

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517230 (CHEMBL4467984) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517232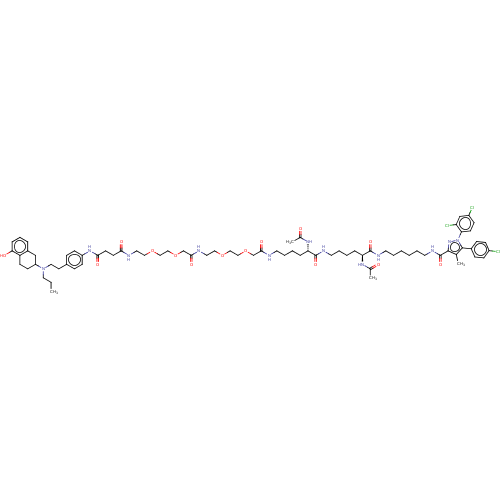 (CHEMBL4546839) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036738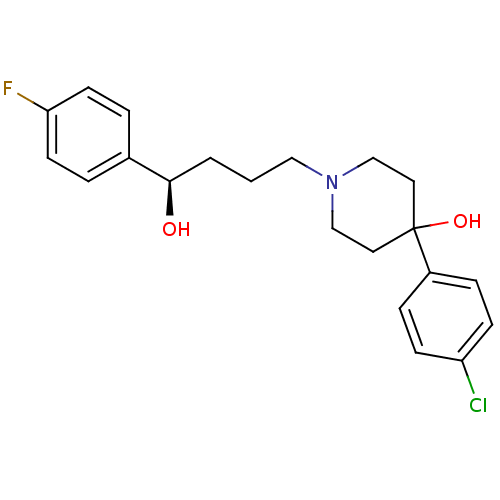 ((R)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50251208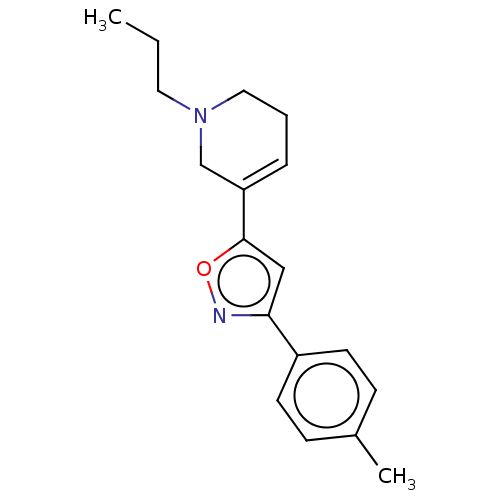 (CHEMBL4088272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517229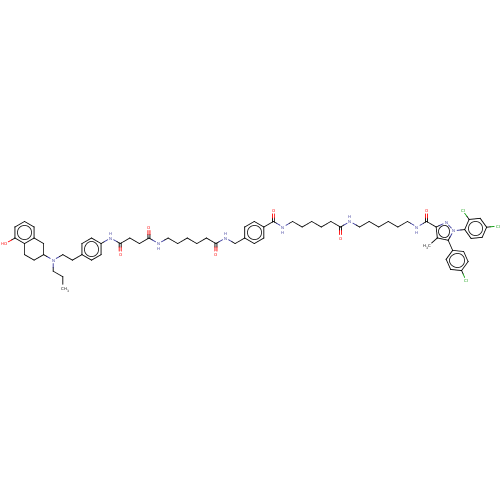 (CHEMBL4541515) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM26113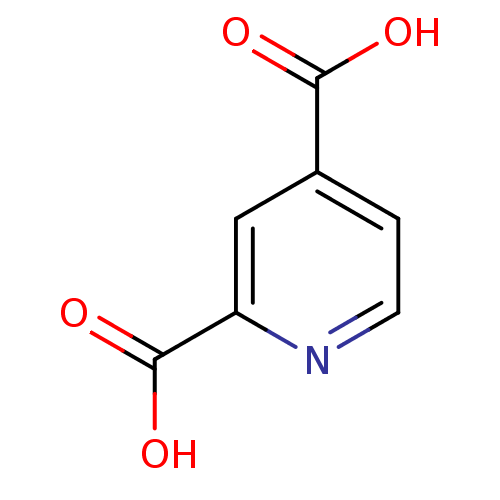 (2,4 PDCA | cid_10365 | pyridine carboxylate, 6a | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517236 (CHEMBL4465127) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-spiperone from DRD2 in rat striatum by scintillation counting | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-pentazocine from sigma1 receptor in guinea pig brain membranes by scintillation counting | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22W8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50064946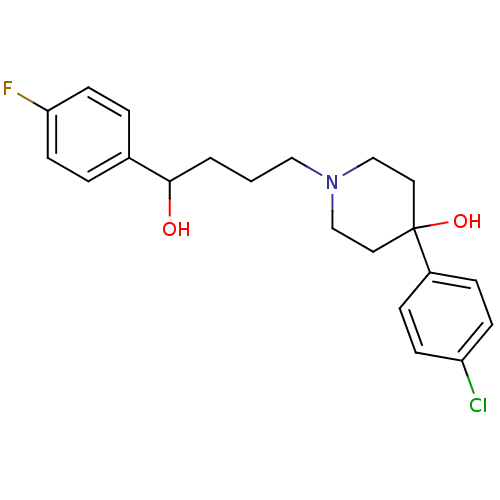 (4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-pentazocine from sigma1 receptor in guinea pig brain membranes by scintillation counting | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50064946 (4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50036734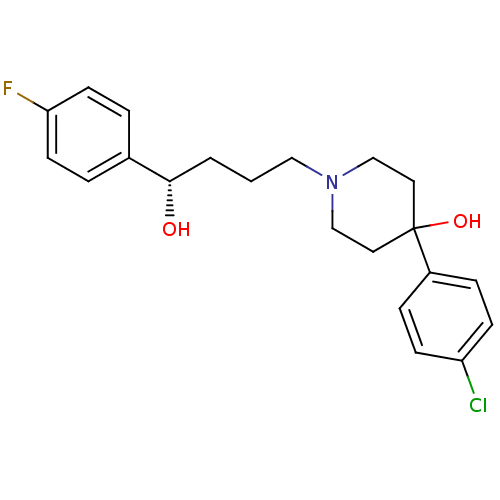 ((S)-4-(4-Chloro-phenyl)-1-[4-(4-fluoro-phenyl)-4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50387918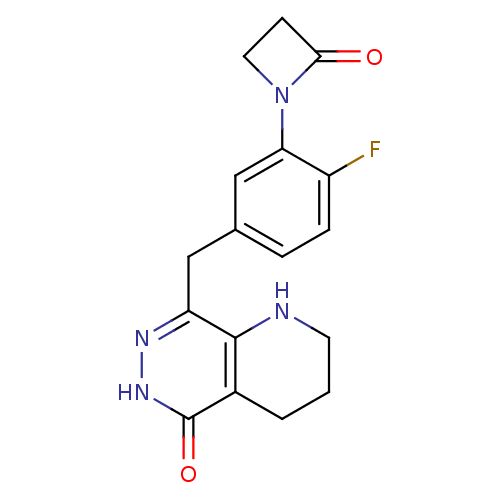 (CHEMBL2058680 | US9283222, 459) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517222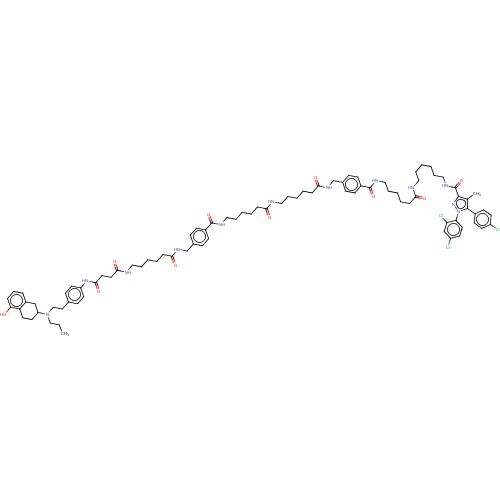 (CHEMBL4579585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082611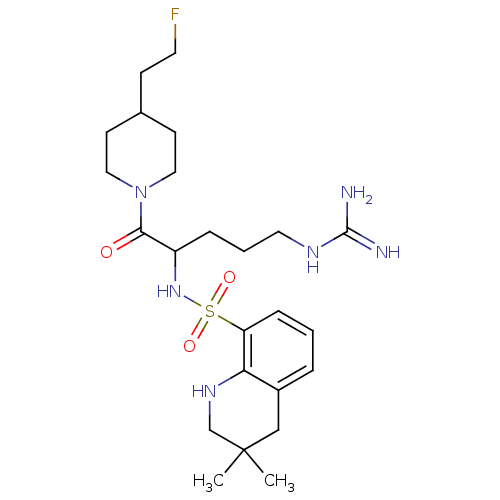 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082575 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-7-OH-DPAT from DRD3 in rat olfactory tubercle by scintillation counting | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082578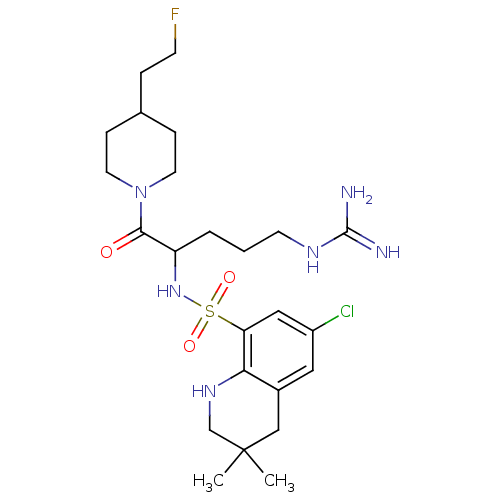 (6-Chloro-3,3-dimethyl-1,2,3,4-tetrahydro-quinoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517233 (CHEMBL4568756) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166735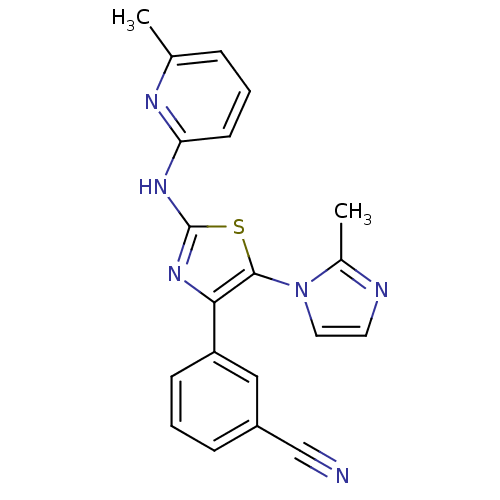 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517221 (CHEMBL4554135) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50082589 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against human thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082579 (6-Chloro-3,3-dimethyl-1,2,3,4-tetrahydro-quinoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50550237 (CHEMBL4747257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50550237 (CHEMBL4747257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-pentazocine from sigma1 receptor in guinea pig brain membranes by scintillation counting | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517223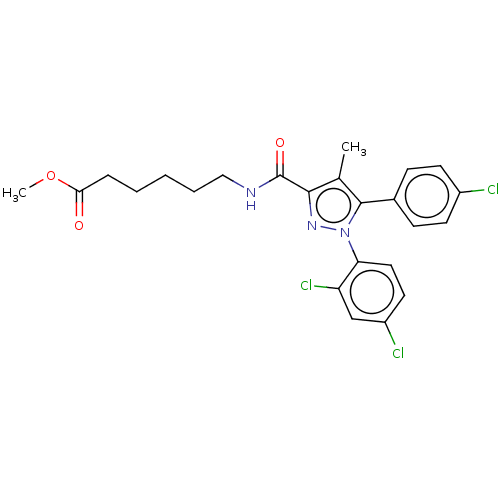 (CHEMBL4471116) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010289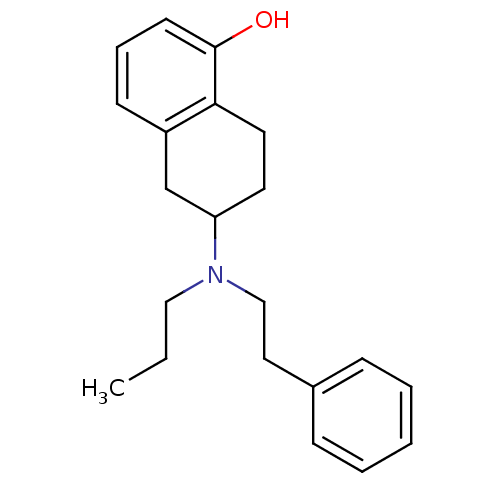 ((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H] spiperone from human D2 dopamine receptor expressed in monkey caudate-putamen membranes | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082583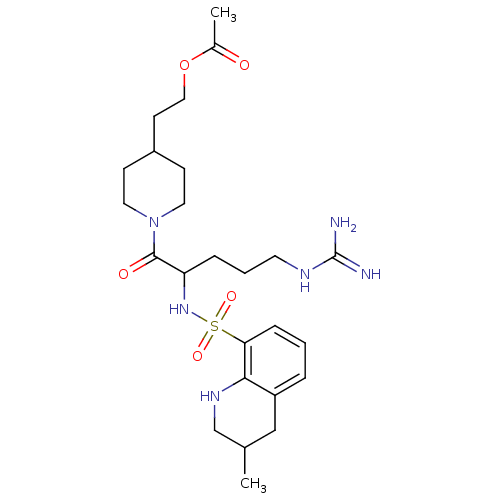 (Acetic acid 2-{1-[5-guanidino-2-(3-methyl-1,2,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50580184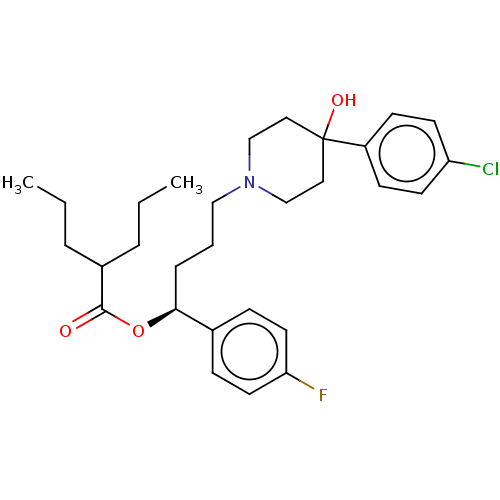 (CHEMBL5079556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by microbeta scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00995 BindingDB Entry DOI: 10.7270/Q2DZ0D56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082589 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166742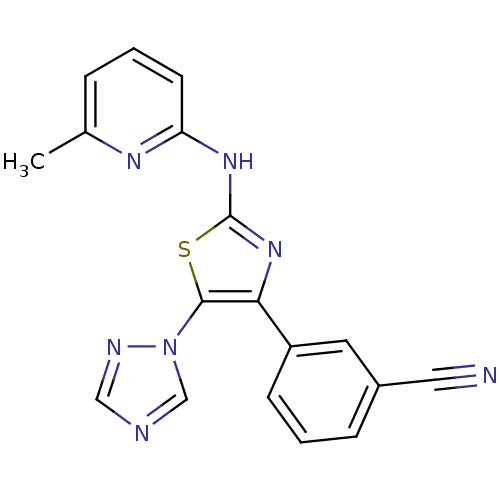 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082577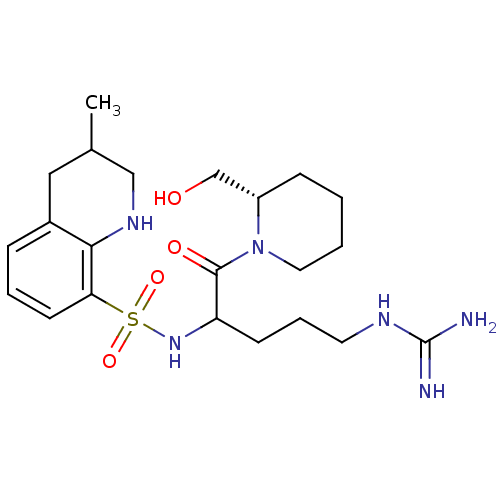 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082598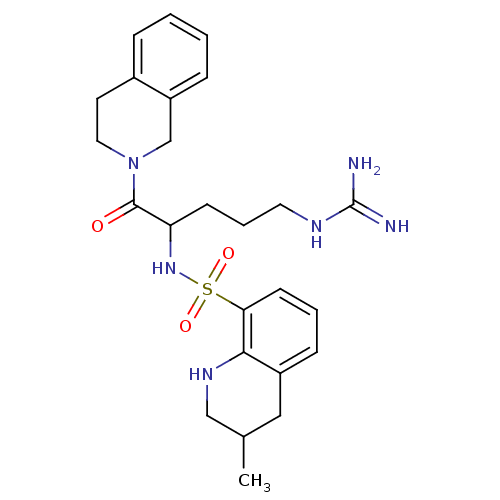 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166739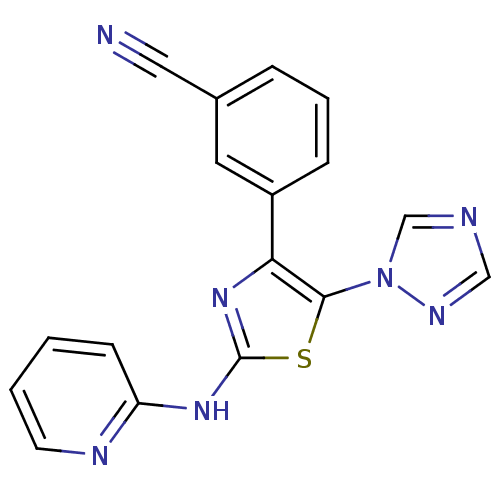 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082612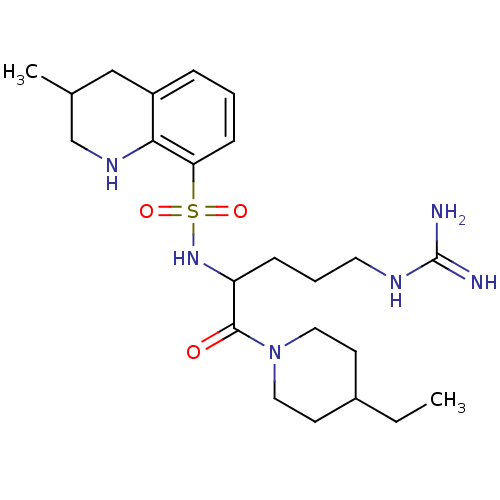 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082580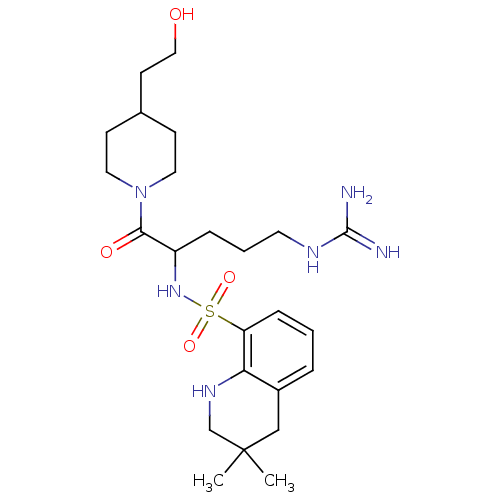 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against human thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082608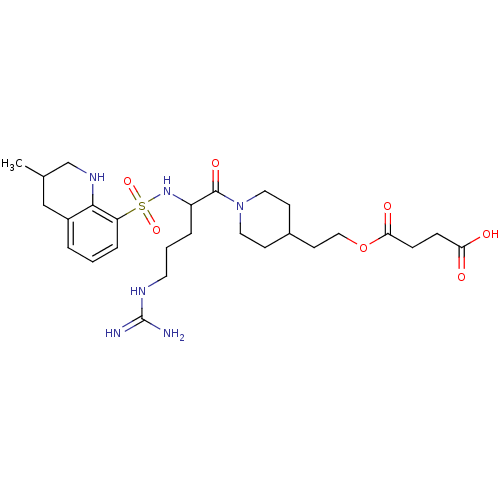 (CHEMBL139718 | Succinic acid mono-(2-{1-[5-guanidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151392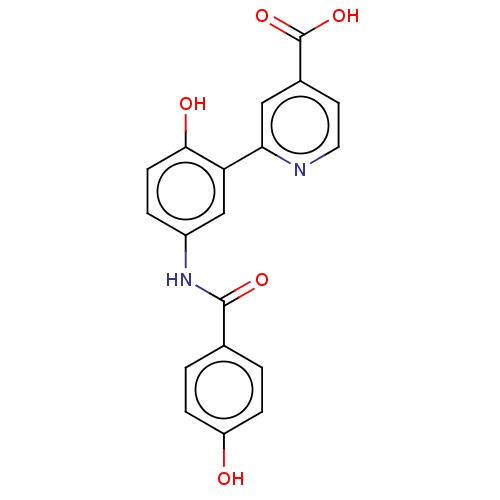 (CHEMBL3775262) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082581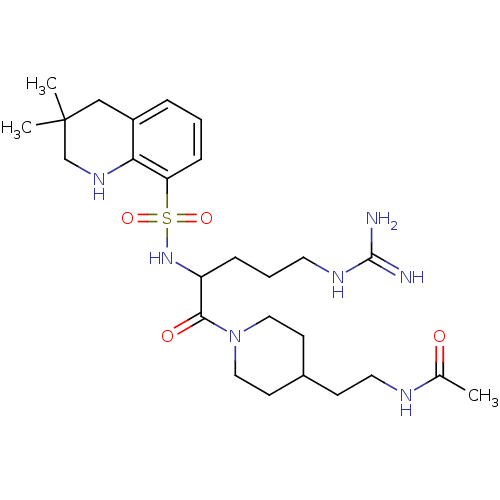 (CHEMBL143203 | N-(2-{1-[2-(3,3-Dimethyl-1,2,3,4-te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082573 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against human thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517226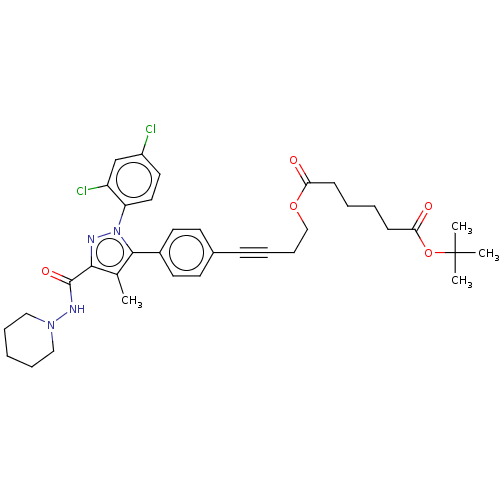 (CHEMBL4449666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082591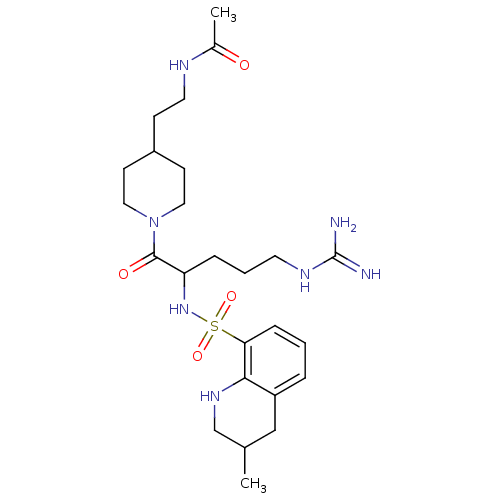 (CHEMBL139606 | N-(2-{1-[5-Guanidino-2-(3-methyl-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1490 total ) | Next | Last >> |