

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521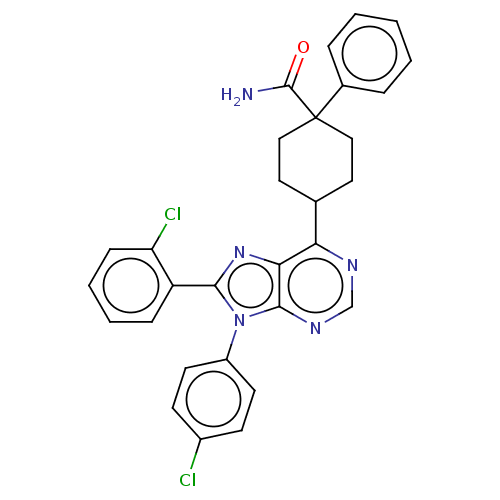 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383388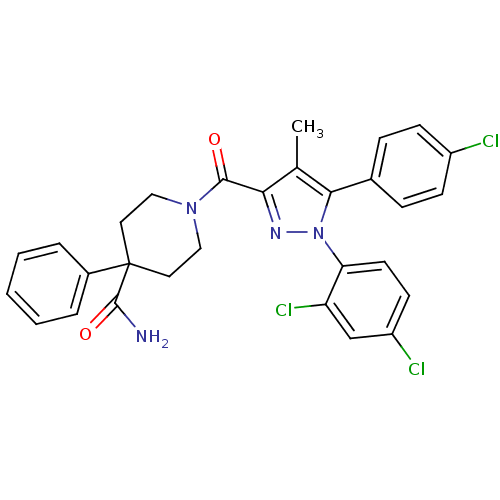 (CHEMBL2030738) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399518 (CHEMBL2180214) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192536 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399523 (CHEMBL2180205) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192536 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399534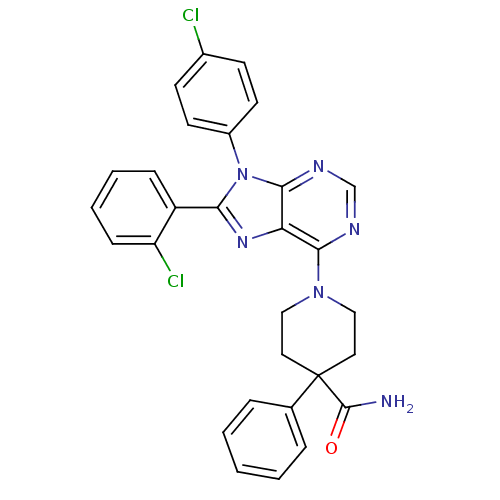 (CHEMBL2180215) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50144618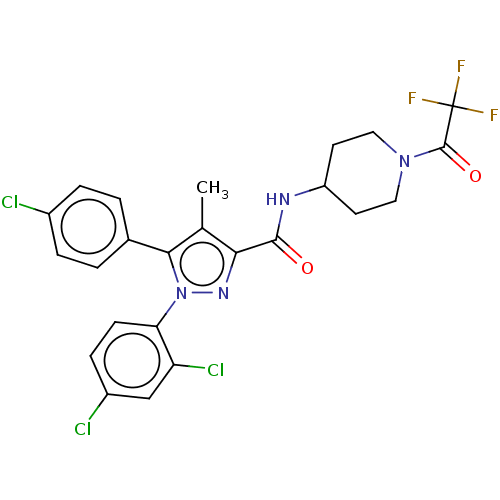 (CHEMBL3758534) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192534 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192534 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192538 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192538 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192530 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50493599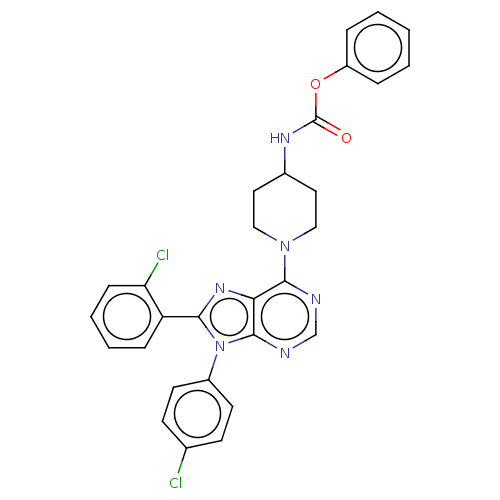 (CHEMBL2436755) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192537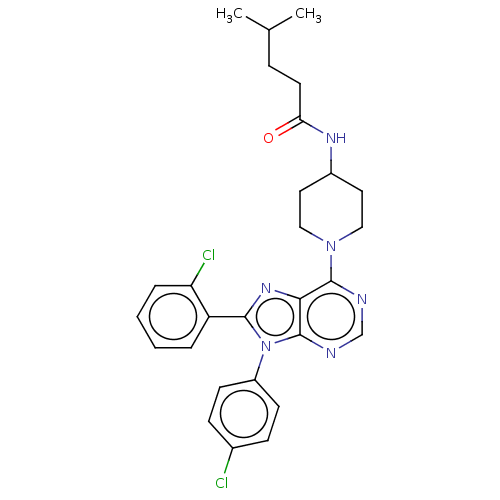 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192537 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383402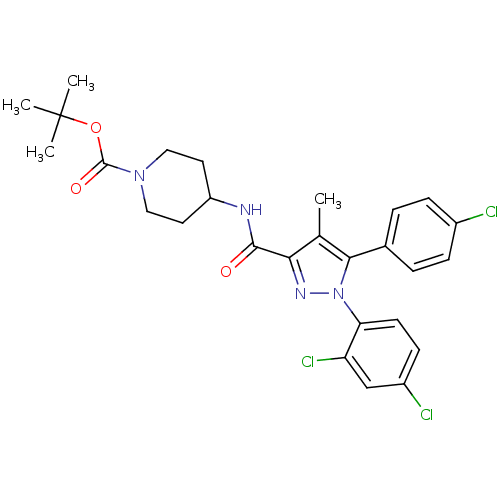 (CHEMBL2030754) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192532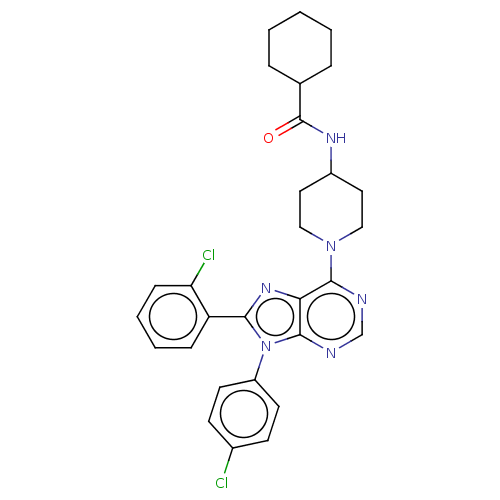 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192532 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383388 (CHEMBL2030738) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383388 (CHEMBL2030738) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192529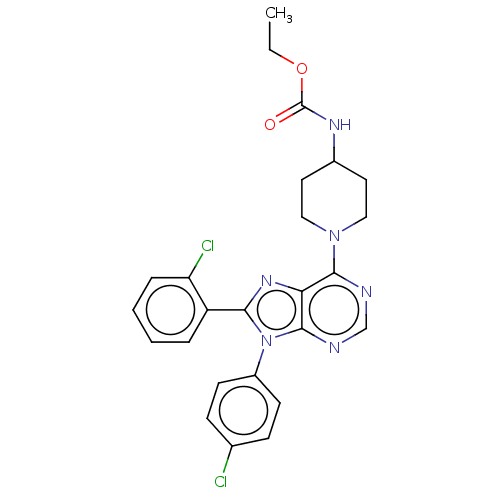 (US9187480, ethyl N-{1-[8-(2-chlorophenyl)-9-(4-chl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192529 (US9187480, ethyl N-{1-[8-(2-chlorophenyl)-9-(4-chl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192535 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192535 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192528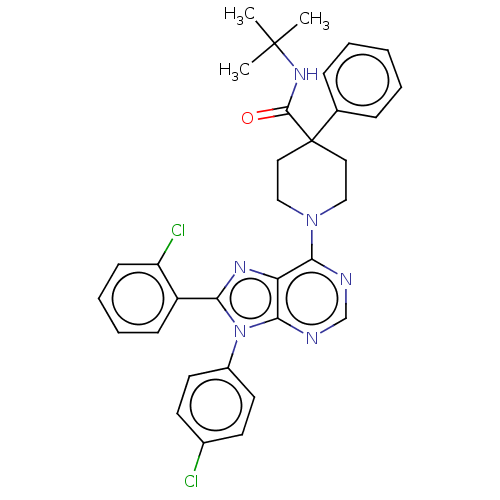 (US9187480, N-tert-butyl-1-[8-(2-chlorophenyl)-9-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192527 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192531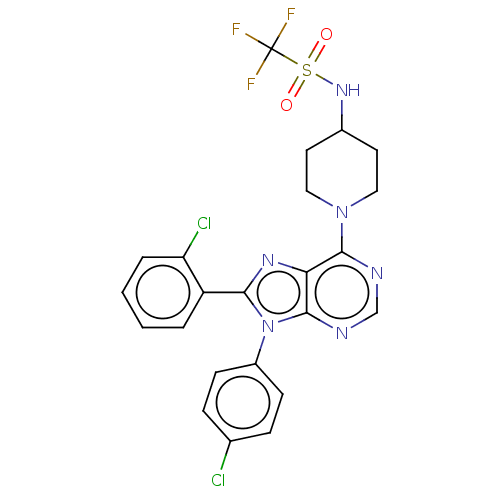 (US9187480, 8-(2-chlorophenyl)-9-(4-chlorophenyl)-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 56: 8066-72 (2013) Article DOI: 10.1021/jm401129n BindingDB Entry DOI: 10.7270/Q2F192NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192531 (US9187480, 8-(2-chlorophenyl)-9-(4-chlorophenyl)-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399531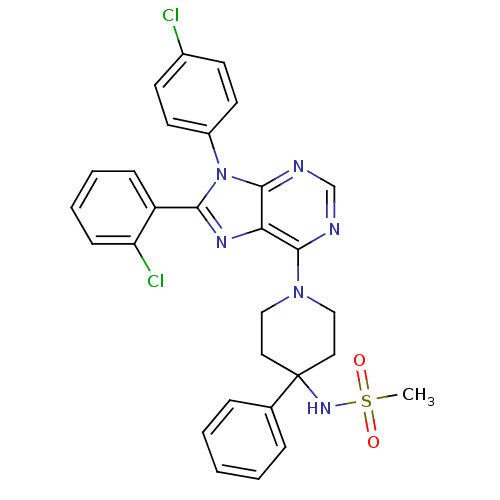 (CHEMBL2180218) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192526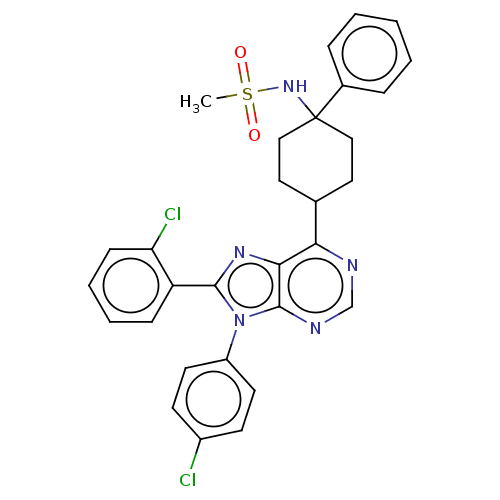 (US9187480, N-{4-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50144619 (CHEMBL3759785) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399533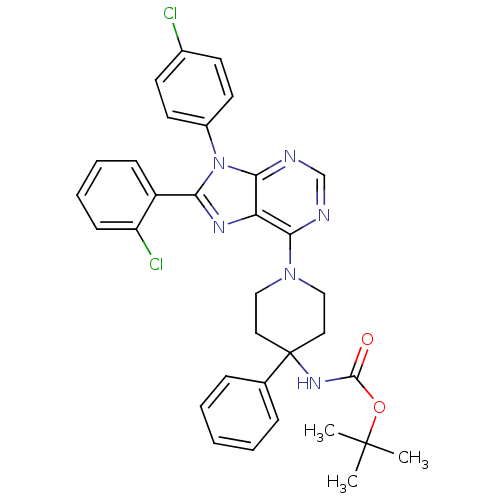 (CHEMBL2180216 | US9187480, tert-butyl N-{1-[8-(2-c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399533 (CHEMBL2180216 | US9187480, tert-butyl N-{1-[8-(2-c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 7.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383386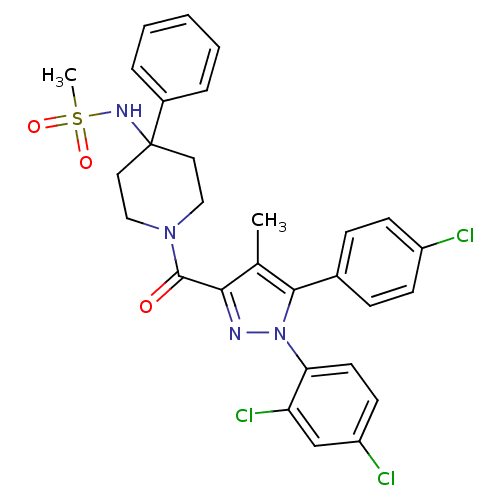 (CHEMBL2029362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50144614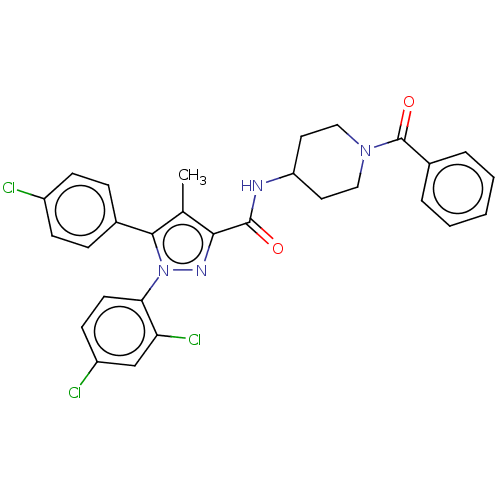 (CHEMBL3758884) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383399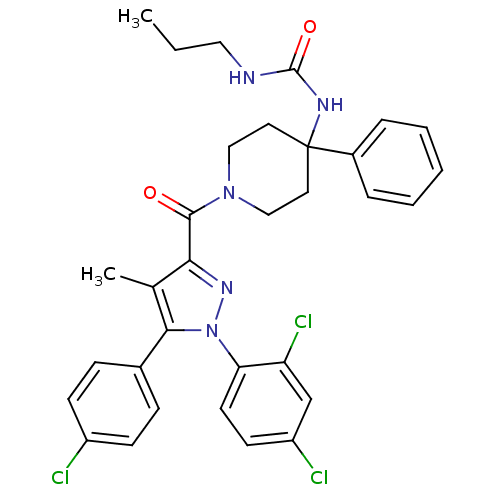 (CHEMBL2030751) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50354809 (CHEMBL1834021) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 21: 5711-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.032 BindingDB Entry DOI: 10.7270/Q2V988HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50144609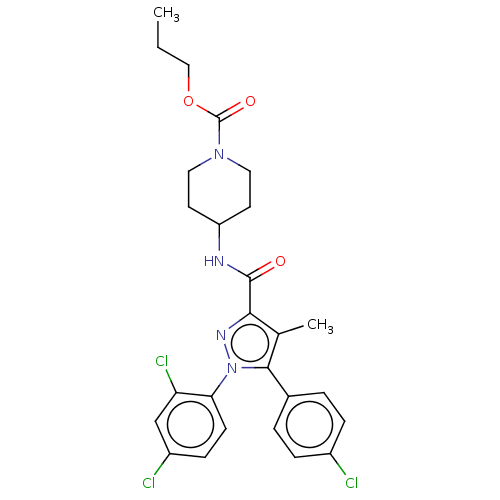 (CHEMBL3759222) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383387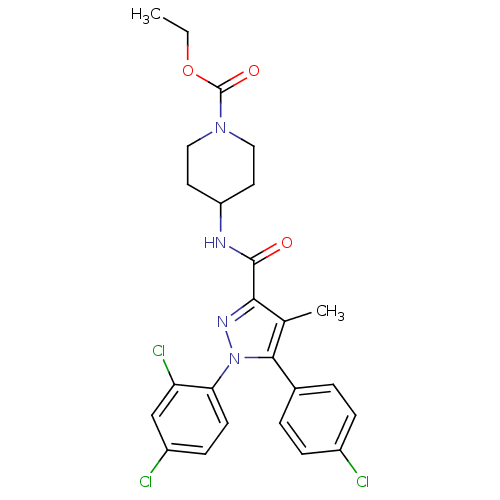 (CHEMBL2030765) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50144615 (CHEMBL3759255) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50144612 (CHEMBL3759254) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 24: 1063-70 (2016) Article DOI: 10.1016/j.bmc.2016.01.033 BindingDB Entry DOI: 10.7270/Q2GB25WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399532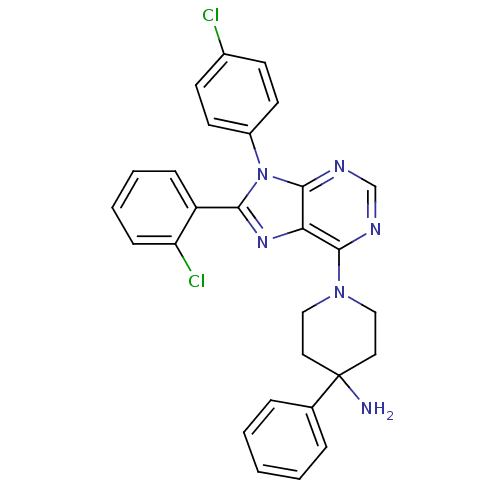 (CHEMBL2180217) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50354809 (CHEMBL1834021) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 21: 5711-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.032 BindingDB Entry DOI: 10.7270/Q2V988HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192525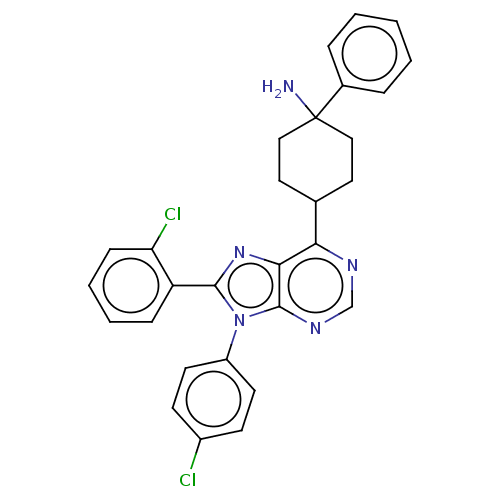 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383413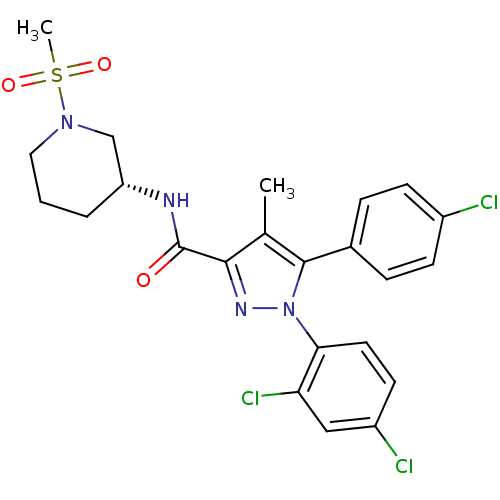 (CHEMBL2030933) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140231 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO-K1 cells after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 21: 5711-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.032 BindingDB Entry DOI: 10.7270/Q2V988HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 247 total ) | Next | Last >> |