

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106483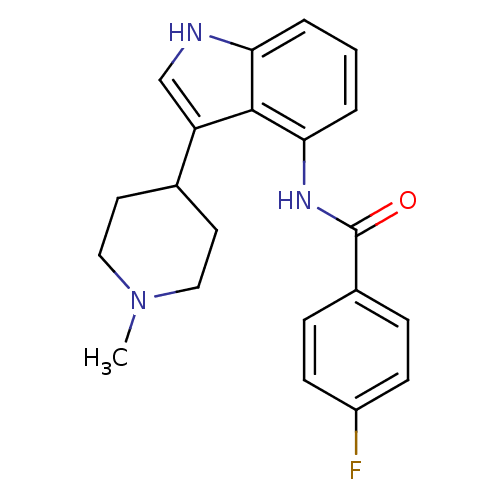 (4-Fluoro-N-[3-(1-methyl-piperidin-4-yl)-1H-indol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061307 (AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106484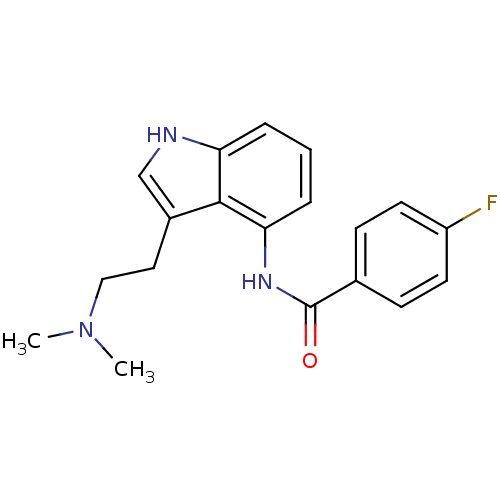 (CHEMBL421287 | N-[3-(2-Dimethylamino-ethyl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor using [3H]-5-HT radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061308 ((3S,4aS,8aS)-2-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1F (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1F receptor was determined | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061305 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1A receptor was determined using [3H]-5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 4 receptor using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 2B receptor using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 2A receptor using [125I]DOI as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1B receptor using [3H]5-HT radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 7 receptor using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor was determined using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 6 receptor using [3H]LSD as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-HT2C receptor was determined using [125I]- DOI as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1E receptor was determined using [3H]5-HT as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat histamine H1 receptor was determined using [3H]pyrilamine as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat dopamine D2 receptor was determined using [3H]raclopride as radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50106482 (CHEMBL339980 | N-[3-(2-Dimethylamino-ethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat dopamine receptor D1 was determined using [3H]SCH-23390 radioligand | J Med Chem 44: 4031-4 (2001) BindingDB Entry DOI: 10.7270/Q22Z168B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50049319 (CHEMBL42407 | Lanepitant | N-[(R)-1-{[Acetyl-(2-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50213827 (CHEMBL351834) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50213826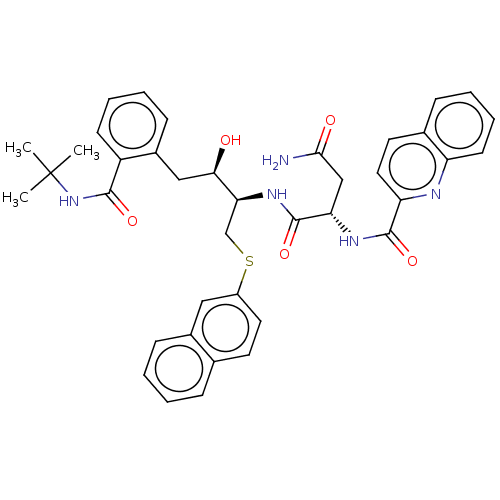 (CHEMBL164045) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101033 (CHEMBL43044 | N-[(R)-1-{[Acetyl-(2-methoxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101037 (CHEMBL47070 | N-[(R)-1-{[Acetyl-(2-methoxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101030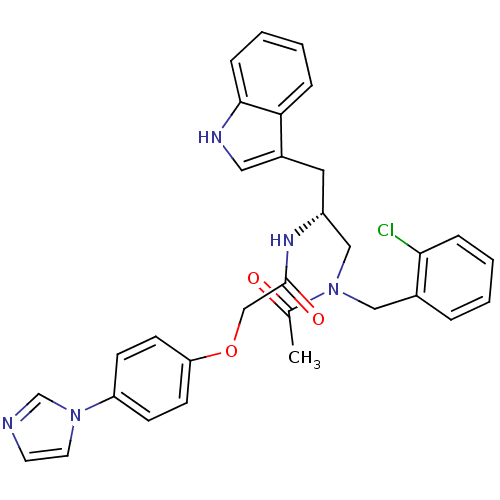 (CHEMBL296013 | N-[(R)-1-{[Acetyl-(2-chloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101029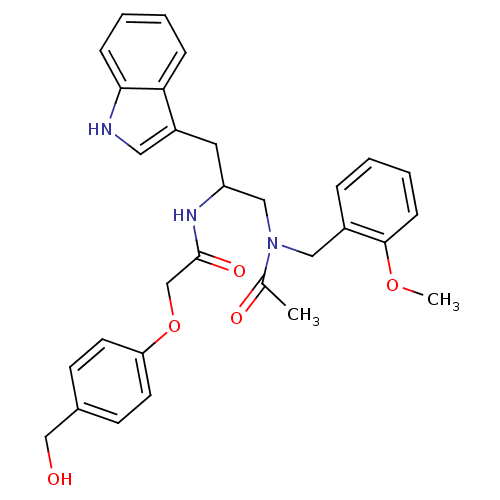 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101031 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286689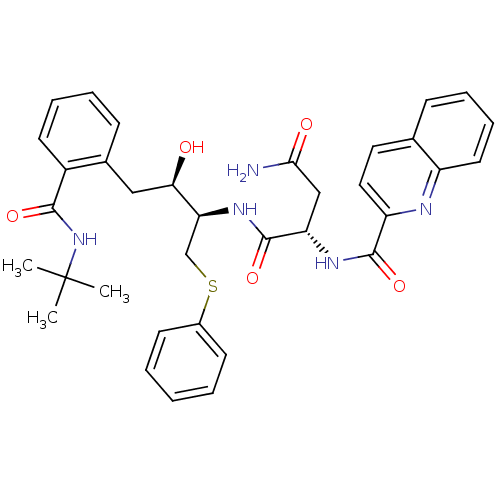 ((S)-N*1*-[(1R,2R)-3-(2-tert-Butylcarbamoyl-phenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286688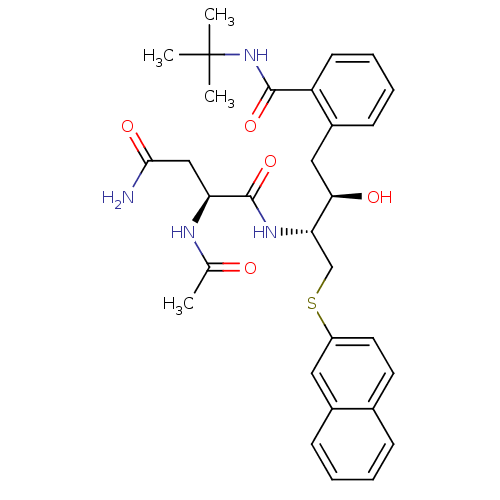 ((S)-2-Acetylamino-N*1*-[(1R,2R)-3-(2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414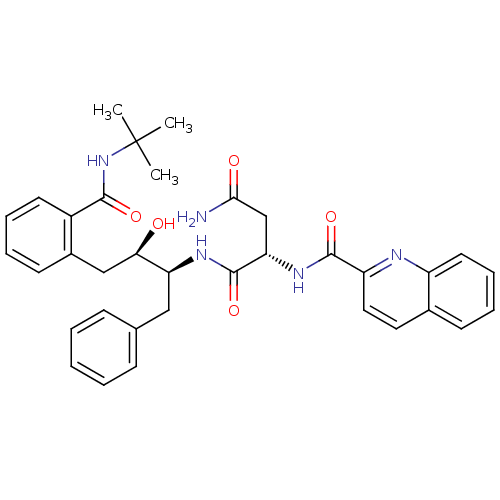 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286691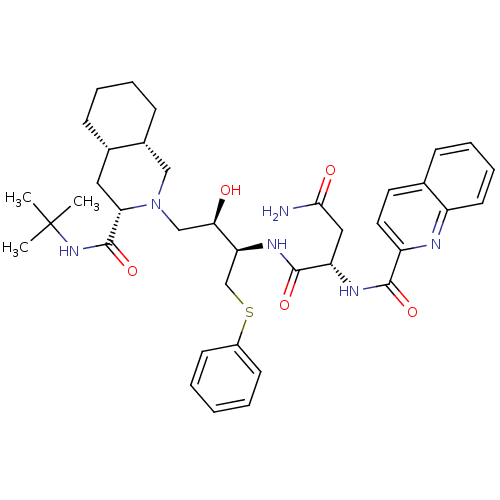 ((S)-N*1*-[(1R,2R)-3-((3S,4aS,8aS)-3-tert-Butylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286690 (CHEMBL164158 | {(S)-1-[(1R,2R)-3-(2-tert-Butylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282667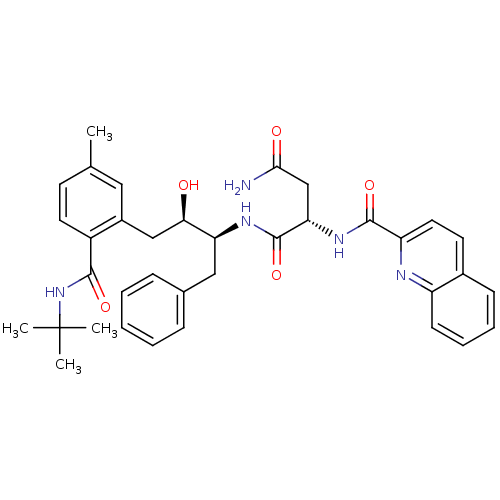 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(2-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101042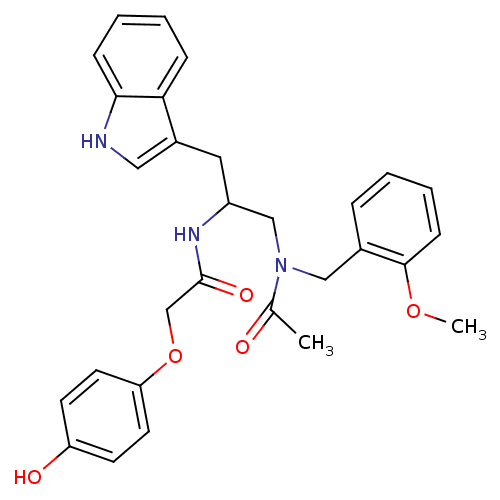 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101034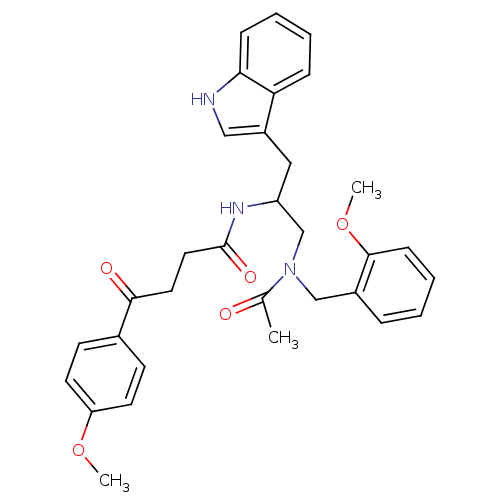 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282663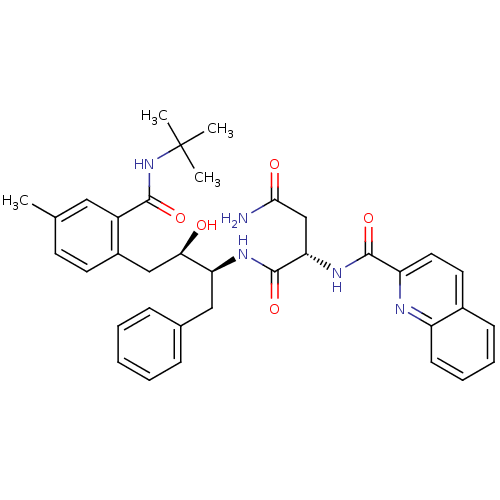 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(2-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282665 ((S)-N*1*-[(1S,2R)-1-Benzyl-3-(1-tert-butylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of HIV-1 protease | Bioorg Med Chem Lett 4: 1385-1390 (1994) Article DOI: 10.1016/S0960-894X(01)80367-X BindingDB Entry DOI: 10.7270/Q2QJ7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101032 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101035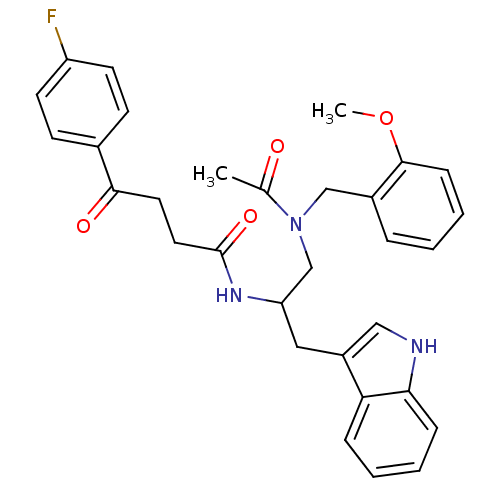 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101040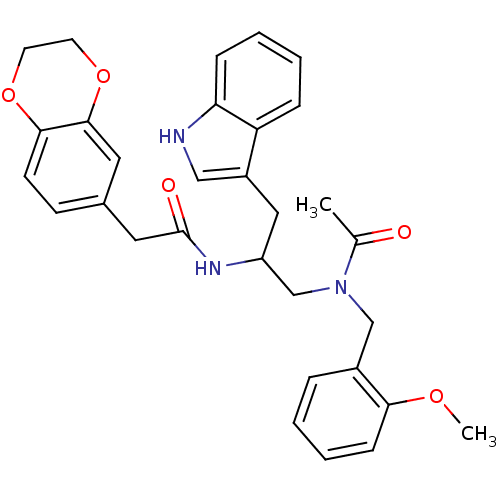 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101038 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101041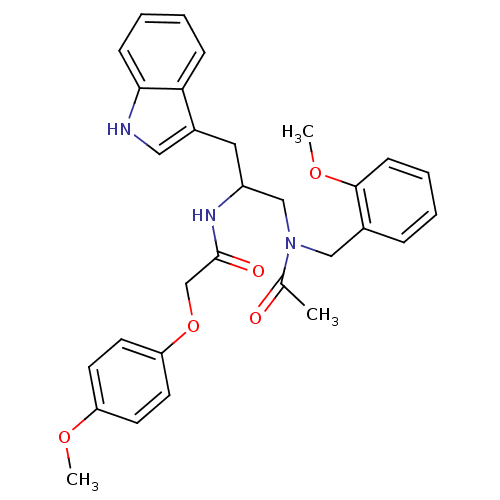 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101036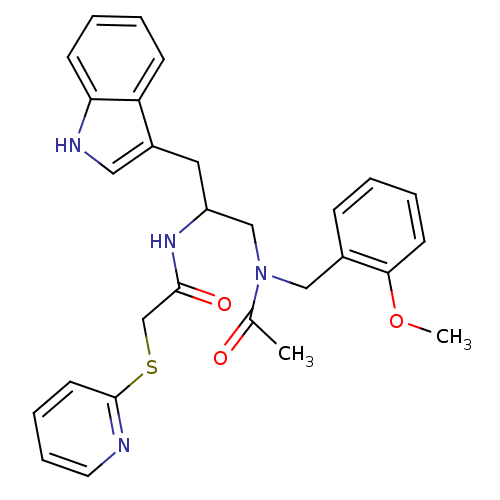 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286684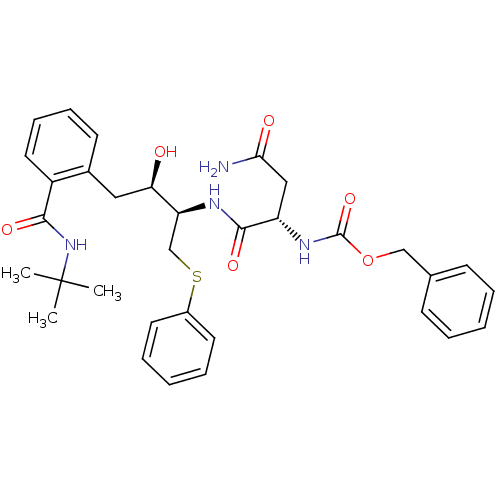 (CHEMBL159378 | {(S)-1-[(1R,2R)-3-(2-tert-Butylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50286687 ((S)-2-Acetylamino-N*1*-[(1R,2R)-3-(2-tert-butylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 715-720 (1995) Article DOI: 10.1016/0960-894X(95)00101-X BindingDB Entry DOI: 10.7270/Q2K937HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50101039 (1N-{2-(1H-3-indolyl)-1-[2-methoxybenzyl(methyl)car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of [125I]-Bolton-Hunter SP to Tachykinin receptor 1 in human IM-9 cell line | Bioorg Med Chem Lett 11: 1643-6 (2001) BindingDB Entry DOI: 10.7270/Q2GQ6X13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |