

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521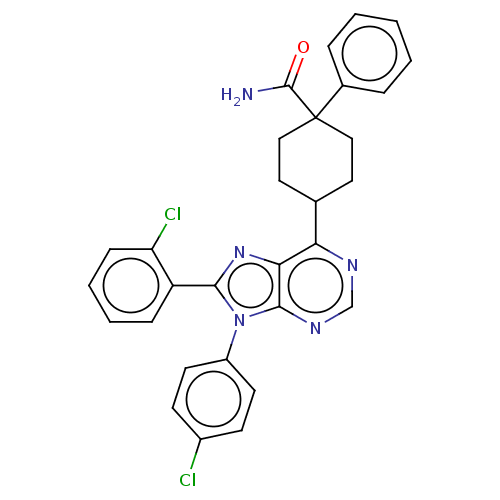 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192536 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192534 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192538 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192530 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192537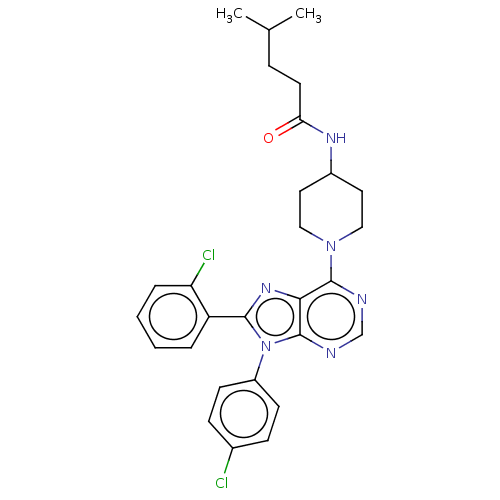 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192532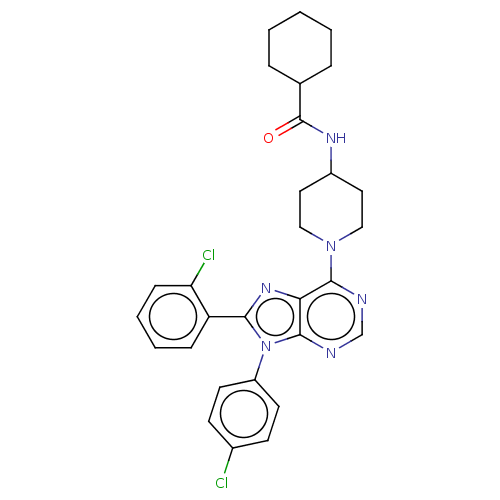 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192529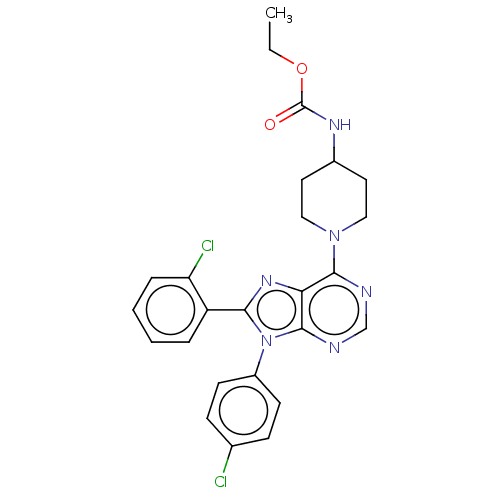 (US9187480, ethyl N-{1-[8-(2-chlorophenyl)-9-(4-chl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192535 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192528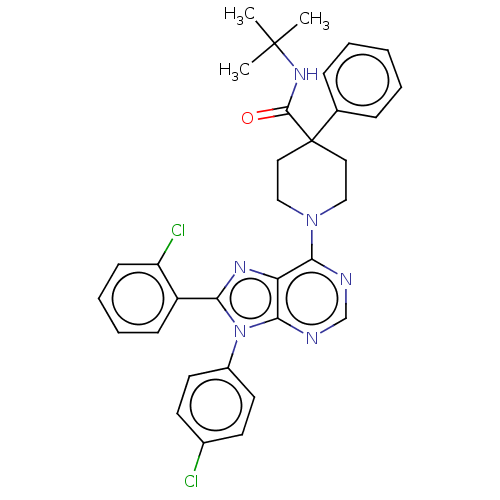 (US9187480, N-tert-butyl-1-[8-(2-chlorophenyl)-9-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192527 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192531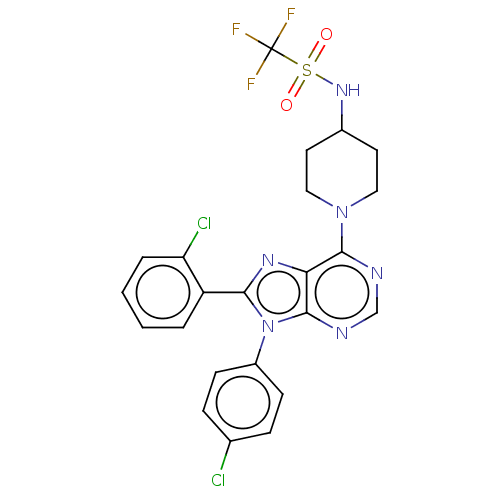 (US9187480, 8-(2-chlorophenyl)-9-(4-chlorophenyl)-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192526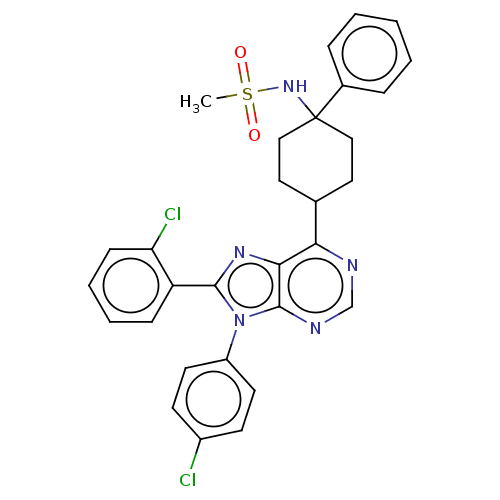 (US9187480, N-{4-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399533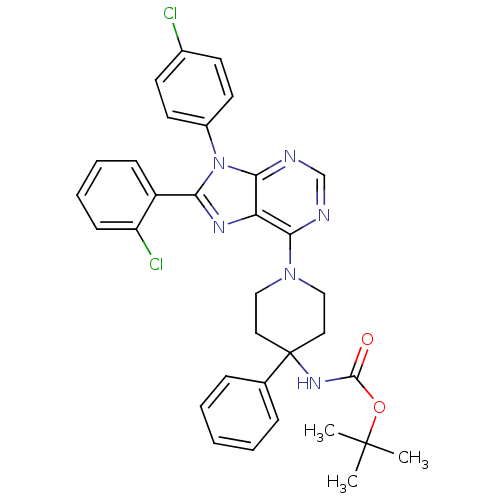 (CHEMBL2180216 | US9187480, tert-butyl N-{1-[8-(2-c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 7.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192525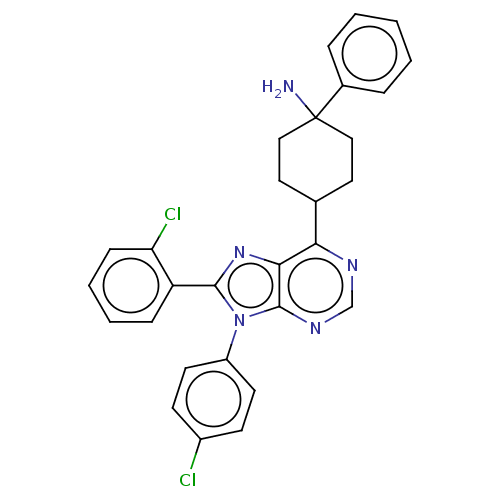 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192524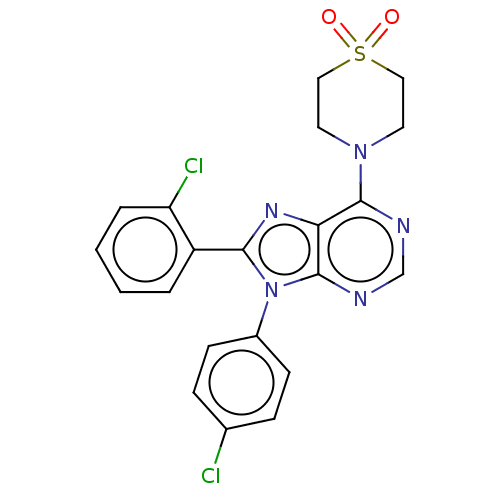 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192533 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399525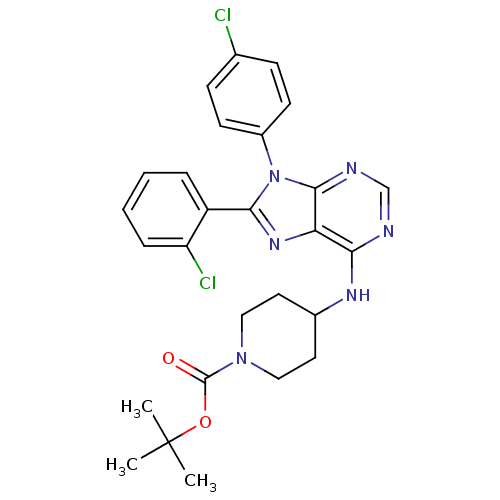 (CHEMBL2180226 | US9187480, tert-butyl 4-{[8-(2-chl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399530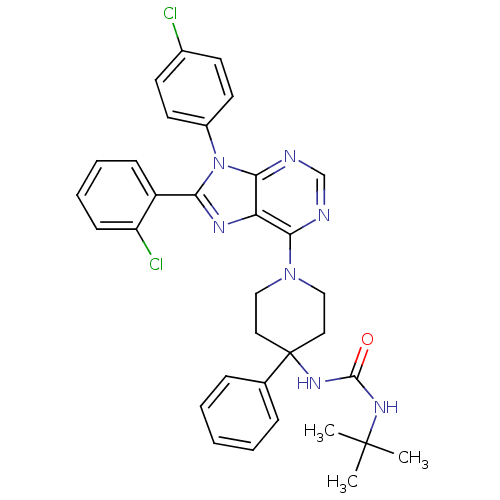 (CHEMBL2180219 | US9187480, 3-tert-butyl-1-{1-[8-(2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 25.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192524 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192522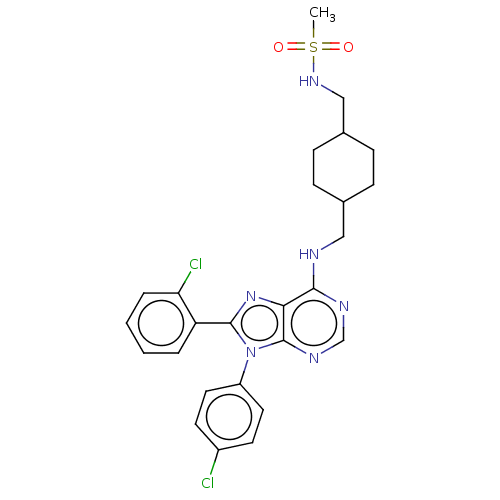 (US9187480, N-{[4-({[8-(2-chlorophenyl)-9-(4-chloro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 44.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192536 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 60.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192535 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 89.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192523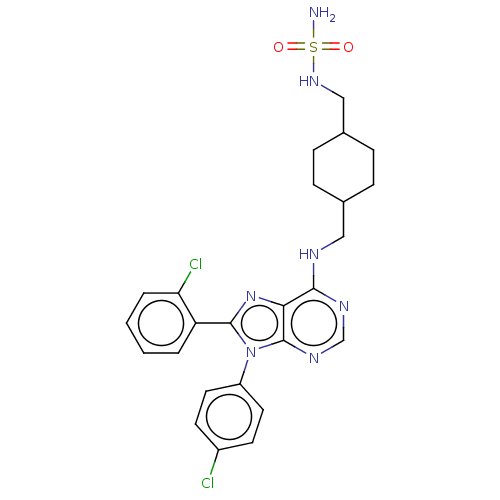 (US9187480, N-{[4-({[8-(2-chlorophenyl)-9-(4-chloro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 94.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399529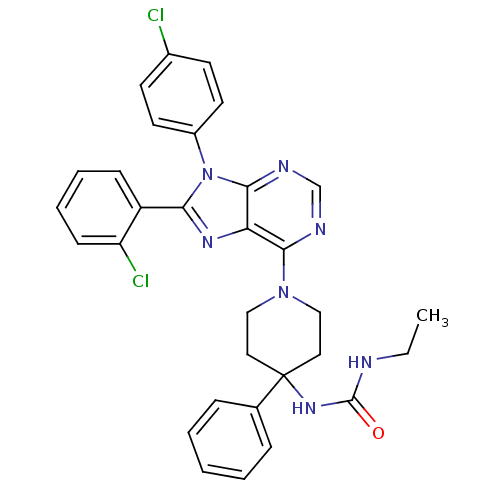 (CHEMBL2180220 | US9187480, 1-{1-[8-(2-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192537 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192522 (US9187480, N-{[4-({[8-(2-chlorophenyl)-9-(4-chloro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192523 (US9187480, N-{[4-({[8-(2-chlorophenyl)-9-(4-chloro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192529 (US9187480, ethyl N-{1-[8-(2-chlorophenyl)-9-(4-chl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192538 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192527 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 726 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192524 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 834 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192532 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 835 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192526 (US9187480, N-{4-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 948 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192533 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192525 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192522 (US9187480, N-{[4-({[8-(2-chlorophenyl)-9-(4-chloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192534 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192531 (US9187480, 8-(2-chlorophenyl)-9-(4-chlorophenyl)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50399529 (CHEMBL2180220 | US9187480, 1-{1-[8-(2-chlorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192521 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192530 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192523 (US9187480, N-{[4-({[8-(2-chlorophenyl)-9-(4-chloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50399525 (CHEMBL2180226 | US9187480, tert-butyl 4-{[8-(2-chl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50399533 (CHEMBL2180216 | US9187480, tert-butyl N-{1-[8-(2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM192528 (US9187480, N-tert-butyl-1-[8-(2-chlorophenyl)-9-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50399530 (CHEMBL2180219 | US9187480, 3-tert-butyl-1-{1-[8-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||