

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262566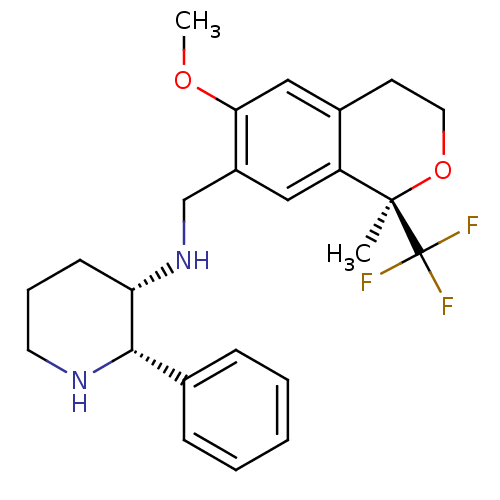 ((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176394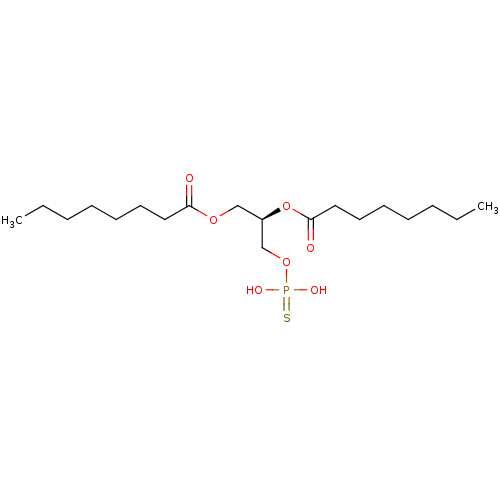 (CHEMBL202361 | octanoic acid (R)-2-octanoyloxy-3-t...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 2 (Homo sapiens (Human)) | BDBM50271765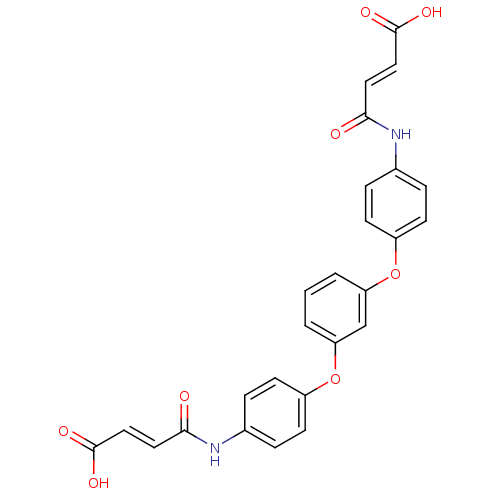 (3-(4-{3-[4-(3-Carboxy-acryloylamino)-phenoxy]-phen...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA2 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262395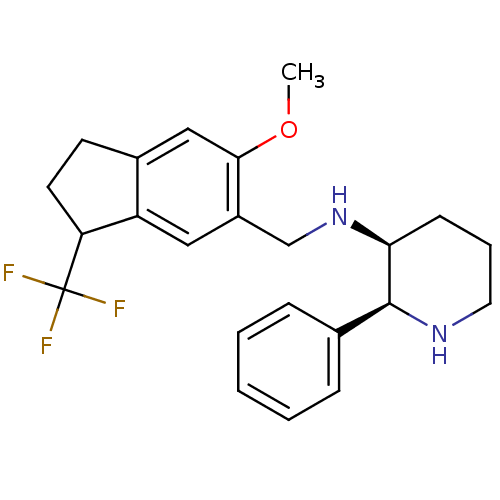 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50067935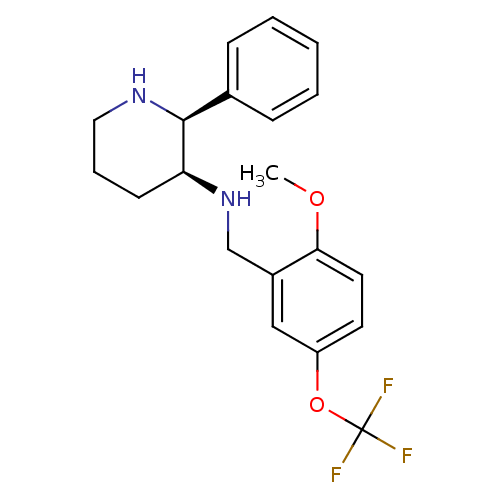 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 2 (Homo sapiens (Human)) | BDBM50271763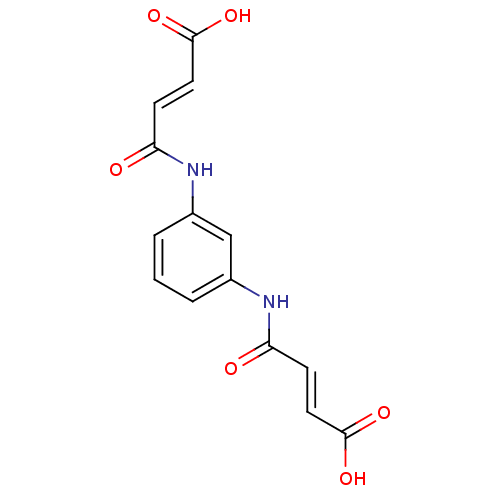 (3-[3-(3-Carboxy-acryloylamino)-phenylcarbamoyl]-ac...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA2 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 2 (Homo sapiens (Human)) | BDBM50271764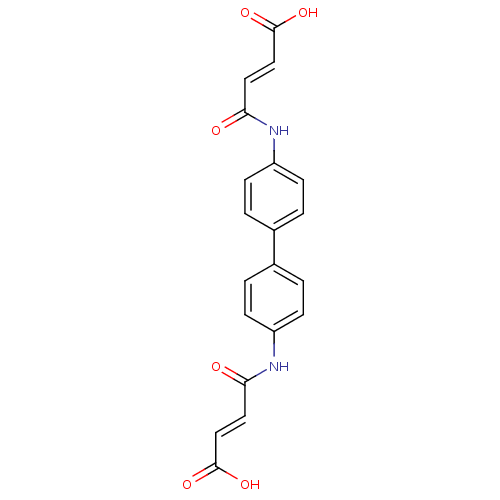 (3-[4'-(3-Carboxy-acryloylamino)-biphenyl-4-ylcarba...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA2 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176391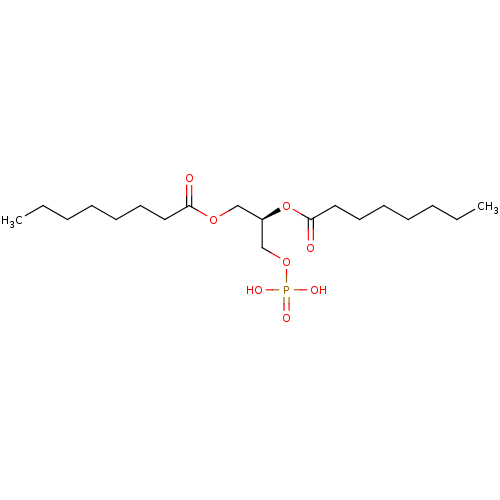 (CHEMBL202185 | octanoic acid (R)-2-octanoyloxy-3-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50271763 (3-[3-(3-Carboxy-acryloylamino)-phenylcarbamoyl]-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176397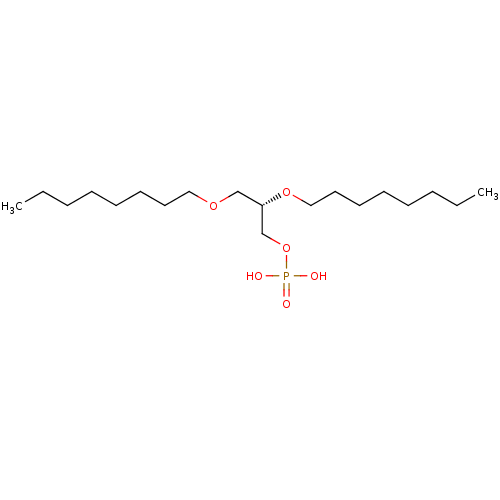 (CHEMBL203986 | nonanoic acid (S)-2-nonanoyloxy-3-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50193515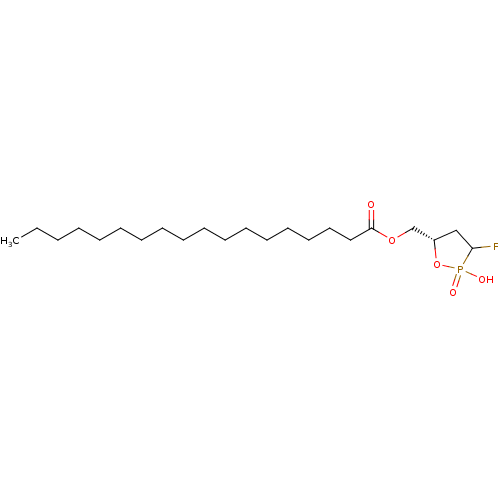 (1-fluoro-(3S)-hydroxyl-4-oleoyloxylbutane 1,3-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA1 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262280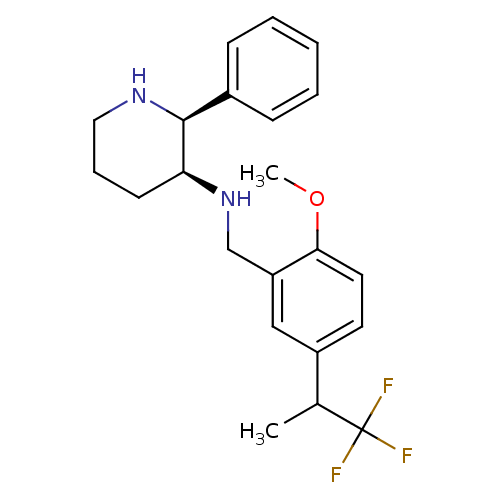 (CHEMBL513351 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Rattus norvegicus) | BDBM50177339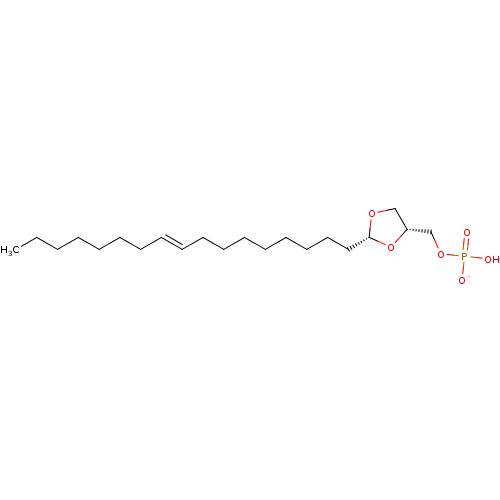 (CHEMBL436763 | potassium ((2R,4R)-2-(heptadec-9-en...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176392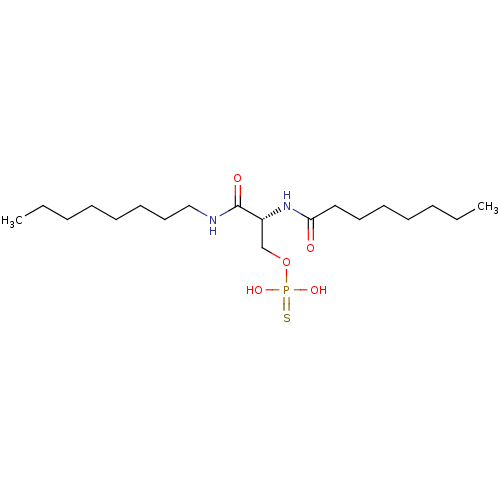 (CHEMBL379248 | thiophosphoric acid (R)-2-octanoyla...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176393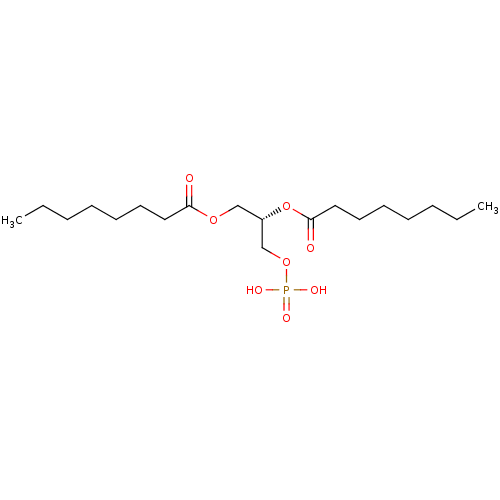 (CHEMBL383095 | octanoic acid (S)-2-octanoyloxy-3-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262510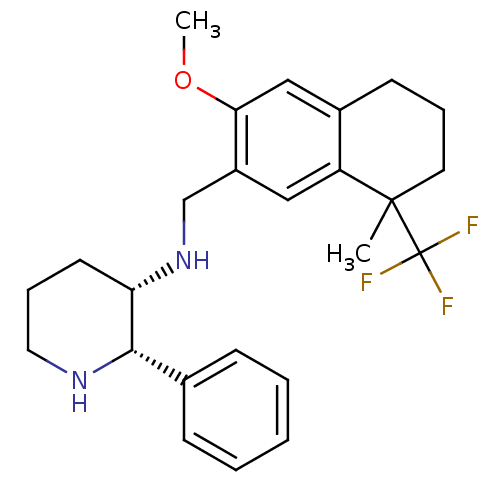 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50176398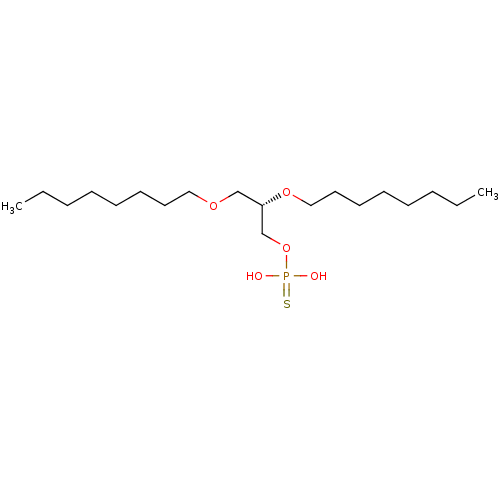 ((S)-O-2,3-bis(octyloxy)propyl O,O-dihydrogen phosp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Rattus norvegicus) | BDBM50177329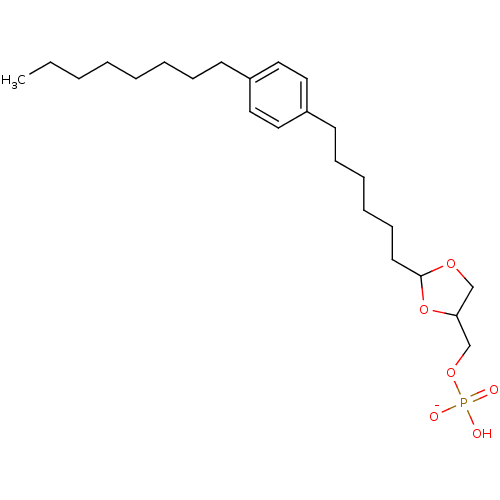 (CHEMBL199380 | potassium (2-(6-(4-octylphenyl)hexy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176396 (CHEMBL204037 | Serine amide phospate derivative | ...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50176393 (CHEMBL383095 | octanoic acid (S)-2-octanoyloxy-3-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Rattus norvegicus) | BDBM50177340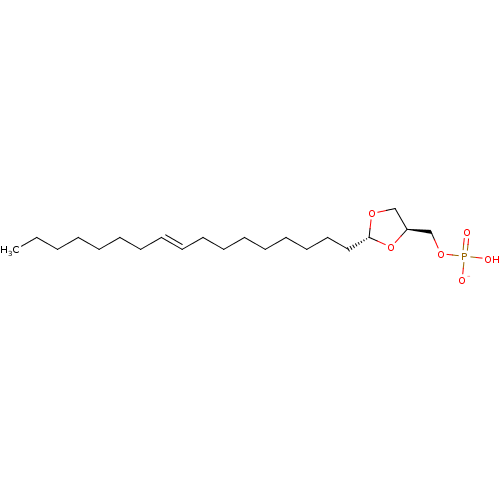 (CHEMBL382116 | potassium ((2R,4S)-2-(heptadec-9-en...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50271765 (3-(4-{3-[4-(3-Carboxy-acryloylamino)-phenoxy]-phen...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA3 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50193518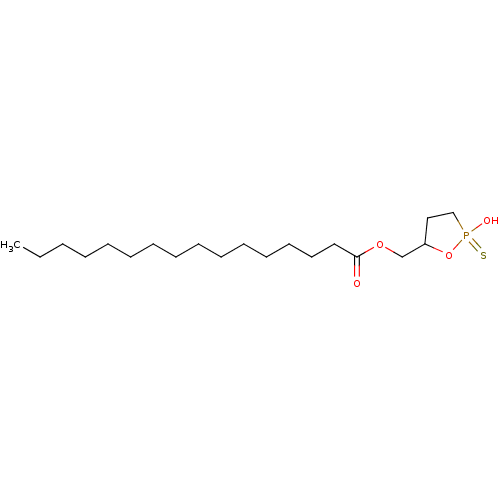 (3-hydroxyl-4-palmitoyloxylbutane 1,3-cyclic phosph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA1 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50241460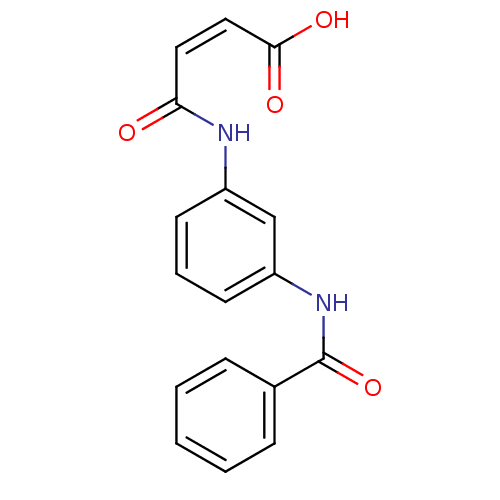 (4-(3-benzamidophenylamino)-4-oxobut-2-enoic acid |...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 317 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA3 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50176394 (CHEMBL202361 | octanoic acid (R)-2-octanoyloxy-3-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50193517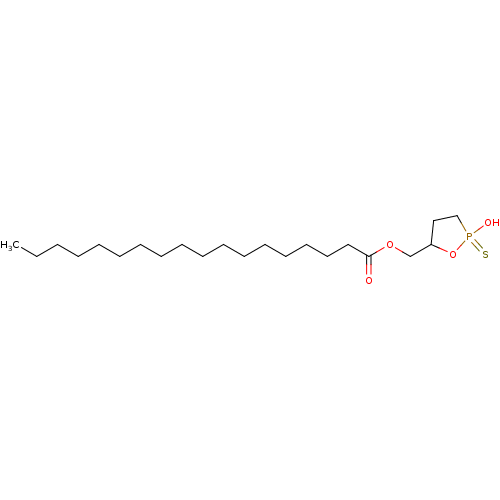 (3-hydroxyl-4-oleoyloxylbutane 1,3-cyclic phosphono...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 403 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA1 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50176391 (CHEMBL202185 | octanoic acid (R)-2-octanoyloxy-3-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262567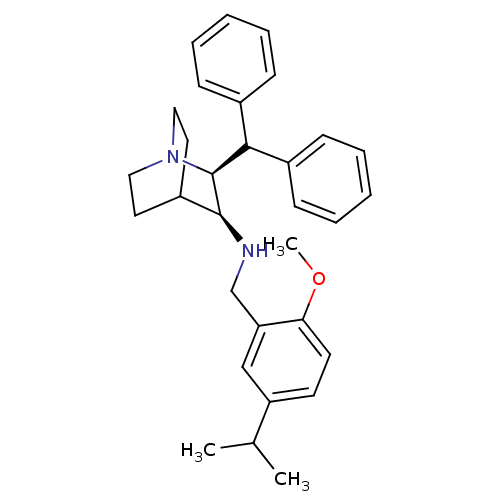 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176395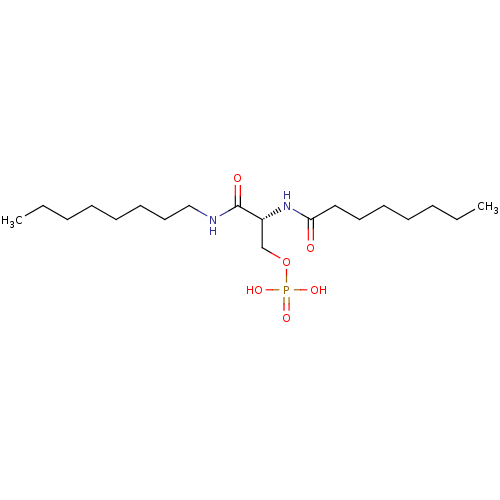 (CHEMBL202251 | phosphoric acid mono-((R)-2-octanoy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 489 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176395 (CHEMBL202251 | phosphoric acid mono-((R)-2-octanoy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 489 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50177336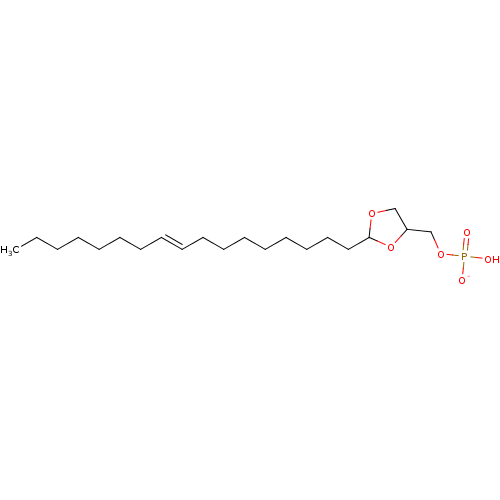 (CHEMBL373007 | potassium (2-(heptadec-9-enyl)-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50193517 (3-hydroxyl-4-oleoyloxylbutane 1,3-cyclic phosphono...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 636 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA3 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50177331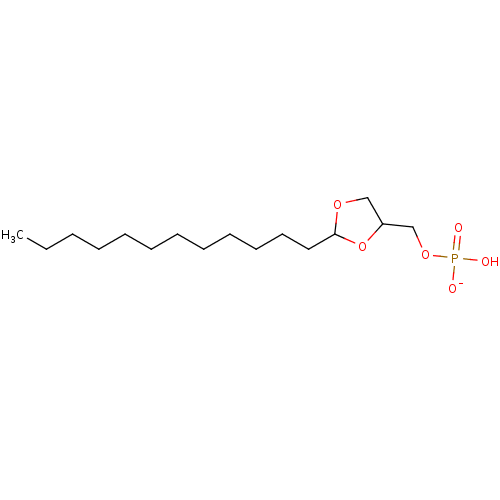 (CHEMBL381470 | potassium (2-dodecyl-1,3-dioxolan-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 652 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262279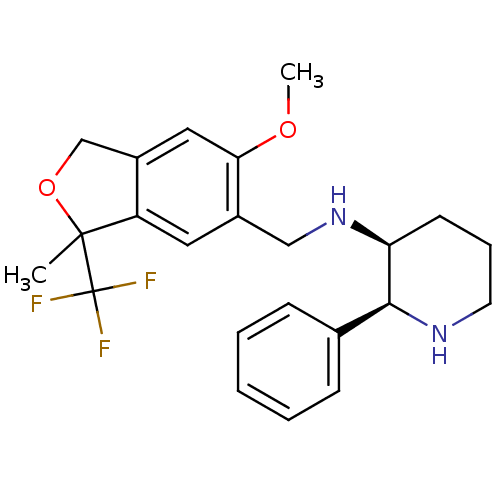 ((2S,3S)-N-((6-methoxy-3-methyl-3-(trifluoromethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 2 (Homo sapiens (Human)) | BDBM50177331 (CHEMBL381470 | potassium (2-dodecyl-1,3-dioxolan-4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA2 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 2 (Homo sapiens (Human)) | BDBM50177331 (CHEMBL381470 | potassium (2-dodecyl-1,3-dioxolan-4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA2 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332461 ((R)-3-carba cyclic-phosphatidic acid | CHEMBL16300...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of recombinant ATX mediated hydrolysis of FS-3 | Bioorg Med Chem Lett 20: 7525-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.115 BindingDB Entry DOI: 10.7270/Q2XD11X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50224198 ((2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50193518 (3-hydroxyl-4-palmitoyloxylbutane 1,3-cyclic phosph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA3 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332460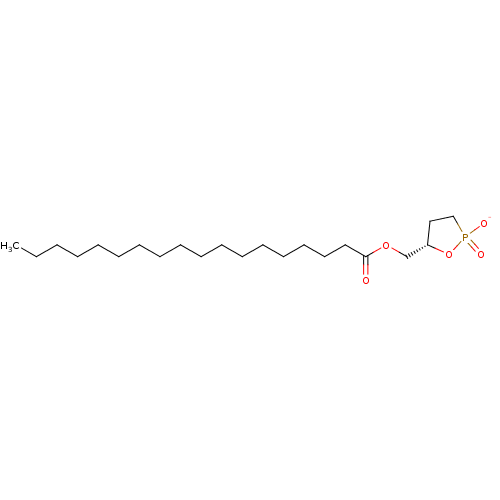 ((S)-carba cyclic-phosphatidic acid | CHEMBL1630084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of recombinant ATX mediated hydrolysis of FS-3 | Bioorg Med Chem Lett 20: 7525-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.115 BindingDB Entry DOI: 10.7270/Q2XD11X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50177329 (CHEMBL199380 | potassium (2-(6-(4-octylphenyl)hexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262337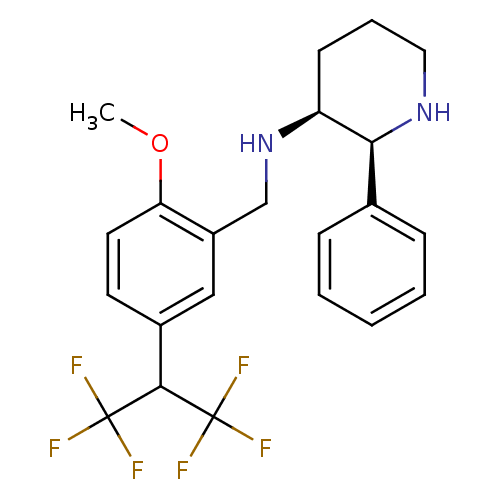 ((2S,3S)-N-(5-(1,1,1,3,3,3-hexafluoropropan-2-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50176392 (CHEMBL379248 | thiophosphoric acid (R)-2-octanoyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50176392 (CHEMBL379248 | thiophosphoric acid (R)-2-octanoyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50193515 (1-fluoro-(3S)-hydroxyl-4-oleoyloxylbutane 1,3-cycl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA3 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262281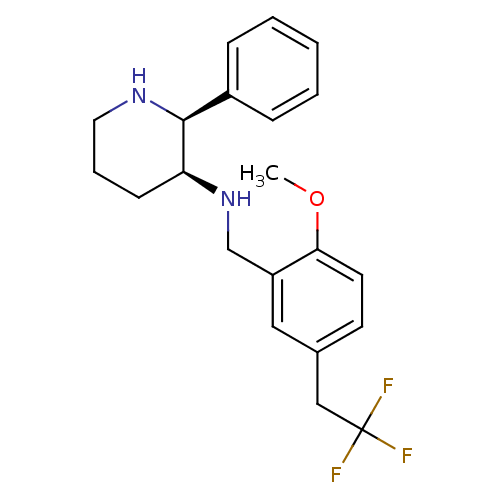 ((2S,3S)-3-[2-Methoxy-5-(2,2,2-trifluoroethyl)benzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50271764 (3-[4'-(3-Carboxy-acryloylamino)-biphenyl-4-ylcarba...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA3 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50271765 (3-(4-{3-[4-(3-Carboxy-acryloylamino)-phenoxy]-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262279 ((2S,3S)-N-((6-methoxy-3-methyl-3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50224198 ((2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 209 total ) | Next | Last >> |