

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10597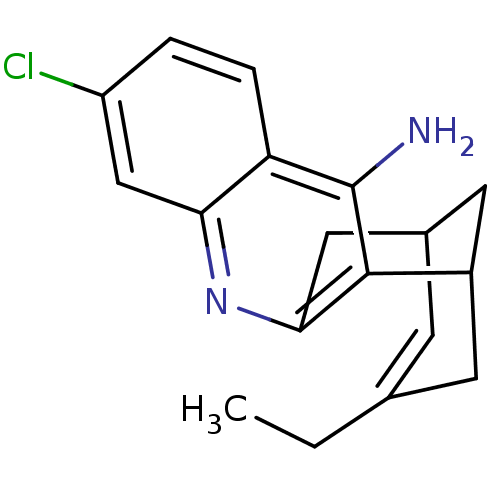 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10595 ((9E)-7-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)hep...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.40 | -46.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10594 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15.7 | -44.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10593 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16.5 | -44.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10595 ((9E)-7-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)hep...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19.5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10593 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20.8 | -43.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10594 ((9E)-N1-(7-(1,2,3,4-Tetrahydroacridin-9-ylamino)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30.8 | -42.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47 | -41.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10597 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 137 | -39.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50160284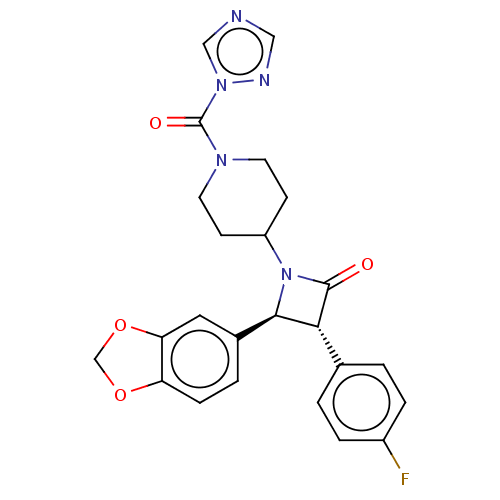 (CHEMBL3785379) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of rat MAGL | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160282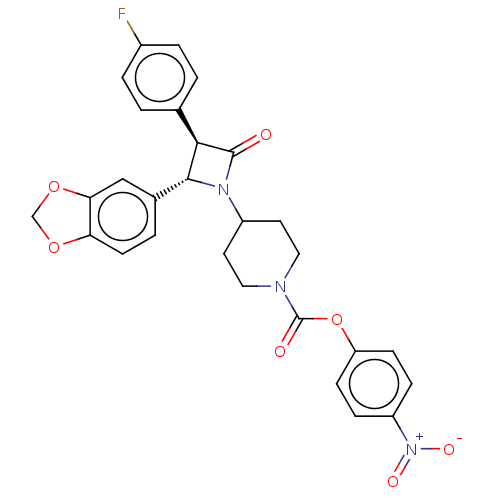 (CHEMBL3785760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50586984 (CHEMBL5084771) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50586985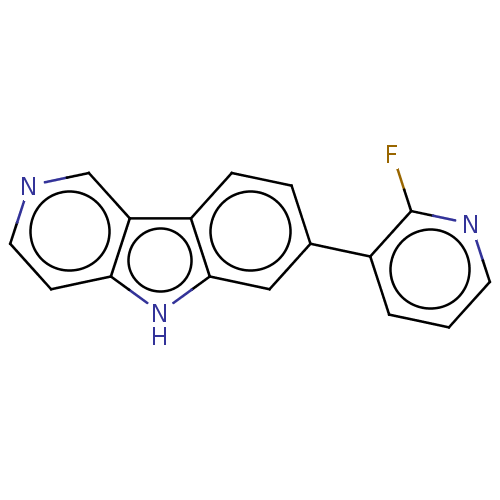 (CHEMBL5082735) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160284 (CHEMBL3785379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160284 (CHEMBL3785379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50586972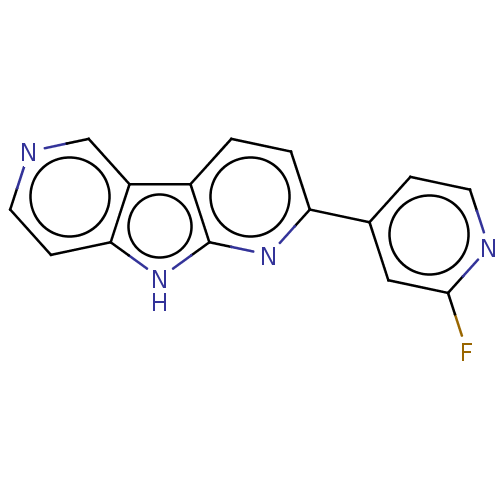 (CHEMBL5088921) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123867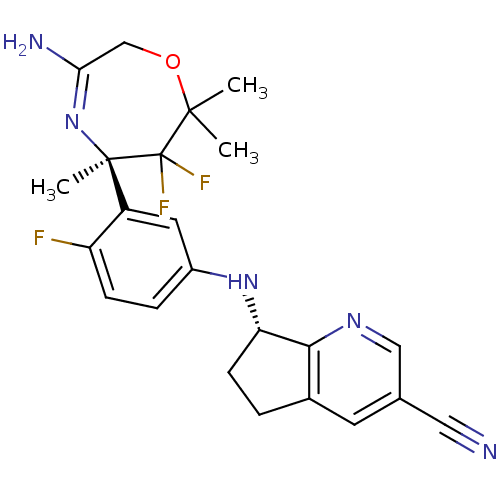 (US8748418, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Mus musculus) | BDBM50586985 (CHEMBL5082735) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [18F]-FEH from MAO-A in mouse brain homogenate incubated for 60 mins by imaging analysis | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM202632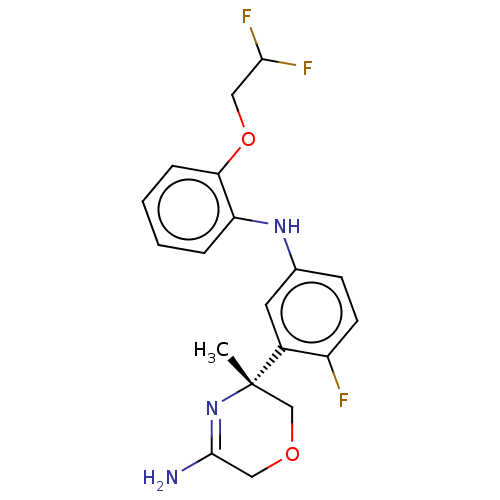 (US9242943, 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SIENA BIOTECH S.P.A.; HOFFMANN-LA ROCHE INC. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US9242943 (2016) BindingDB Entry DOI: 10.7270/Q2833QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50160284 (CHEMBL3785379) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of MAGL in rat brain membranes preincubated for 20 mins followed by fluorophosphonate-rhodamine addition measured after 30 mins by competi... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123862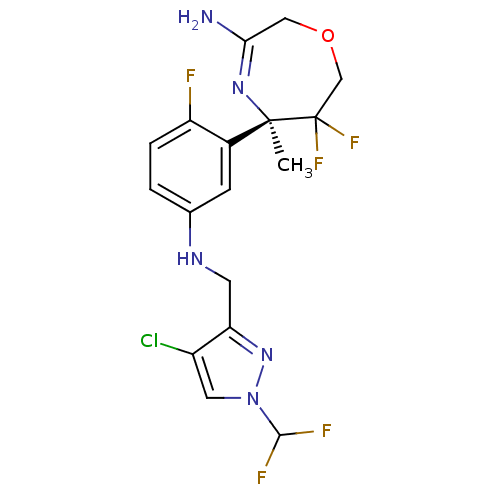 (US8748418, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM130199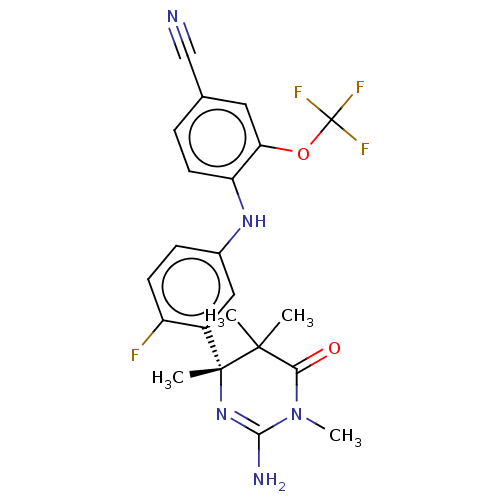 (US8815881, 161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 4 |
Hoffmann-La Roche Inc.; Siena Biotech S.p.A. US Patent | Assay Description BACE2 enzyme ectodomain (derived from plasmid pET17b-T7-hu proBACE2) was prepared as described in Ostermann et al., Crystal Structure of Human BACE2 ... | US Patent US8815881 (2014) BindingDB Entry DOI: 10.7270/Q2NZ86B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160280 (CHEMBL3787340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM130206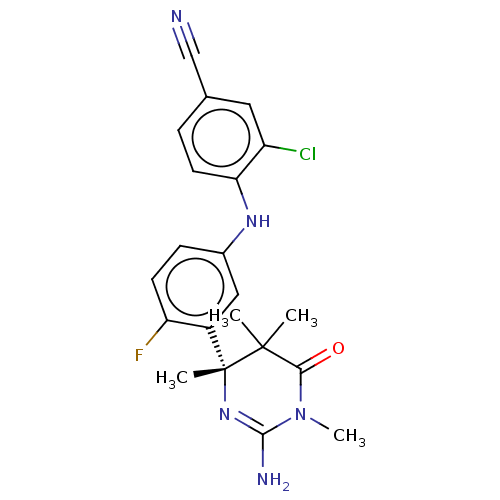 (US8815881, 168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | 4 |
Hoffmann-La Roche Inc.; Siena Biotech S.p.A. US Patent | Assay Description BACE2 enzyme ectodomain (derived from plasmid pET17b-T7-hu proBACE2) was prepared as described in Ostermann et al., Crystal Structure of Human BACE2 ... | US Patent US8815881 (2014) BindingDB Entry DOI: 10.7270/Q2NZ86B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50586975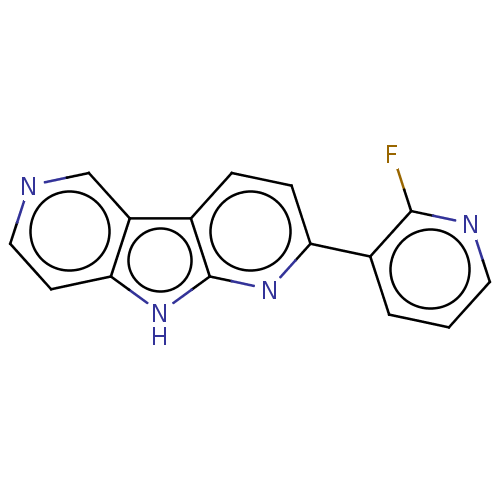 (CHEMBL5081662) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202636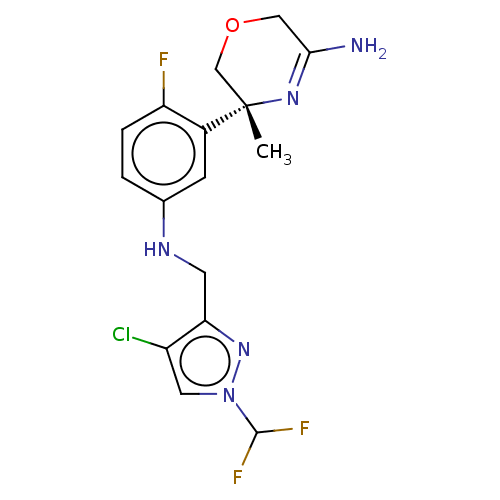 (US9242943, 45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SIENA BIOTECH S.P.A.; HOFFMANN-LA ROCHE INC. US Patent | Assay Description DNA of the human APP wt gene (APP695) were used to assess the potency of the compounds in a cellular assay. The cells were seeded in 96-well microtit... | US Patent US9242943 (2016) BindingDB Entry DOI: 10.7270/Q2833QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM202615 (US9242943, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
SIENA BIOTECH S.P.A.; HOFFMANN-LA ROCHE INC. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US9242943 (2016) BindingDB Entry DOI: 10.7270/Q2833QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50586986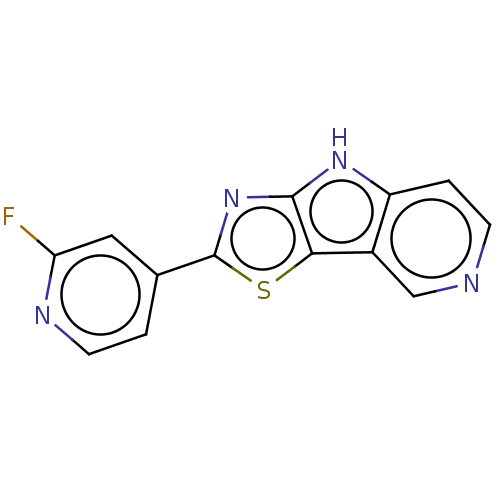 (CHEMBL5091624) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM130215 (US8815881, 150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | 4 |
Hoffmann-La Roche Inc.; Siena Biotech S.p.A. US Patent | Assay Description BACE2 enzyme ectodomain (derived from plasmid pET17b-T7-hu proBACE2) was prepared as described in Ostermann et al., Crystal Structure of Human BACE2 ... | US Patent US8815881 (2014) BindingDB Entry DOI: 10.7270/Q2NZ86B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50399931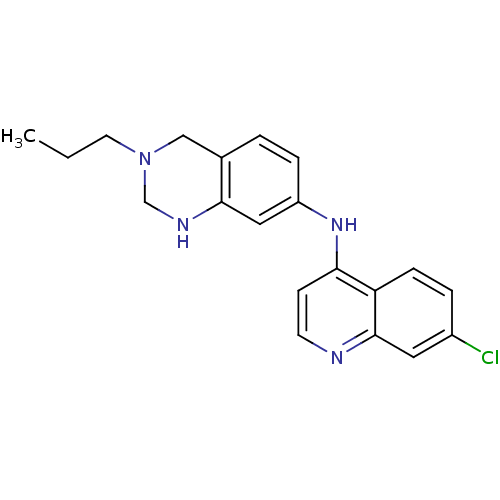 (CHEMBL2181207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in rat after 2 to 3 days by patch clamp assay | J Med Chem 55: 10387-404 (2012) Article DOI: 10.1021/jm300831b BindingDB Entry DOI: 10.7270/Q2N017PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160277 (CHEMBL3785536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123857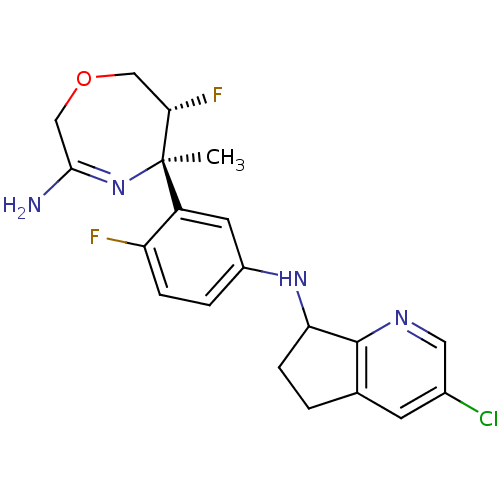 (US8748418, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123869 (US8748418, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM123862 (US8748418, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123864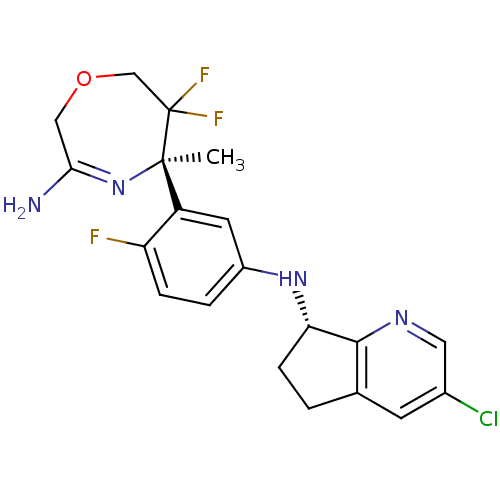 (US8748418, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160283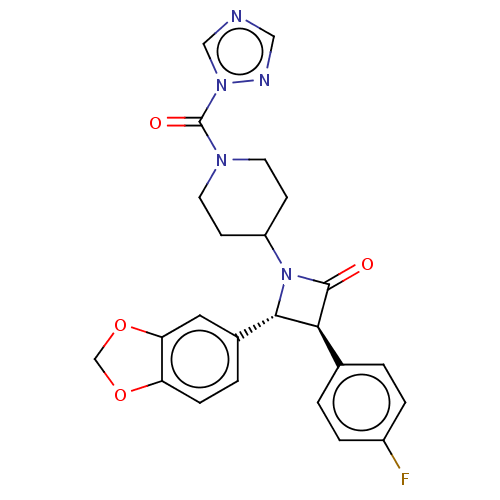 (CHEMBL3787346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM130218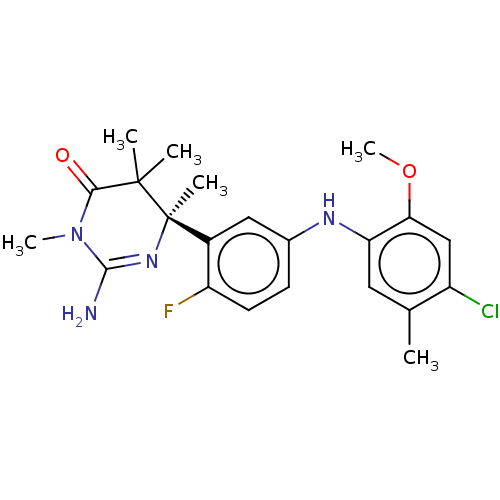 (US8815881, 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | 4 |
Hoffmann-La Roche Inc.; Siena Biotech S.p.A. US Patent | Assay Description BACE2 enzyme ectodomain (derived from plasmid pET17b-T7-hu proBACE2) was prepared as described in Ostermann et al., Crystal Structure of Human BACE2 ... | US Patent US8815881 (2014) BindingDB Entry DOI: 10.7270/Q2NZ86B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123858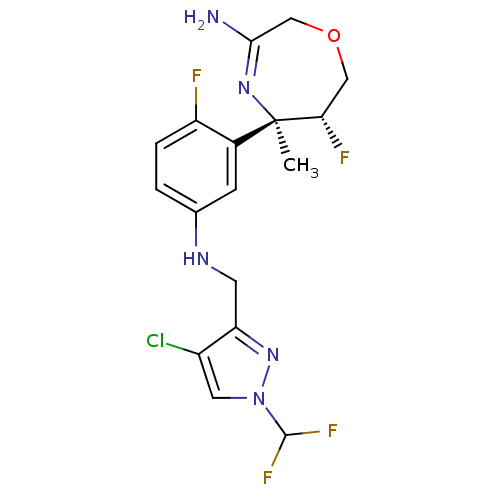 (US8748418, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50586979 (CHEMBL5084917) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM130203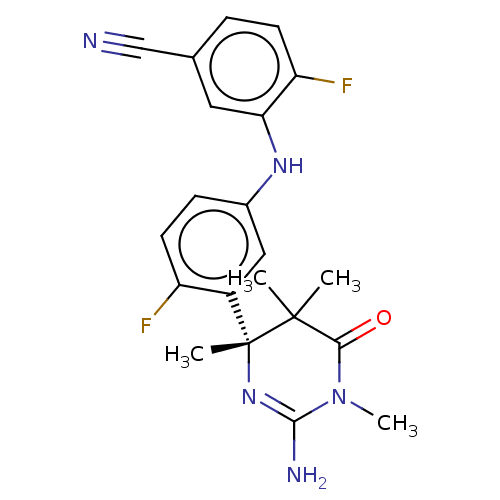 (US8815881, 165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | 4 |
Hoffmann-La Roche Inc.; Siena Biotech S.p.A. US Patent | Assay Description BACE2 enzyme ectodomain (derived from plasmid pET17b-T7-hu proBACE2) was prepared as described in Ostermann et al., Crystal Structure of Human BACE2 ... | US Patent US8815881 (2014) BindingDB Entry DOI: 10.7270/Q2NZ86B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM123870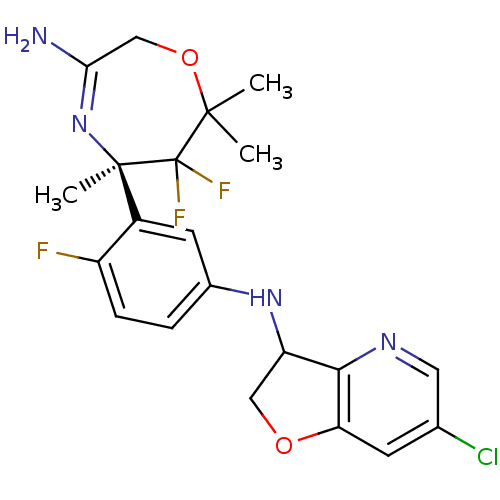 (US8748418, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM123858 (US8748418, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202619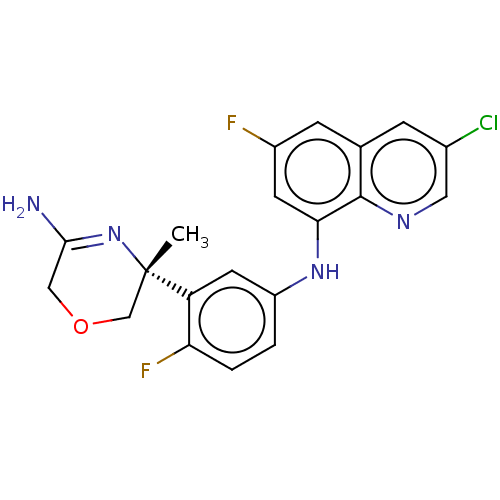 (US9242943, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
SIENA BIOTECH S.P.A.; HOFFMANN-LA ROCHE INC. US Patent | Assay Description DNA of the human APP wt gene (APP695) were used to assess the potency of the compounds in a cellular assay. The cells were seeded in 96-well microtit... | US Patent US9242943 (2016) BindingDB Entry DOI: 10.7270/Q2833QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Mus musculus) | BDBM50586984 (CHEMBL5084771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [18F]-FEH from MAO-A in mouse brain homogenate incubated for 60 mins by imaging analysis | Citation and Details Article DOI: 10.1016/j.bmc.2021.116528 BindingDB Entry DOI: 10.7270/Q2PC3698 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM123870 (US8748418, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate an... | US Patent US8748418 (2014) BindingDB Entry DOI: 10.7270/Q2C53JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50160279 (CHEMBL3787224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human MAGL expressed in Escherichia coli using 2-arachidonoyl-[3H]-glycerol as substrate incubated f... | J Med Chem 59: 2612-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01812 BindingDB Entry DOI: 10.7270/Q21R6SC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM130201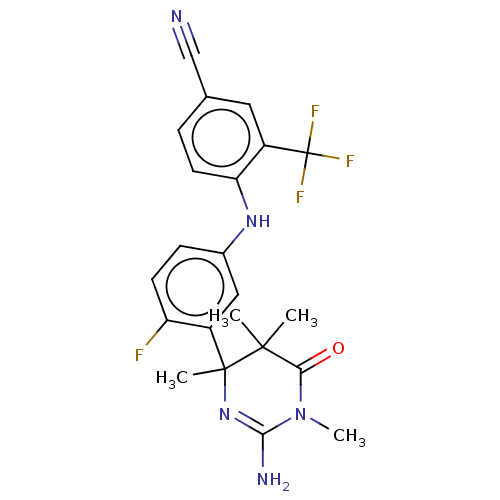 (US8815881, 163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | 4 |
Hoffmann-La Roche Inc.; Siena Biotech S.p.A. US Patent | Assay Description BACE2 enzyme ectodomain (derived from plasmid pET17b-T7-hu proBACE2) was prepared as described in Ostermann et al., Crystal Structure of Human BACE2 ... | US Patent US8815881 (2014) BindingDB Entry DOI: 10.7270/Q2NZ86B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 246 total ) | Next | Last >> |