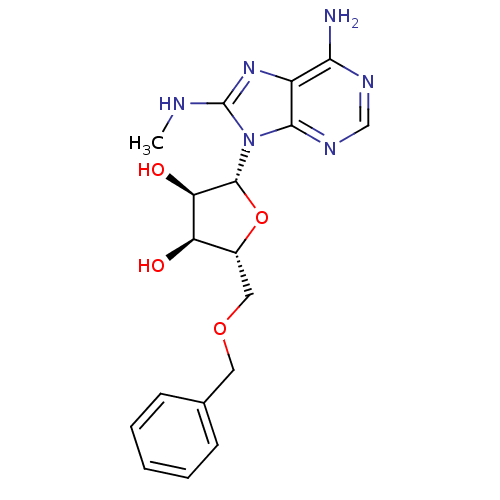
((2R,3R,4S,5R)-2-(6-Amino-8-methylaminopurin-9-yl)-...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H22N6O4/c1-20-18-23-12-15(19)21-9-22-16(12)24(18)17-14(26)13(25)11(28-17)8-27-7-10-5-3-2-4-6-10/h2-6,9,11,13-14,17,25-26H,7-8H2,1H3,(H,20,23)(H2,19,21,22)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32375
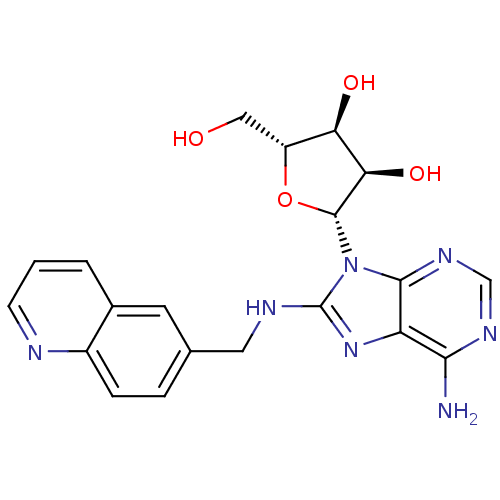
(adenosine-derived inhibitor (Grp78), 9 | adenosine...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(NCc3ccc4ncccc4c3)nc12 |r| Show InChI InChI=1S/C20H21N7O4/c21-17-14-18(25-9-24-17)27(19-16(30)15(29)13(8-28)31-19)20(26-14)23-7-10-3-4-12-11(6-10)2-1-5-22-12/h1-6,9,13,15-16,19,28-30H,7-8H2,(H,23,26)(H2,21,24,25)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.29E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32376
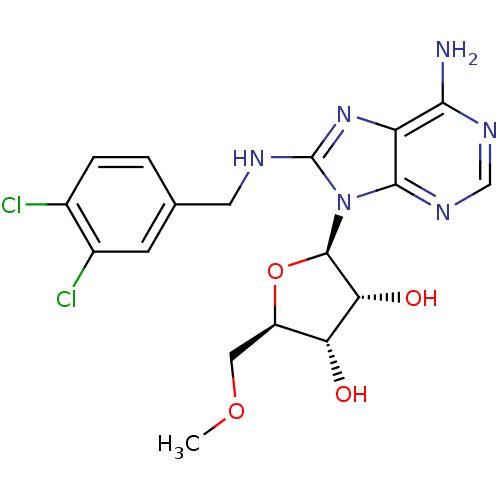
(adenosine-derived inhibitor (Grp78), 11 | adenosin...)Show SMILES COC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1c(NCc2ccc(Cl)c(Cl)c2)nc2c(N)ncnc12 |r| Show InChI InChI=1S/C18H20Cl2N6O4/c1-29-6-11-13(27)14(28)17(30-11)26-16-12(15(21)23-7-24-16)25-18(26)22-5-8-2-3-9(19)10(20)4-8/h2-4,7,11,13-14,17,27-28H,5-6H2,1H3,(H,22,25)(H2,21,23,24)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82128
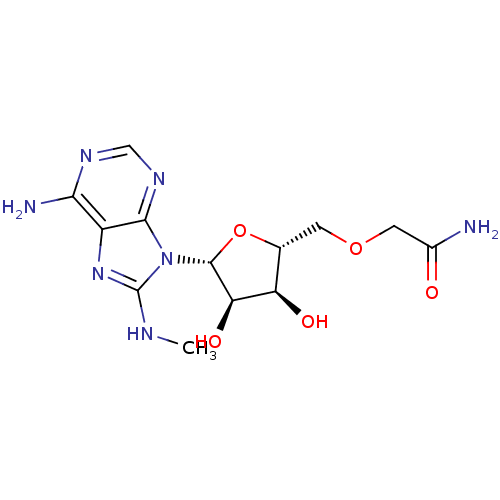
(2-[(2R,3S,4R,5R)-5-(6-Amino-8-methylaminopurin-9-y...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCC(N)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H19N7O5/c1-16-13-19-7-10(15)17-4-18-11(7)20(13)12-9(23)8(22)5(25-12)2-24-3-6(14)21/h4-5,8-9,12,22-23H,2-3H2,1H3,(H2,14,21)(H,16,19)(H2,15,17,18)/t5-,8-,9-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.85E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364235

(CHEMBL1952280)Show SMILES CN(Cc1ccccc1)C(=O)N1CC(C1)O[C@@H](c1ccc(Cl)cc1)c1cccnc1Cl |r| Show InChI InChI=1S/C24H23Cl2N3O2/c1-28(14-17-6-3-2-4-7-17)24(30)29-15-20(16-29)31-22(18-9-11-19(25)12-10-18)21-8-5-13-27-23(21)26/h2-13,20,22H,14-16H2,1H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82126

((2R,3R,4S,5R)-2-(6-Amino-8-methylaminopurin-9-yl)-...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCC2CCCCC2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H28N6O4/c1-20-18-23-12-15(19)21-9-22-16(12)24(18)17-14(26)13(25)11(28-17)8-27-7-10-5-3-2-4-6-10/h9-11,13-14,17,25-26H,2-8H2,1H3,(H,20,23)(H2,19,21,22)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.07E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32373
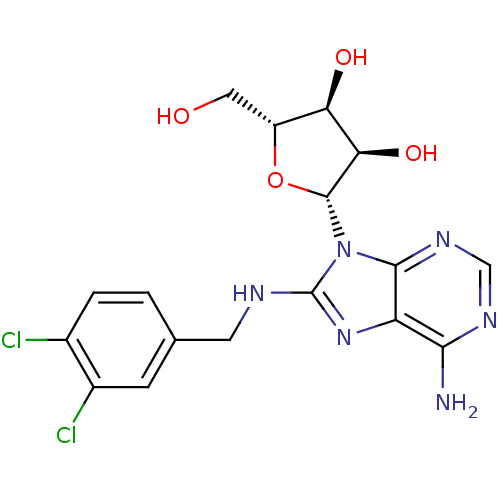
(adenosine-derived inhibitor (Grp78), 8 | adenosine...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(NCc3ccc(Cl)c(Cl)c3)nc12 |r| Show InChI InChI=1S/C17H18Cl2N6O4/c18-8-2-1-7(3-9(8)19)4-21-17-24-11-14(20)22-6-23-15(11)25(17)16-13(28)12(27)10(5-26)29-16/h1-3,6,10,12-13,16,26-28H,4-5H2,(H,21,24)(H2,20,22,23)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.31E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82129
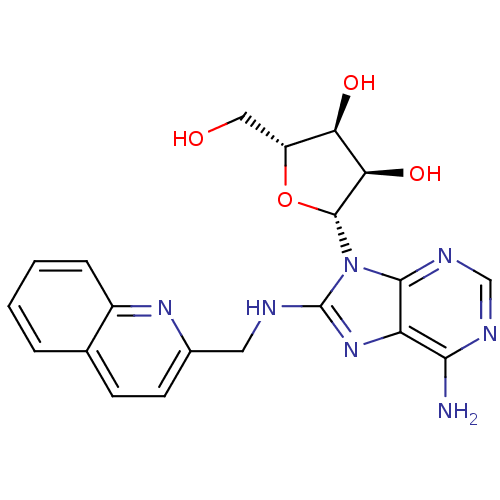
((2R,3R,4S,5R)-2-{6-Amino-8-[(quinolin-2-ylmethyl)a...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(NCc3ccc4ccccc4n3)nc12 |r| Show InChI InChI=1S/C20H21N7O4/c21-17-14-18(24-9-23-17)27(19-16(30)15(29)13(8-28)31-19)20(26-14)22-7-11-6-5-10-3-1-2-4-12(10)25-11/h1-6,9,13,15-16,19,28-30H,7-8H2,(H,22,26)(H2,21,23,24)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.32E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32370
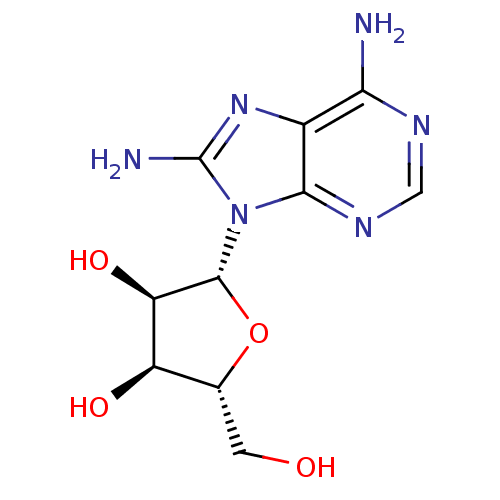
(adenosine-derived inhibitor (Grp78), 2 | adenosine...)Show SMILES Nc1nc2c(N)ncnc2n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O4/c11-7-4-8(14-2-13-7)16(10(12)15-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,15)(H2,11,13,14)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4.46E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50296484

((S)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 19: 4241-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.097
BindingDB Entry DOI: 10.7270/Q28S4PZC |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32371
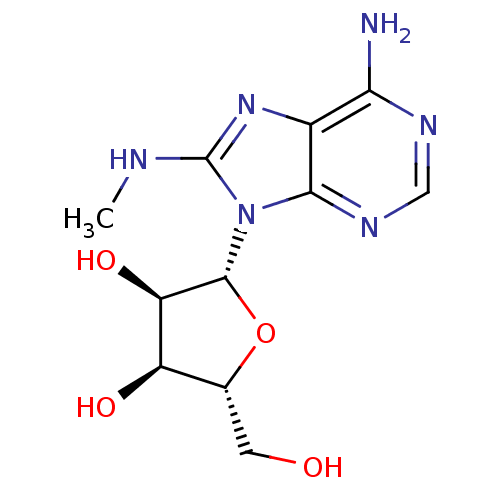
(adenosine-derived inhibitor (Grp78), 3 | adenosine...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16N6O4/c1-13-11-16-5-8(12)14-3-15-9(5)17(11)10-7(20)6(19)4(2-18)21-10/h3-4,6-7,10,18-20H,2H2,1H3,(H,13,16)(H2,12,14,15)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM16590
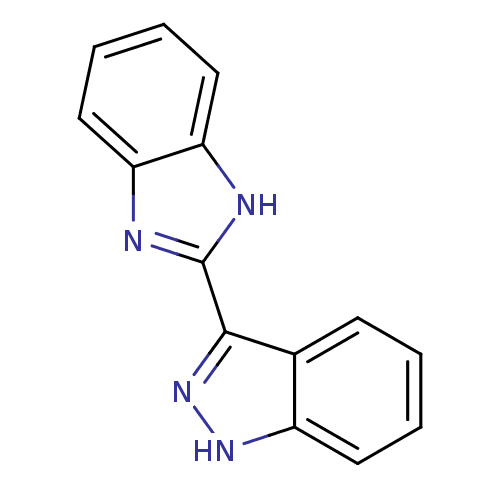
(2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...)Show InChI InChI=1S/C14H10N4/c1-2-6-10-9(5-1)13(18-17-10)14-15-11-7-3-4-8-12(11)16-14/h1-8H,(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8.58E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd
| Assay Description
For Ki determinations a matrix of inhibitor and substrate concentrations were tested. Inhibitor concentrations were tested from four times IC50 with ... |
Bioorg Med Chem 14: 1792-804 (2006)
Article DOI: 10.1016/j.bmc.2005.10.022
BindingDB Entry DOI: 10.7270/Q2154F9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50296485

((R)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 22: 901-6 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.032
BindingDB Entry DOI: 10.7270/Q2D79BVH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50296485

((R)-{3-[(4-Chlorophenyl)-(2-chloropyridin-3-yl)met...)Show SMILES Clc1ccc(cc1)[C@@H](OC1CN(C1)C(=O)N1CCCCC1)c1cccnc1Cl |r| Show InChI InChI=1S/C21H23Cl2N3O2/c22-16-8-6-15(7-9-16)19(18-5-4-10-24-20(18)23)28-17-13-26(14-17)21(27)25-11-2-1-3-12-25/h4-10,17,19H,1-3,11-14H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 19: 4241-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.097
BindingDB Entry DOI: 10.7270/Q28S4PZC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50047247

(1,3-Dibutyl-7-(2-oxo-propyl)-3,7-dihydro-purine-2,...)Show InChI InChI=1S/C16H24N4O3/c1-4-6-8-19-14-13(18(11-17-14)10-12(3)21)15(22)20(16(19)23)9-7-5-2/h11H,4-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191378

(CHEMBL212163 | sodium (R,E)-6-((6,8-dihydro-5H-imi...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCOCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-8(12-16(11)9(6-21-12)13(18)19)3-7-4-15-1-2-20-5-10(15)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b8-3+/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191377

((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CNCCn2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-9(12-17(11)10(6-21-12)13(19)20)4-7-3-8-5-14-1-2-16(8)15-7/h3-4,6,12,14H,1-2,5H2,(H,19,20)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191389
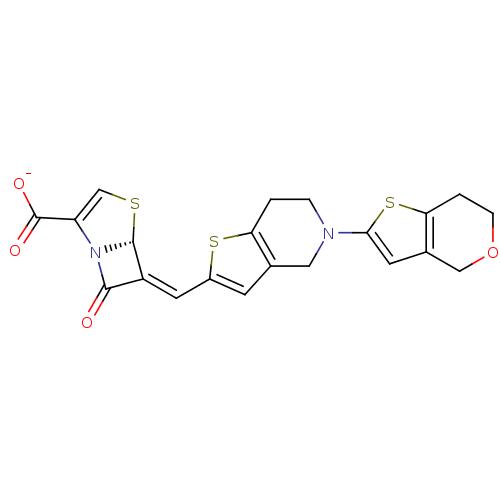
(6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(CCc2s1)c1cc2COCCc2s1 |t:3| Show InChI InChI=1S/C21H18N2O4S3/c24-19-14(20-23(19)15(10-28-20)21(25)26)7-13-5-11-8-22(3-1-16(11)29-13)18-6-12-9-27-4-2-17(12)30-18/h5-7,10,20H,1-4,8-9H2,(H,25,26)/p-1/b14-7-/t20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191390
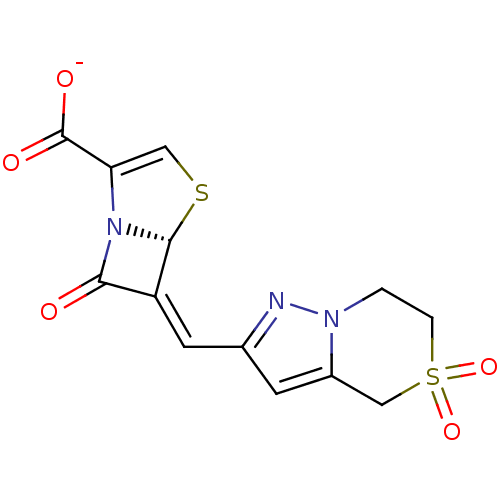
((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CS(=O)(=O)CCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O5S2/c17-11-9(12-16(11)10(5-22-12)13(18)19)4-7-3-8-6-23(20,21)2-1-15(8)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191379

((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CSCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191379

((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CSCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473208

(CHEMBL20531)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C21H28N4O6S/c1-5-7-11-23-19-18(20(26)24(21(23)27)12-8-6-2)25(14-22-19)32(28,29)15-9-10-16(30-3)17(13-15)31-4/h9-10,13-14H,5-8,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473209
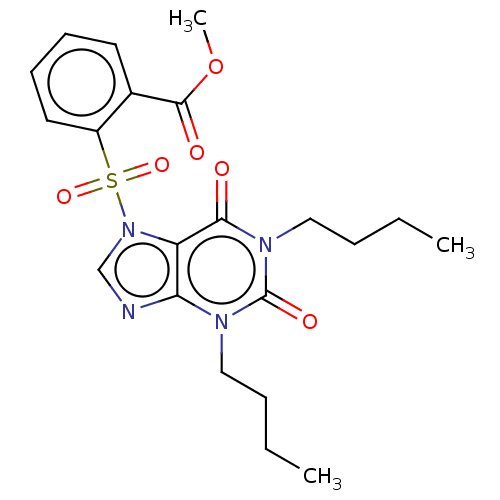
(CHEMBL20958)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)c1ccccc1C(=O)OC Show InChI InChI=1S/C21H26N4O6S/c1-4-6-12-23-18-17(19(26)24(21(23)28)13-7-5-2)25(14-22-18)32(29,30)16-11-9-8-10-15(16)20(27)31-3/h8-11,14H,4-7,12-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191386

((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CCCCn2n1 |t:3| Show InChI InChI=1S/C14H13N3O3S/c18-12-10(13-17(12)11(7-21-13)14(19)20)6-8-5-9-3-1-2-4-16(9)15-8/h5-7,13H,1-4H2,(H,19,20)/p-1/b10-6-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191383

((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2CCSCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-8(12-16(11)9(5-21-12)13(18)19)3-7-4-15-1-2-20-6-10(15)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b8-3-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191381

((5R,6Z)-6-{[5-(4-methoxybenzyl)-4,5,6,7-tetrahydro...)Show SMILES COc1ccc(CN2CCc3sc(\C=C4/[C@H]5SC=C(N5C4=O)C([O-])=O)cc3C2)cc1 |c:17| Show InChI InChI=1S/C22H20N2O4S2/c1-28-15-4-2-13(3-5-15)10-23-7-6-19-14(11-23)8-16(30-19)9-17-20(25)24-18(22(26)27)12-29-21(17)24/h2-5,8-9,12,21H,6-7,10-11H2,1H3,(H,26,27)/p-1/b17-9-/t21-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191390

((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CS(=O)(=O)CCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O5S2/c17-11-9(12-16(11)10(5-22-12)13(18)19)4-7-3-8-6-23(20,21)2-1-15(8)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM11439

(4-({5-[(4-aminocyclohexyl)amino]-3-bromopyrazolo[1...)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2cc(N[C@H]3CC[C@H](N)CC3)nc3c(Br)cnn23)cc1Cl |r,wU:18.18,wD:15.14,(-9.87,7.79,;-8.54,7.02,;-8.54,5.48,;-7.2,7.79,;-8.29,8.88,;-6.11,8.88,;-5.87,7.02,;-5.87,5.48,;-4.53,4.71,;-3.2,5.48,;-1.87,4.71,;-1.87,3.17,;-3.2,2.4,;-3.2,.86,;-4.53,.09,;-5.87,.86,;-5.87,2.4,;-7.2,3.17,;-8.53,2.4,;-9.87,3.17,;-8.53,.86,;-7.2,.09,;-1.87,.09,;-.53,.86,;.93,.39,;1.41,-1.08,;1.84,1.63,;.93,2.88,;-.53,2.4,;-3.2,7.02,;-4.53,7.79,;-4.53,9.33,)| Show InChI InChI=1S/C20H25BrClN7O2S/c1-28(2)32(30,31)17-8-7-14(9-16(17)22)26-19-10-18(25-13-5-3-12(23)4-6-13)27-20-15(21)11-24-29(19)20/h7-13,26H,3-6,23H2,1-2H3,(H,25,27)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 15: 863-7 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.073
BindingDB Entry DOI: 10.7270/Q2ZK5DWP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191383

((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2CCSCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-8(12-16(11)9(5-21-12)13(18)19)3-7-4-15-1-2-20-6-10(15)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b8-3-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191386

((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CCCCn2n1 |t:3| Show InChI InChI=1S/C14H13N3O3S/c18-12-10(13-17(12)11(7-21-13)14(19)20)6-8-5-9-3-1-2-4-16(9)15-8/h5-7,13H,1-4H2,(H,19,20)/p-1/b10-6-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191377

((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CNCCn2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-9(12-17(11)10(6-21-12)13(19)20)4-7-3-8-5-14-1-2-16(8)15-7/h3-4,6,12,14H,1-2,5H2,(H,19,20)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473206

(CHEMBL20951)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C20H26N4O4S/c1-3-5-12-22-18-17(19(25)23(20(22)26)13-6-4-2)24(15-21-18)29(27,28)14-16-10-8-7-9-11-16/h7-11,15H,3-6,12-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191385
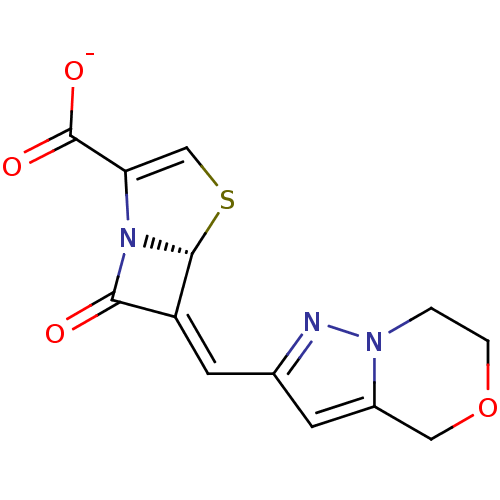
((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2COCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191389

(6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(CCc2s1)c1cc2COCCc2s1 |t:3| Show InChI InChI=1S/C21H18N2O4S3/c24-19-14(20-23(19)15(10-28-20)21(25)26)7-13-5-11-8-22(3-1-16(11)29-13)18-6-12-9-27-4-2-17(12)30-18/h5-7,10,20H,1-4,8-9H2,(H,25,26)/p-1/b14-7-/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM11429
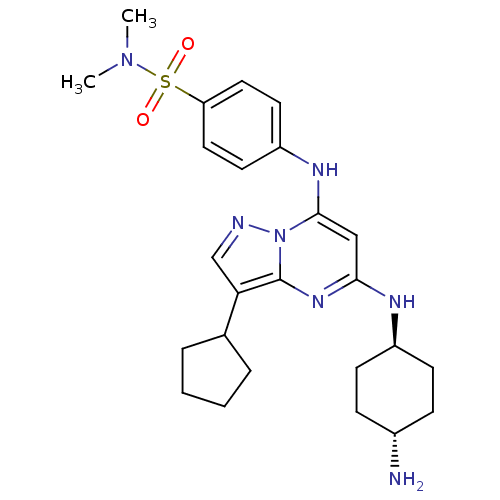
(4-({5-[(4-aminocyclohexyl)amino]-3-cyclopentylpyra...)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2cc(N[C@H]3CC[C@H](N)CC3)nc3c(cnn23)C2CCCC2)cc1 |r,wU:18.18,wD:15.14,(-8.53,5.48,;-8.53,7.02,;-9.87,7.79,;-7.2,7.79,;-8.29,8.88,;-6.11,8.88,;-5.87,7.02,;-5.87,5.48,;-4.53,4.71,;-3.2,5.48,;-1.87,4.71,;-1.87,3.17,;-3.2,2.4,;-3.2,.86,;-4.53,.09,;-5.87,.86,;-5.87,2.4,;-7.2,3.17,;-8.53,2.4,;-9.87,3.17,;-8.53,.86,;-7.2,.09,;-1.87,.09,;-.53,.86,;.93,.39,;1.84,1.63,;.93,2.88,;-.53,2.4,;1.41,-1.08,;.6,-2.39,;1.6,-3.56,;3.03,-2.97,;2.91,-1.44,;-3.2,7.02,;-4.53,7.79,)| Show InChI InChI=1S/C25H35N7O2S/c1-31(2)35(33,34)21-13-11-20(12-14-21)29-24-15-23(28-19-9-7-18(26)8-10-19)30-25-22(16-27-32(24)25)17-5-3-4-6-17/h11-19,29H,3-10,26H2,1-2H3,(H,28,30)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 15: 863-7 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.073
BindingDB Entry DOI: 10.7270/Q2ZK5DWP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191387

((5R),(6Z)-6-(7-methyl-5,6,7,8-tetrahydroimidazo[1,...)Show SMILES CN1CCn2cc(\C=C3/[C@H]4SC=C(N4C3=O)C([O-])=O)nc2C1 |c:11| Show InChI InChI=1S/C14H14N4O3S/c1-16-2-3-17-5-8(15-11(17)6-16)4-9-12(19)18-10(14(20)21)7-22-13(9)18/h4-5,7,13H,2-3,6H2,1H3,(H,20,21)/p-1/b9-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473212
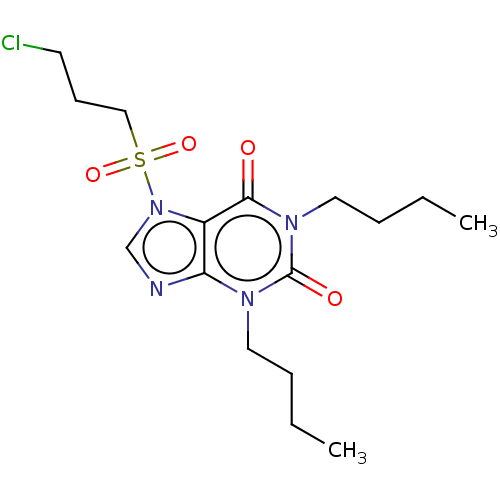
(CHEMBL418740)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)CCCCl Show InChI InChI=1S/C16H25ClN4O4S/c1-3-5-9-19-14-13(15(22)20(16(19)23)10-6-4-2)21(12-18-14)26(24,25)11-7-8-17/h12H,3-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191380
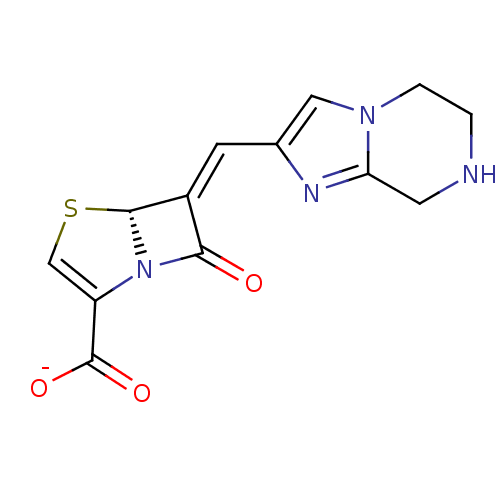
(CHEMBL379440 | sodium (R,E)-7-oxo-6-((5,6,7,8-tetr...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCNCc2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-8(12-17(11)9(6-21-12)13(19)20)3-7-5-16-2-1-14-4-10(16)15-7/h3,5-6,12,14H,1-2,4H2,(H,19,20)/p-1/b8-3+/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data