

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50364856 (CHEMBL1950167) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain | Bioorg Med Chem Lett 22: 1633-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.126 BindingDB Entry DOI: 10.7270/Q2D21Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279404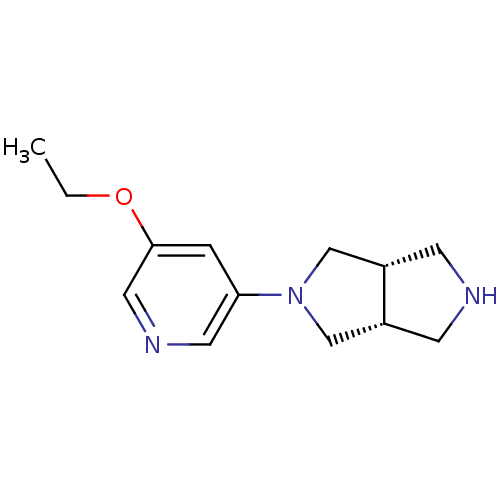 (CHEMBL447388 | cis-2-(5-Ethoxy-3-pyridinyl)octahyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279405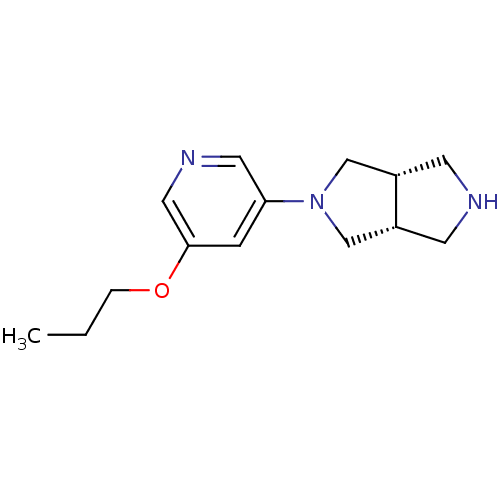 (CHEMBL488772 | cis-3-(5-Propyloxy-3-pyridinyl)-3,7...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50278878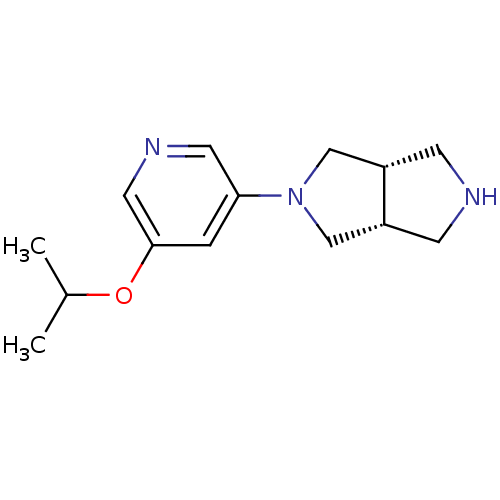 (CHEMBL502567 | cis-3-(5-Isopropyloxy-3-pyridinyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50278877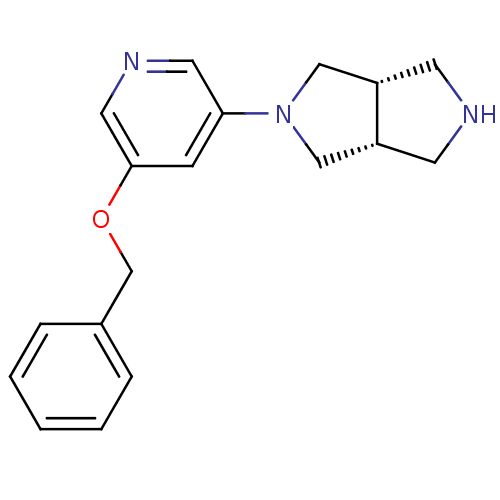 (CHEMBL524911 | cis-2-(5-(Benzyloxy)pyridin-3-yl)oc...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279402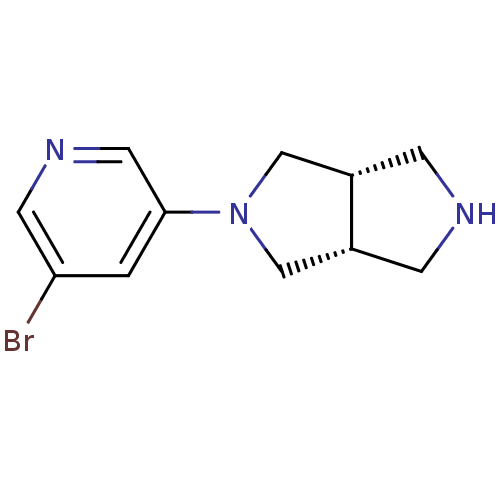 (CHEMBL486511 | cis-2-(5-Bromopyridin-3-yl)-octahyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279403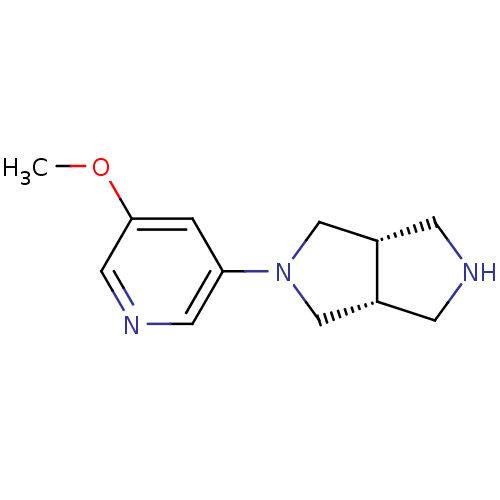 (CHEMBL486512 | cis-2-(5-Methoxy-3-pyridinyl)octahy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50303527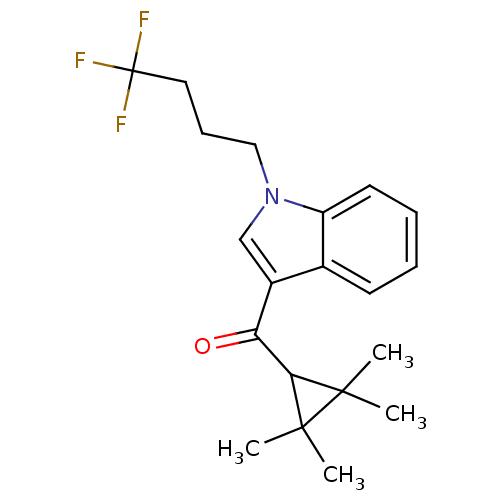 ((2,2,3,3-Tetramethylcyclopropyl)(1-(4,4,4-trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50364875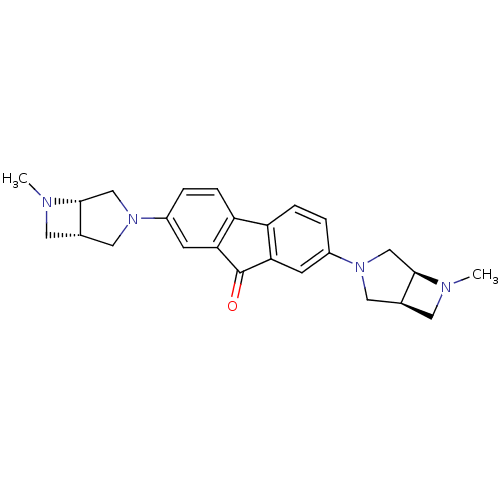 (CHEMBL1950156) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain | Bioorg Med Chem Lett 22: 1633-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.126 BindingDB Entry DOI: 10.7270/Q2D21Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279369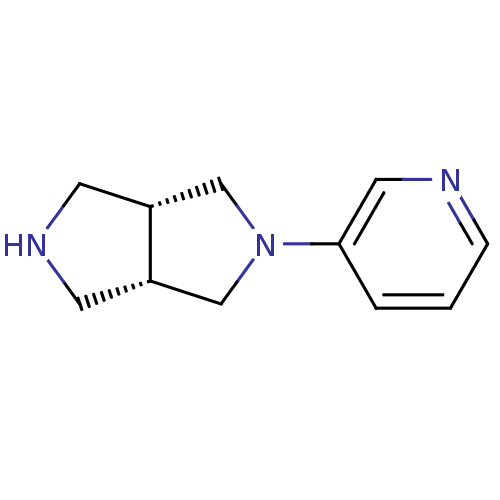 (CHEMBL460562 | cis-2-(3-Pyridinyl)octahydropyrrolo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21301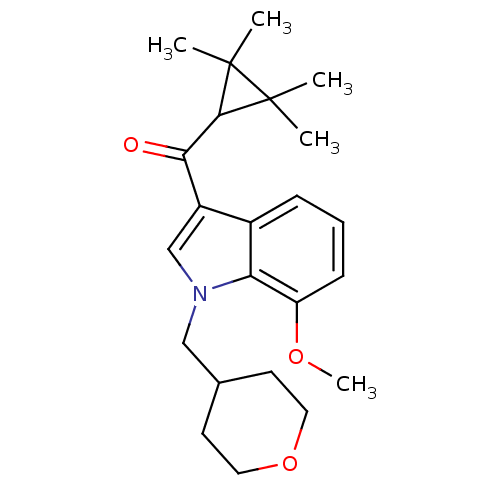 (7-methoxy-1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50303556 ((1-((Tetrahydrofuran-3-yl)methyl)-1H-indol-3-yl)(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21291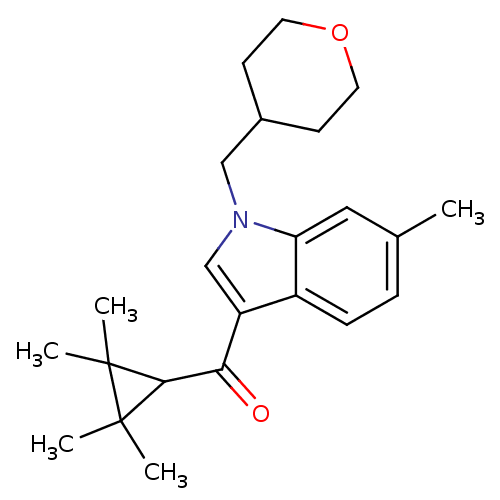 (6-methyl-1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetrameth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM139889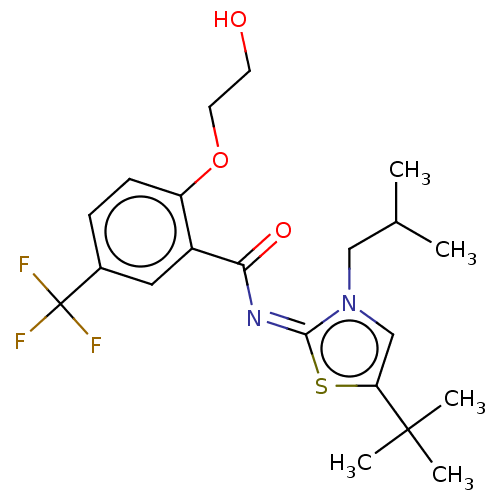 (US8895592, 19) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The CB1 and CB2 radioligand binding assays described herein are utilized to ascertain the selectivity of compounds of the present application for bin... | US Patent US8895592 (2014) BindingDB Entry DOI: 10.7270/Q2CV4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50278881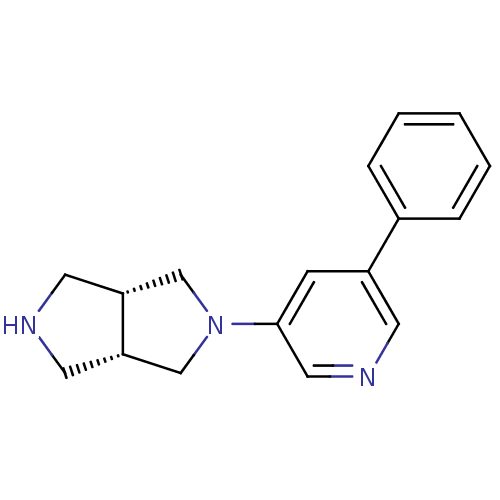 (CHEMBL496667 | cis-2-(5-Phenylpyridin-3-yl)-octahy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50303551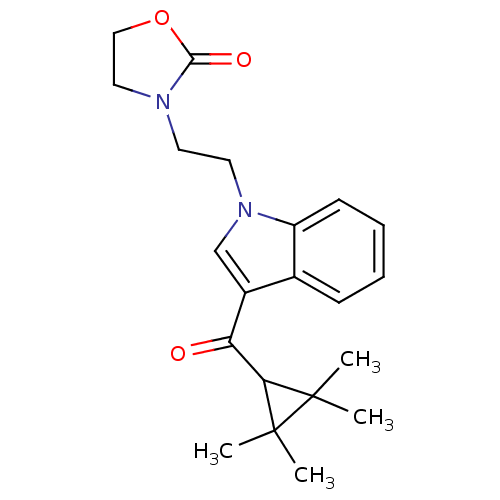 (3-(2-(3-(2,2,3,3-Tetramethylcyclopropanecarbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279371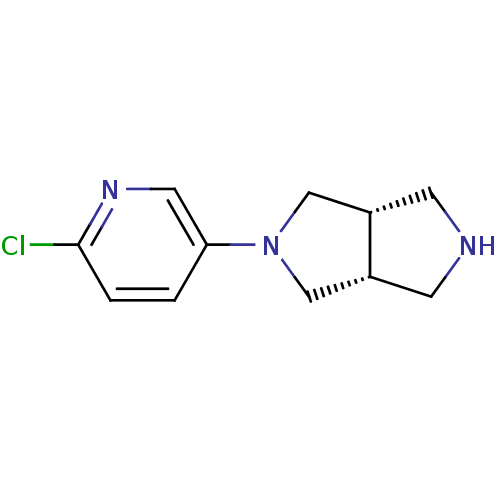 (CHEMBL484831 | cis-2-(6-Chloro-3-pyridinyl)octahyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21284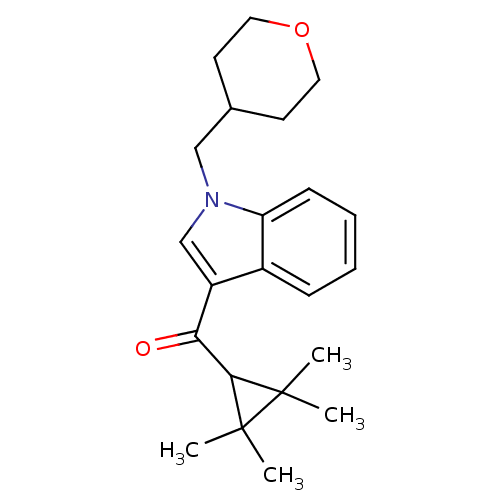 (1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21284 (1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50279059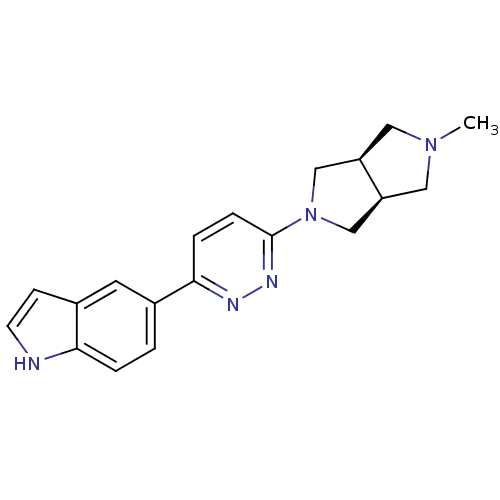 (CHEMBL496851 | cis-5-[6-(5-Methyl-hexahydro-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]A585539 from alpha7 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50279401 (CHEMBL498943 | cis-5-(6-Bromo-pyridin-3-yl)-hexahy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane | J Med Chem 52: 4126-41 (2009) Article DOI: 10.1021/jm900249k BindingDB Entry DOI: 10.7270/Q2GT5P3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50364879 (CHEMBL1950160) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain | Bioorg Med Chem Lett 22: 1633-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.126 BindingDB Entry DOI: 10.7270/Q2D21Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133276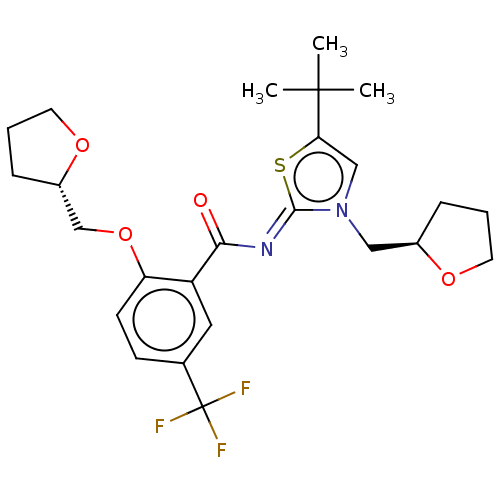 (US8846730, 23) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133287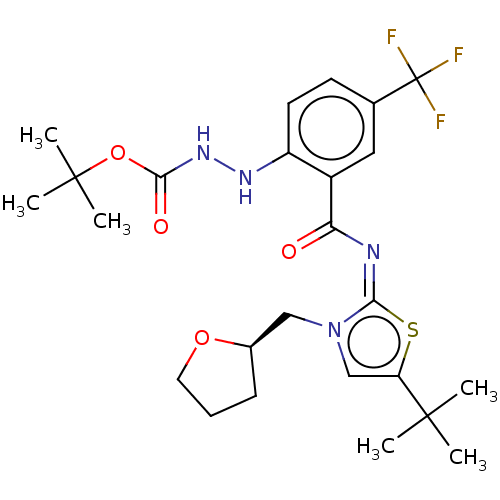 (US8846730, 40) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM133313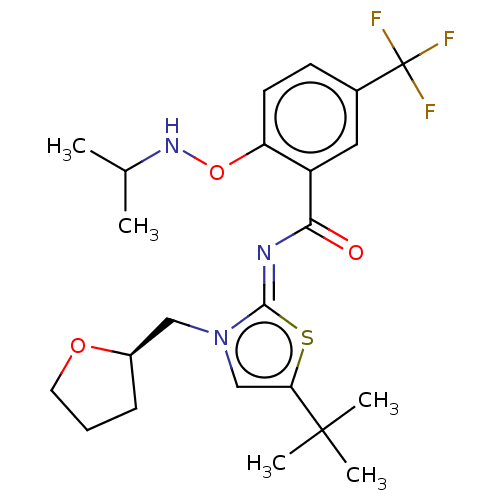 (US8846730, 79) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133307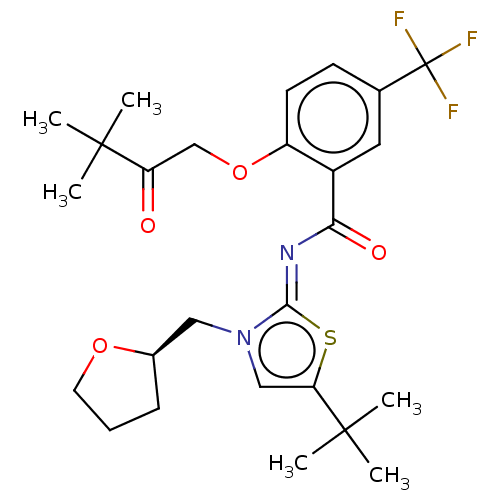 (US8846730, 72) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM135877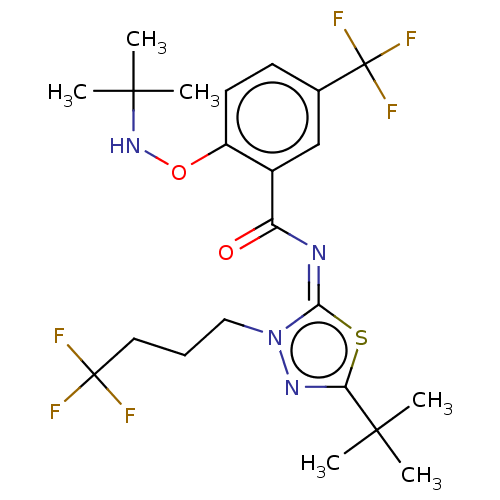 (US8859596, 164) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50364874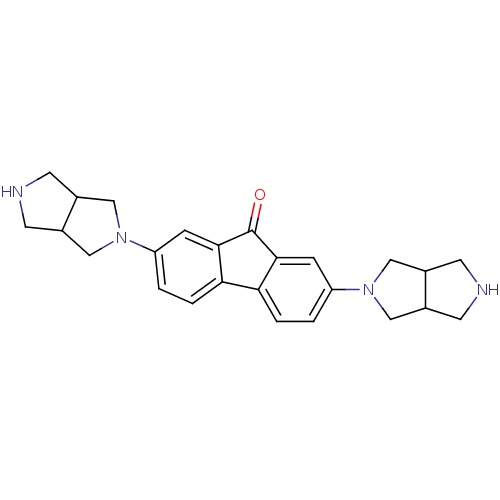 (CHEMBL1950155) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain | Bioorg Med Chem Lett 22: 1633-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.126 BindingDB Entry DOI: 10.7270/Q2D21Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135855 (US8859596, 141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.320 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM133287 (US8846730, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135909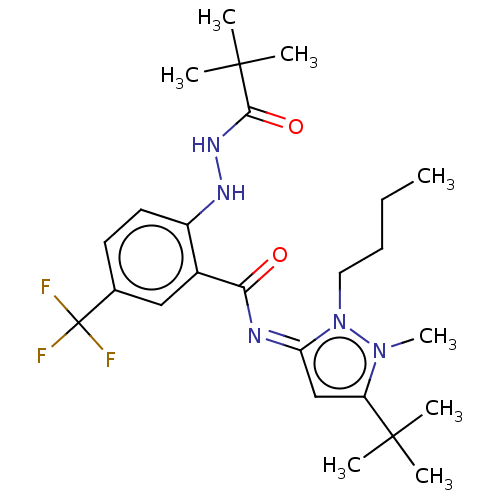 (US8859596, 198) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135905 (US8859596, 193) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50303534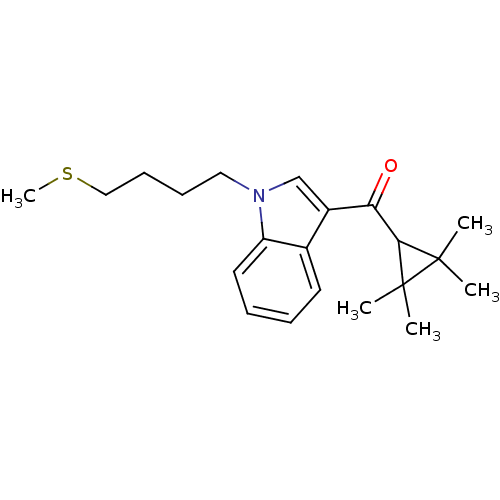 ((1-(4-(Methylthio)butyl)-1H-indol-3-yl)(2,2,3,3-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21334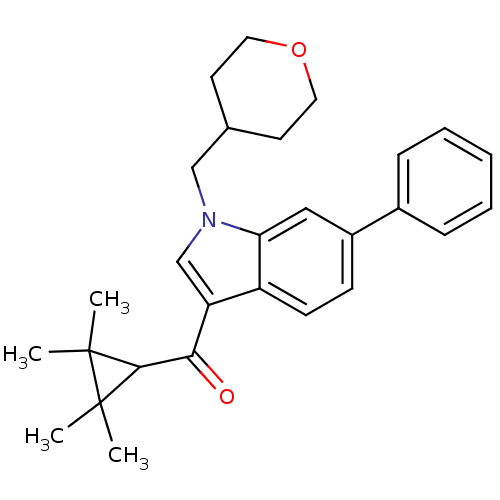 (1-(oxan-4-ylmethyl)-6-phenyl-3-[(2,2,3,3-tetrameth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50303558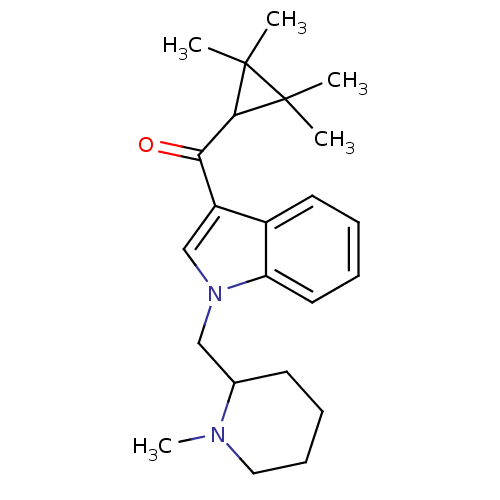 ((1-((1-Methylpiperidin-2-yl)methyl)-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM135905 (US8859596, 193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133315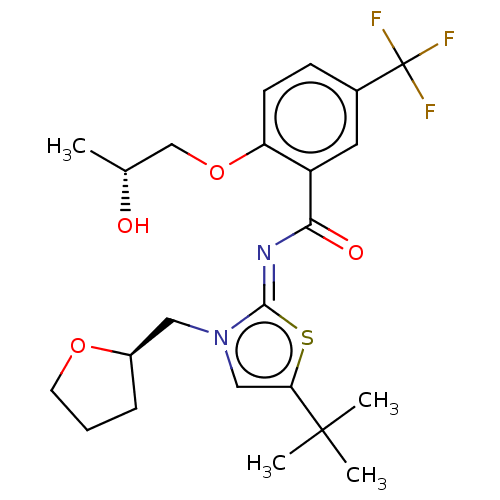 (US8846730, 81) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM9807 (US8846730, 50) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133275 (US8846730, 22) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM133276 (US8846730, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM133316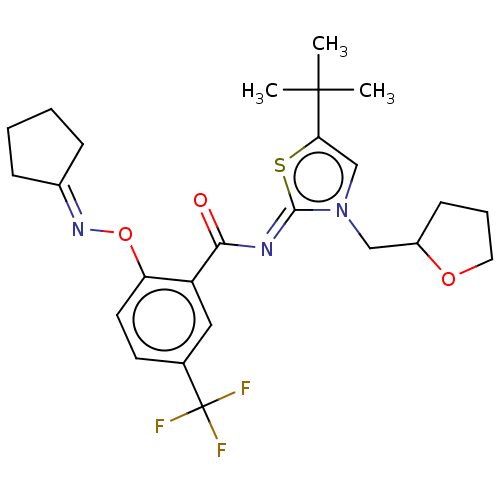 (US8846730, 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133313 (US8846730, 79) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM133308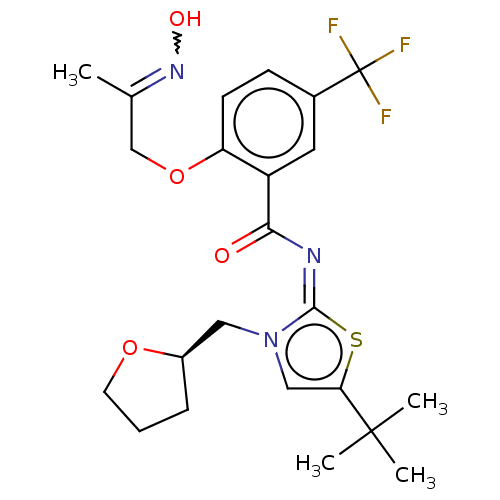 (US8846730, 73) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21300 (6-methoxy-1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50364869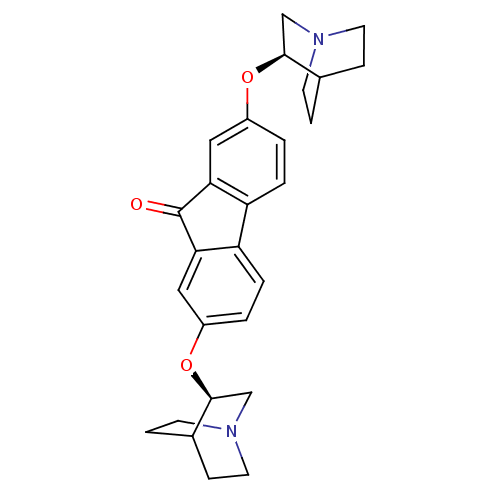 (CHEMBL1950150) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain | Bioorg Med Chem Lett 22: 1633-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.126 BindingDB Entry DOI: 10.7270/Q2D21Z2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM135882 (US8859596, 169) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM135875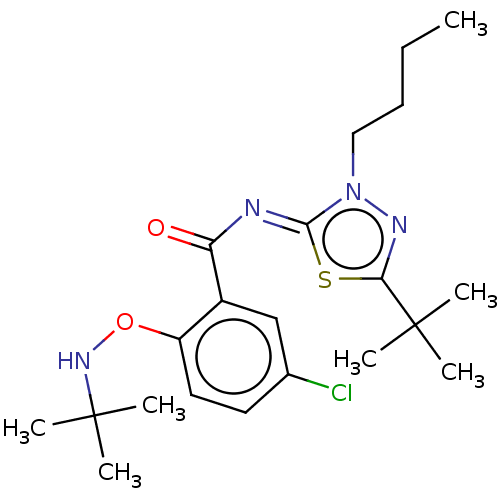 (US8859596, 162) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM9651 (US8846730, 4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8846730 (2014) BindingDB Entry DOI: 10.7270/Q2028Q6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM135882 (US8859596, 169) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
AbbVie Inc. US Patent | Assay Description HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz... | US Patent US8859596 (2014) BindingDB Entry DOI: 10.7270/Q2W66JHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1508 total ) | Next | Last >> |