 Found 835 hits with Last Name = 'garrett' and Initial = 'm'
Found 835 hits with Last Name = 'garrett' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50017309
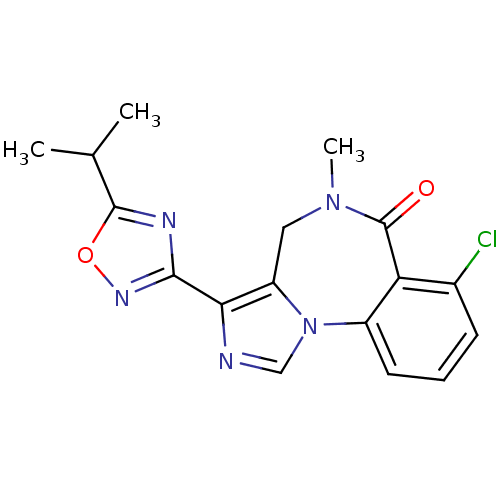
(7-Chloro-3-(5-isopropyl-[1,2,4]oxadiazol-3-yl)-5-m...)Show SMILES CC(C)c1nc(no1)-c1ncn-2c1CN(C)C(=O)c1c(Cl)cccc-21 Show InChI InChI=1S/C17H16ClN5O2/c1-9(2)16-20-15(21-25-16)14-12-7-22(3)17(24)13-10(18)5-4-6-11(13)23(12)8-19-14/h4-6,8-9H,7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50001765

(8-chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]tri...)Show SMILES Cc1nnc2CN=C(c3ccccc3Cl)c3cc(Cl)ccc3-n12 |t:6| Show InChI InChI=1S/C17H12Cl2N4/c1-10-21-22-16-9-20-17(12-4-2-3-5-14(12)19)13-8-11(18)6-7-15(13)23(10)16/h2-8H,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50243981
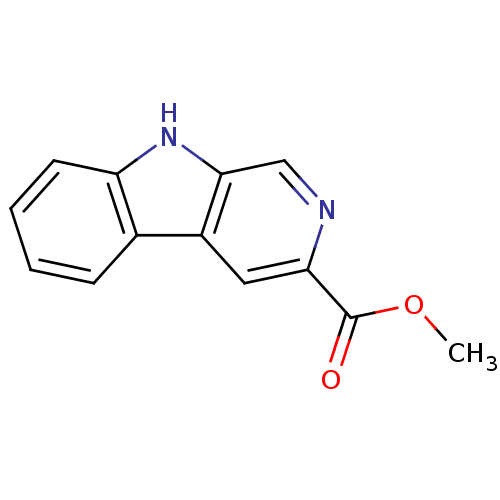
(Beta CCM | CHEMBL453066 | Methyl Beta-carboline-3 ...)Show InChI InChI=1S/C13H10N2O2/c1-17-13(16)11-6-9-8-4-2-3-5-10(8)15-12(9)7-14-11/h2-7,15H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM26267
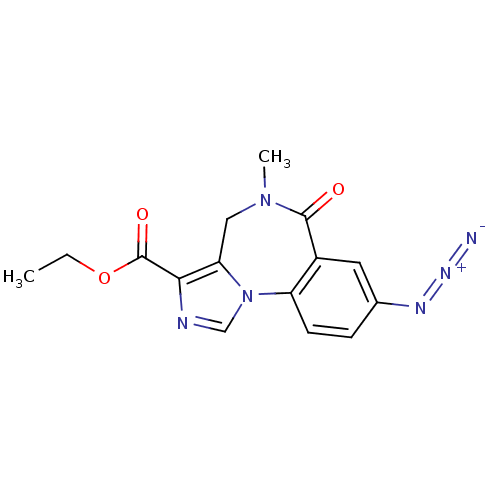
(RO-154513 | Ro15-4513 | [3H]Ro15-4513 | ethyl 12-a...)Show SMILES CCOC(=O)c1ncn-2c1CN(C)C(=O)c1cc(ccc-21)N=[N+]=[N-] Show InChI InChI=1S/C15H14N6O3/c1-3-24-15(23)13-12-7-20(2)14(22)10-6-9(18-19-16)4-5-11(10)21(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM25878
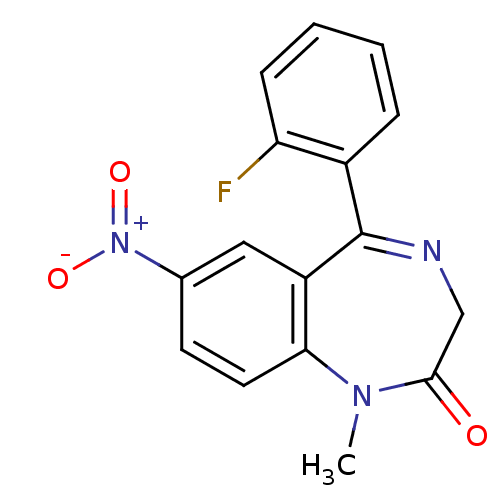
(5-(2-fluorophenyl)-1-methyl-7-nitro-2,3-dihydro-1H...)Show SMILES CN1c2ccc(cc2C(=NCC1=O)c1ccccc1F)[N+]([O-])=O |c:9| Show InChI InChI=1S/C16H12FN3O3/c1-19-14-7-6-10(20(22)23)8-12(14)16(18-9-15(19)21)11-4-2-3-5-13(11)17/h2-8H,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM81951
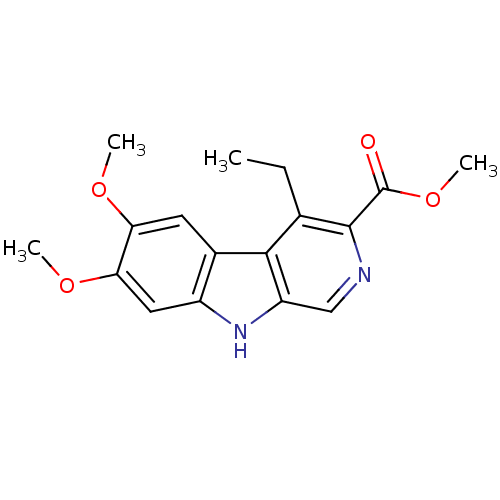
(CAS_104999 | DMCM | NSC_104999)Show InChI InChI=1S/C17H18N2O4/c1-5-9-15-10-6-13(21-2)14(22-3)7-11(10)19-12(15)8-18-16(9)17(20)23-4/h6-8,19H,5H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM50017320
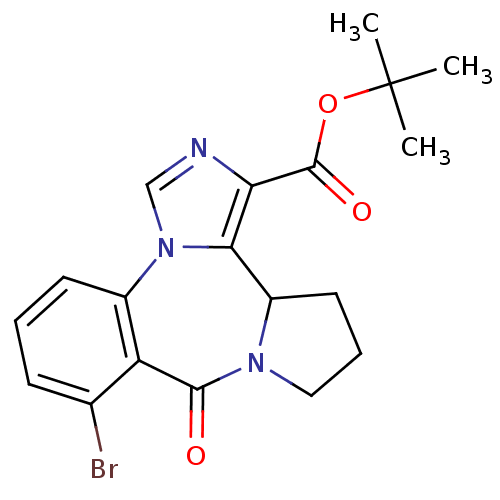
(8-Bromo-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11b-tria...)Show SMILES CC(C)(C)OC(=O)c1ncn-2c1C1CCCN1C(=O)c1c(Br)cccc-21 Show InChI InChI=1S/C19H20BrN3O3/c1-19(2,3)26-18(25)15-16-13-8-5-9-22(13)17(24)14-11(20)6-4-7-12(14)23(16)10-21-15/h4,6-7,10,13H,5,8-9H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM26266
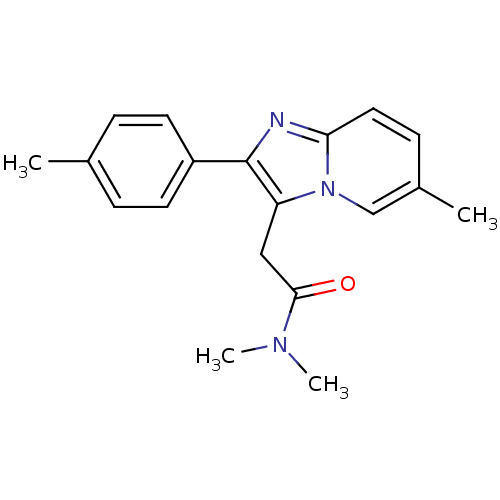
(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 20.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50026756
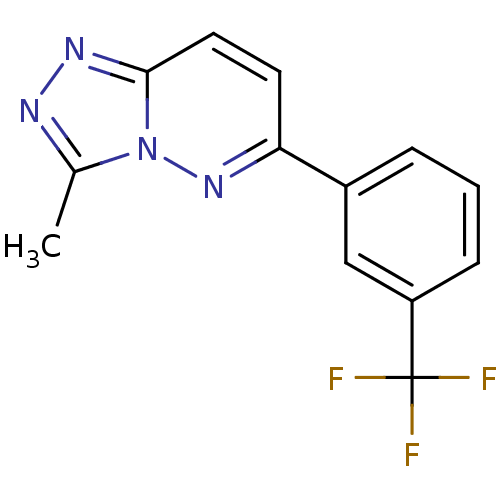
(3-Methyl-6-(3-trifluoromethyl-phenyl)-[1,2,4]triaz...)Show InChI InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 89.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM81951

(CAS_104999 | DMCM | NSC_104999)Show InChI InChI=1S/C17H18N2O4/c1-5-9-15-10-6-13(21-2)14(22-3)7-11(10)19-12(15)8-18-16(9)17(20)23-4/h6-8,19H,5H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM26263
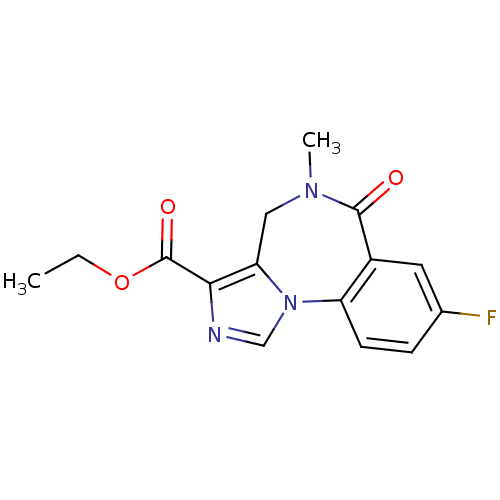
(Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...)Show InChI InChI=1S/C15H14FN3O3/c1-3-22-15(21)13-12-7-18(2)14(20)10-6-9(16)4-5-11(10)19(12)8-17-13/h4-6,8H,3,7H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM50017309

(7-Chloro-3-(5-isopropyl-[1,2,4]oxadiazol-3-yl)-5-m...)Show SMILES CC(C)c1nc(no1)-c1ncn-2c1CN(C)C(=O)c1c(Cl)cccc-21 Show InChI InChI=1S/C17H16ClN5O2/c1-9(2)16-20-15(21-25-16)14-12-7-22(3)17(24)13-10(18)5-4-6-11(13)23(12)8-19-14/h4-6,8-9H,7H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM50243981

(Beta CCM | CHEMBL453066 | Methyl Beta-carboline-3 ...)Show InChI InChI=1S/C13H10N2O2/c1-17-13(16)11-6-9-8-4-2-3-5-10(8)15-12(9)7-14-11/h2-7,15H,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM50001765

(8-chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]tri...)Show SMILES Cc1nnc2CN=C(c3ccccc3Cl)c3cc(Cl)ccc3-n12 |t:6| Show InChI InChI=1S/C17H12Cl2N4/c1-10-21-22-16-9-20-17(12-4-2-3-5-14(12)19)13-8-11(18)6-7-15(13)23(10)16/h2-8H,9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM26266

(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM25878

(5-(2-fluorophenyl)-1-methyl-7-nitro-2,3-dihydro-1H...)Show SMILES CN1c2ccc(cc2C(=NCC1=O)c1ccccc1F)[N+]([O-])=O |c:9| Show InChI InChI=1S/C16H12FN3O3/c1-19-14-7-6-10(20(22)23)8-12(14)16(18-9-15(19)21)11-4-2-3-5-13(11)17/h2-8H,9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(Homo sapiens (Human)) | BDBM50026756

(3-Methyl-6-(3-trifluoromethyl-phenyl)-[1,2,4]triaz...)Show InChI InChI=1S/C13H9F3N4/c1-8-17-18-12-6-5-11(19-20(8)12)9-3-2-4-10(7-9)13(14,15)16/h2-7H,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 49: 253-9 (1996)
BindingDB Entry DOI: 10.7270/Q2639N8C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50555401
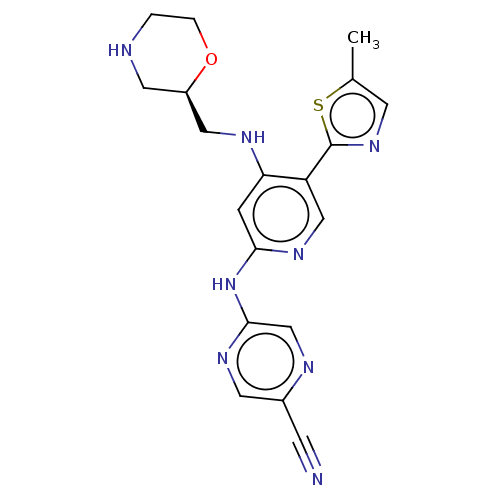
(CHEMBL4742233)Show SMILES Cc1cnc(s1)-c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50555385
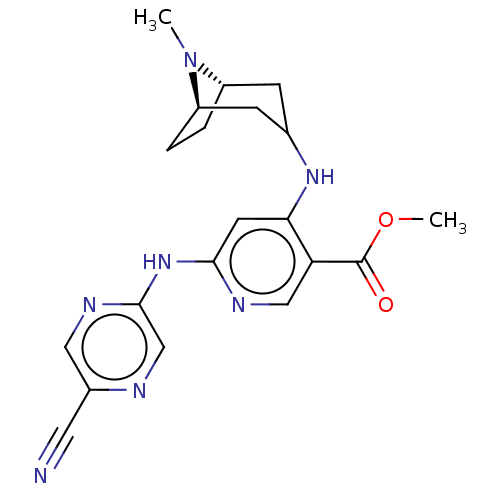
(CHEMBL4753307)Show SMILES [H][C@]12CC[C@]([H])(CC(C1)Nc1cc(Nc3cnc(cn3)C#N)ncc1C(=O)OC)N2C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131
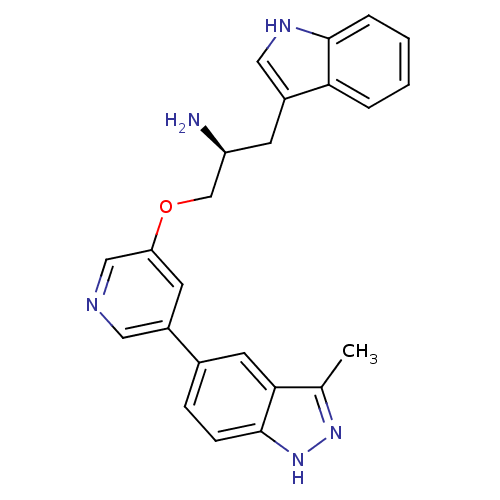
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Astex
| Assay Description
The purified PKB beta enzyme was assayed with a peptide substrate and test compound in the presence of 30 uM ATP/ [gamma-33P]ATP in 96-well plates. I... |
J Mol Biol 367: 882-94 (2007)
Article DOI: 10.1016/j.jmb.2007.01.004
BindingDB Entry DOI: 10.7270/Q29Z934H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328998
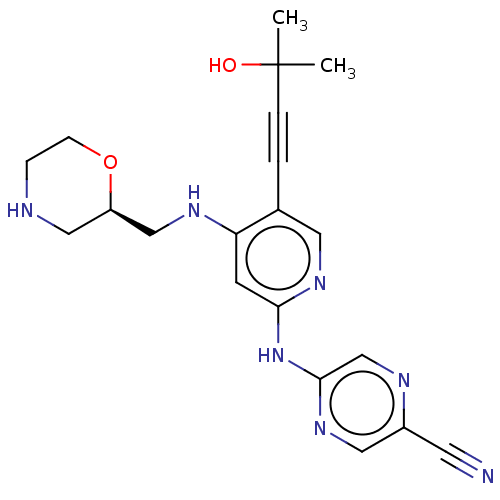
(1137478-52-4 | US11787792, Compound Y-158 | US9663...)Show SMILES CC(C)(O)C#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| Show InChI InChI=1S/C20H23N7O2/c1-20(2,28)4-3-14-9-25-18(27-19-13-23-15(8-21)10-26-19)7-17(14)24-12-16-11-22-5-6-29-16/h7,9-10,13,16,22,28H,5-6,11-12H2,1-2H3,(H2,24,25,26,27)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50307954
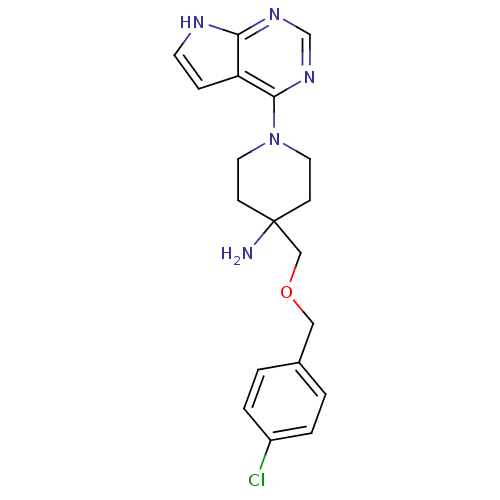
(4-((4-Chlorobenzyloxy)methyl)-1-(7H-pyrrolo[2,3-d]...)Show SMILES NC1(COCc2ccc(Cl)cc2)CCN(CC1)c1ncnc2[nH]ccc12 Show InChI InChI=1S/C19H22ClN5O/c20-15-3-1-14(2-4-15)11-26-12-19(21)6-9-25(10-7-19)18-16-5-8-22-17(16)23-13-24-18/h1-5,8,13H,6-7,9-12,21H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of PKB in human U87MG cells assessed as GSK3beta phosphorylation by ELISA |
J Med Chem 53: 2239-49 (2010)
Article DOI: 10.1021/jm901788j
BindingDB Entry DOI: 10.7270/Q23B608V |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50237622
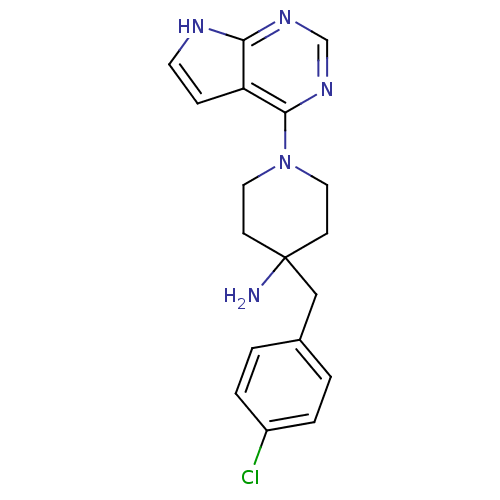
(4-(4-Chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4...)Show InChI InChI=1S/C18H20ClN5/c19-14-3-1-13(2-4-14)11-18(20)6-9-24(10-7-18)17-15-5-8-21-16(15)22-12-23-17/h1-5,8,12H,6-7,9-11,20H2,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of PKB in human U87MG cells assessed as GSK3beta phosphorylation by ELISA |
J Med Chem 53: 2239-49 (2010)
Article DOI: 10.1021/jm901788j
BindingDB Entry DOI: 10.7270/Q23B608V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C]
(Homo sapiens (Human)) | BDBM6266
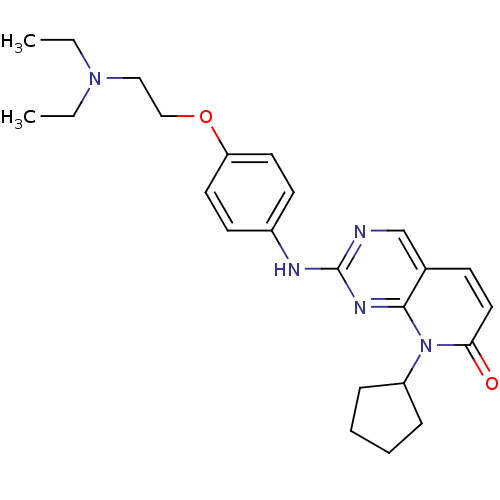
(8-Cyclopentyl-2-[4-(2-diethylaminoethoxy)phenylami...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3ccc(=O)n(C4CCCC4)c3n2)cc1 Show InChI InChI=1S/C24H31N5O2/c1-3-28(4-2)15-16-31-21-12-10-19(11-13-21)26-24-25-17-18-9-14-22(30)29(23(18)27-24)20-7-5-6-8-20/h9-14,17,20H,3-8,15-16H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... |
J Med Chem 43: 4606-16 (2000)
Article DOI: 10.1021/jm000271k
BindingDB Entry DOI: 10.7270/Q25B00N4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50555402
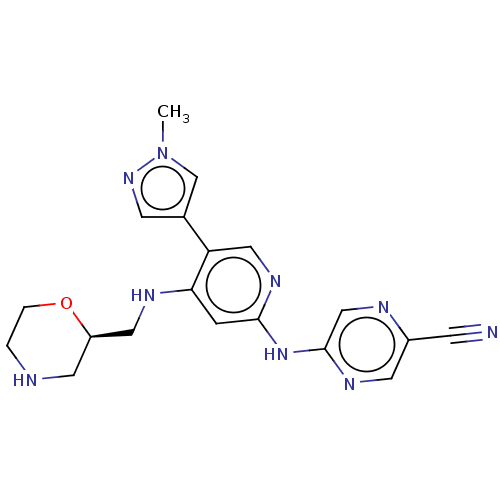
(CHEMBL4790962)Show SMILES Cn1cc(cn1)-c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50401629

(CHEMBL2203839)Show SMILES CNc1cc(Nc2cnc(C#N)c(O[C@@H]3CCNC3)n2)ncc1-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H21N9O/c1-21-15-5-17(24-9-14(15)12-7-25-28(2)11-12)26-18-10-23-16(6-20)19(27-18)29-13-3-4-22-8-13/h5,7,9-11,13,22H,3-4,8H2,1-2H3,(H2,21,24,26,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH as substrate after 1 hr by microfluidic assay in presence of ATP |
J Med Chem 55: 10229-40 (2012)
Article DOI: 10.1021/jm3012933
BindingDB Entry DOI: 10.7270/Q2N87BZM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50401628

(CHEMBL2203840)Show SMILES CNc1cc(Nc2cnc(C#N)c(O[C@H]3CCNC3)n2)ncc1-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H21N9O/c1-21-15-5-17(24-9-14(15)12-7-25-28(2)11-12)26-18-10-23-16(6-20)19(27-18)29-13-3-4-22-8-13/h5,7,9-11,13,22H,3-4,8H2,1-2H3,(H2,21,24,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH as substrate after 1 hr by microfluidic assay in presence of ATP |
J Med Chem 55: 10229-40 (2012)
Article DOI: 10.1021/jm3012933
BindingDB Entry DOI: 10.7270/Q2N87BZM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
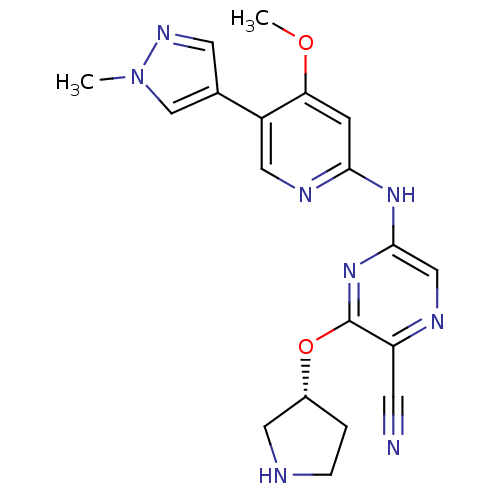
(Homo sapiens (Human)) | BDBM50401627
(CHEMBL2203844)Show SMILES COc1cc(Nc2cnc(C#N)c(O[C@@H]3CCNC3)n2)ncc1-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H20N8O2/c1-27-11-12(7-24-27)14-9-23-17(5-16(14)28-2)25-18-10-22-15(6-20)19(26-18)29-13-3-4-21-8-13/h5,7,9-11,13,21H,3-4,8H2,1-2H3,(H,23,25,26)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH as substrate after 1 hr by microfluidic assay in presence of ATP |
J Med Chem 55: 10229-40 (2012)
Article DOI: 10.1021/jm3012933
BindingDB Entry DOI: 10.7270/Q2N87BZM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50555384
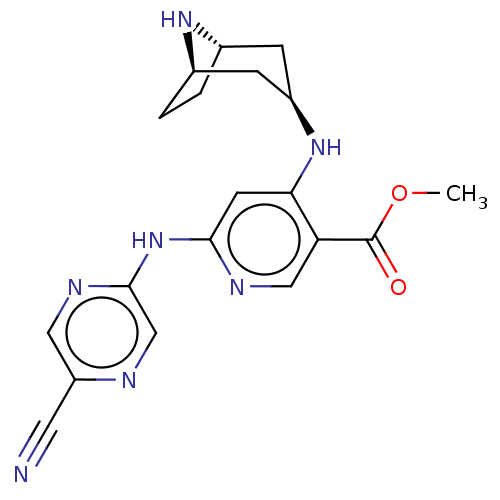
(CHEMBL4789854)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(Nc3cnc(cn3)C#N)ncc1C(=O)OC)N2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328995
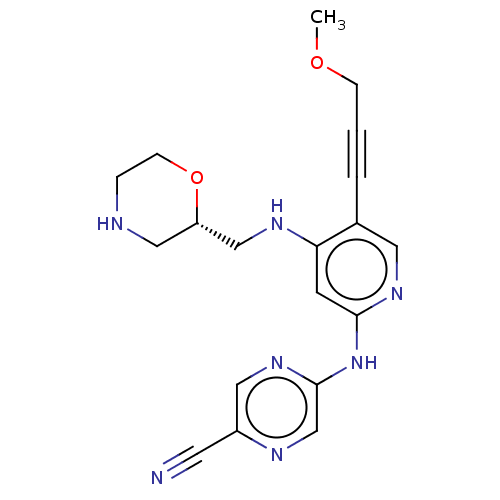
(1137478-47-7 | US11787792, Compound Y-154 | US9663...)Show SMILES COCC#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C19H21N7O2/c1-27-5-2-3-14-9-24-18(26-19-13-22-15(8-20)10-25-19)7-17(14)23-12-16-11-21-4-6-28-16/h7,9-10,13,16,21H,4-6,11-12H2,1H3,(H2,23,24,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited
US Patent
| Assay Description
CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... |
US Patent US9663503 (2017)
BindingDB Entry DOI: 10.7270/Q2T43W7G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328995

(1137478-47-7 | US11787792, Compound Y-154 | US9663...)Show SMILES COCC#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C19H21N7O2/c1-27-5-2-3-14-9-24-18(26-19-13-22-15(8-20)10-25-19)7-17(14)23-12-16-11-21-4-6-28-16/h7,9-10,13,16,21H,4-6,11-12H2,1H3,(H2,23,24,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328993
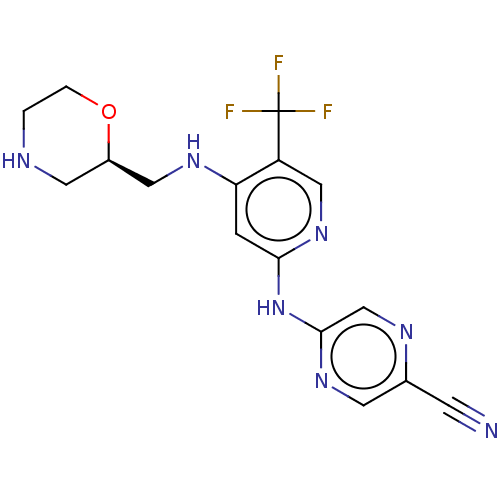
(5-[[4-[[(2R)-morpholin-2-yl]methylamino]-5-(triflu...)Show SMILES FC(F)(F)c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| Show InChI InChI=1S/C16H16F3N7O/c17-16(18,19)12-8-25-14(26-15-9-22-10(4-20)5-24-15)3-13(12)23-7-11-6-21-1-2-27-11/h3,5,8-9,11,21H,1-2,6-7H2,(H2,23,24,25,26)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328993

(5-[[4-[[(2R)-morpholin-2-yl]methylamino]-5-(triflu...)Show SMILES FC(F)(F)c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| Show InChI InChI=1S/C16H16F3N7O/c17-16(18,19)12-8-25-14(26-15-9-22-10(4-20)5-24-15)3-13(12)23-7-11-6-21-1-2-27-11/h3,5,8-9,11,21H,1-2,6-7H2,(H2,23,24,25,26)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328993

(5-[[4-[[(2R)-morpholin-2-yl]methylamino]-5-(triflu...)Show SMILES FC(F)(F)c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| Show InChI InChI=1S/C16H16F3N7O/c17-16(18,19)12-8-25-14(26-15-9-22-10(4-20)5-24-15)3-13(12)23-7-11-6-21-1-2-27-11/h3,5,8-9,11,21H,1-2,6-7H2,(H2,23,24,25,26)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited
US Patent
| Assay Description
CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... |
US Patent US9663503 (2017)
BindingDB Entry DOI: 10.7270/Q2T43W7G |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50307955
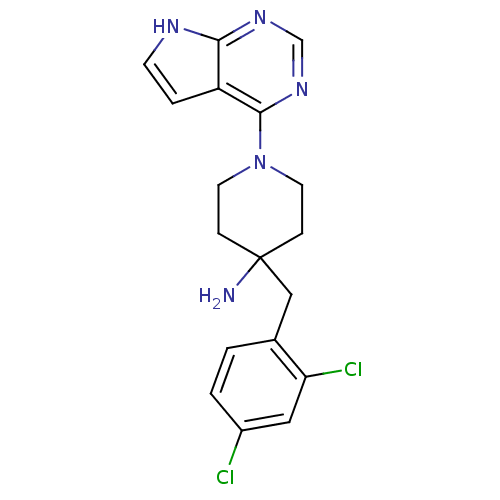
(4-(2,4-Dichlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimid...)Show SMILES NC1(Cc2ccc(Cl)cc2Cl)CCN(CC1)c1ncnc2[nH]ccc12 Show InChI InChI=1S/C18H19Cl2N5/c19-13-2-1-12(15(20)9-13)10-18(21)4-7-25(8-5-18)17-14-3-6-22-16(14)23-11-24-17/h1-3,6,9,11H,4-5,7-8,10,21H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of PKB in human PC3M cells assessed as GSK3beta phosphorylation by ELISA |
J Med Chem 53: 2239-49 (2010)
Article DOI: 10.1021/jm901788j
BindingDB Entry DOI: 10.7270/Q23B608V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50555409
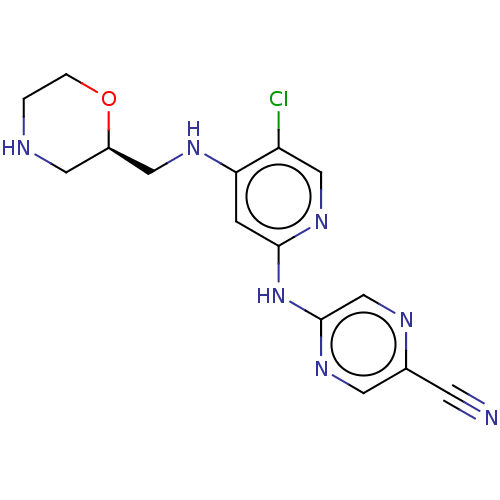
(CHEMBL4754960)Show SMILES Clc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328997
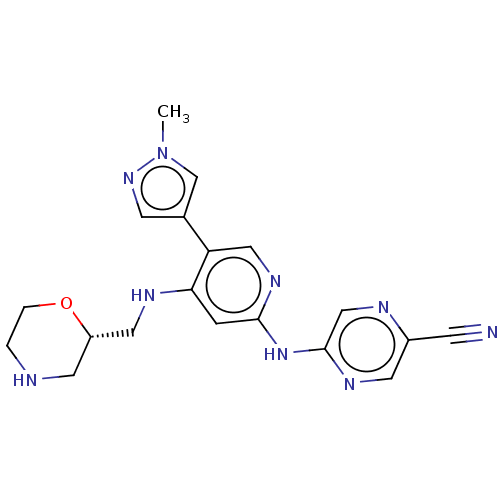
(1137478-45-5 | US11787792, Compound Y-152 | US9663...)Show SMILES Cn1cc(cn1)-c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C19H21N9O/c1-28-12-13(6-26-28)16-10-25-18(27-19-11-22-14(5-20)7-24-19)4-17(16)23-9-15-8-21-2-3-29-15/h4,6-7,10-12,15,21H,2-3,8-9H2,1H3,(H2,23,24,25,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328997

(1137478-45-5 | US11787792, Compound Y-152 | US9663...)Show SMILES Cn1cc(cn1)-c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C19H21N9O/c1-28-12-13(6-26-28)16-10-25-18(27-19-11-22-14(5-20)7-24-19)4-17(16)23-9-15-8-21-2-3-29-15/h4,6-7,10-12,15,21H,2-3,8-9H2,1H3,(H2,23,24,25,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited
US Patent
| Assay Description
CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... |
US Patent US9663503 (2017)
BindingDB Entry DOI: 10.7270/Q2T43W7G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328996
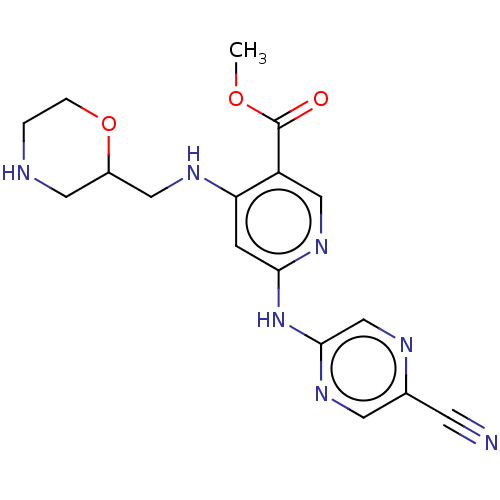
(1137477-07-6 | US11787792, Compound Y-081 | US9663...)Show InChI InChI=1S/C17H19N7O3/c1-26-17(25)13-9-23-15(24-16-10-20-11(5-18)6-22-16)4-14(13)21-8-12-7-19-2-3-27-12/h4,6,9-10,12,19H,2-3,7-8H2,1H3,(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited
US Patent
| Assay Description
CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... |
US Patent US9663503 (2017)
BindingDB Entry DOI: 10.7270/Q2T43W7G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328998

(1137478-52-4 | US11787792, Compound Y-158 | US9663...)Show SMILES CC(C)(O)C#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| Show InChI InChI=1S/C20H23N7O2/c1-20(2,28)4-3-14-9-25-18(27-19-13-23-15(8-21)10-26-19)7-17(14)24-12-16-11-22-5-6-29-16/h7,9-10,13,16,22,28H,5-6,11-12H2,1-2H3,(H2,24,25,26,27)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328998

(1137478-52-4 | US11787792, Compound Y-158 | US9663...)Show SMILES CC(C)(O)C#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@H]1CNCCO1 |r| Show InChI InChI=1S/C20H23N7O2/c1-20(2,28)4-3-14-9-25-18(27-19-13-23-15(8-21)10-26-19)7-17(14)24-12-16-11-22-5-6-29-16/h7,9-10,13,16,22,28H,5-6,11-12H2,1-2H3,(H2,24,25,26,27)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited
US Patent
| Assay Description
CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... |
US Patent US9663503 (2017)
BindingDB Entry DOI: 10.7270/Q2T43W7G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328996

(1137477-07-6 | US11787792, Compound Y-081 | US9663...)Show InChI InChI=1S/C17H19N7O3/c1-26-17(25)13-9-23-15(24-16-10-20-11(5-18)6-22-16)4-14(13)21-8-12-7-19-2-3-27-12/h4,6,9-10,12,19H,2-3,7-8H2,1H3,(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50555405
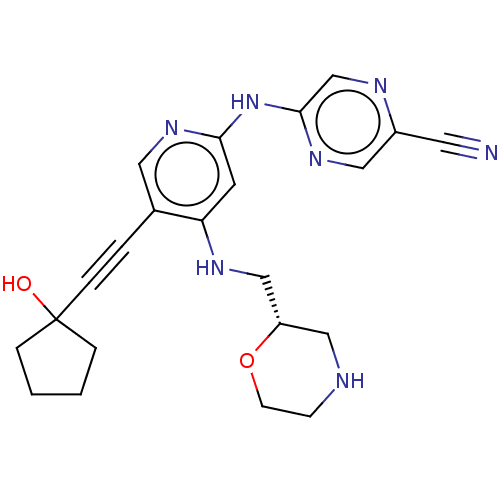
(CHEMBL4784718)Show SMILES OC1(CCCC1)C#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of human CHK1 using ATP as substrate by DELFIA |
J Med Chem 54: 8328-42 (2011)
Article DOI: 10.1021/jm2007326
BindingDB Entry DOI: 10.7270/Q2GH9JDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
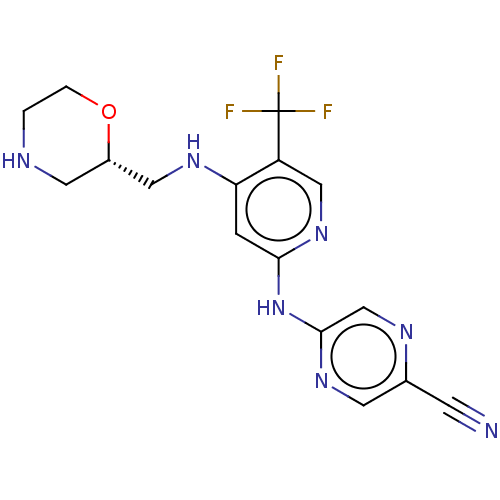
(Homo sapiens (Human)) | BDBM328994
(5-[[4-[[(2S)-morpholin-2-yl]methylamino]-5-(triflu...)Show SMILES FC(F)(F)c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C16H16F3N7O/c17-16(18,19)12-8-25-14(26-15-9-22-10(4-20)5-24-15)3-13(12)23-7-11-6-21-1-2-27-11/h3,5,8-9,11,21H,1-2,6-7H2,(H2,23,24,25,26)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited
US Patent
| Assay Description
CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... |
US Patent US9663503 (2017)
BindingDB Entry DOI: 10.7270/Q2T43W7G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328994

(5-[[4-[[(2S)-morpholin-2-yl]methylamino]-5-(triflu...)Show SMILES FC(F)(F)c1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C16H16F3N7O/c17-16(18,19)12-8-25-14(26-15-9-22-10(4-20)5-24-15)3-13(12)23-7-11-6-21-1-2-27-11/h3,5,8-9,11,21H,1-2,6-7H2,(H2,23,24,25,26)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM328996

(1137477-07-6 | US11787792, Compound Y-081 | US9663...)Show InChI InChI=1S/C17H19N7O3/c1-26-17(25)13-9-23-15(24-16-10-20-11(5-18)6-22-16)4-14(13)21-8-12-7-19-2-3-27-12/h4,6,9-10,12,19H,2-3,7-8H2,1H3,(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM329000
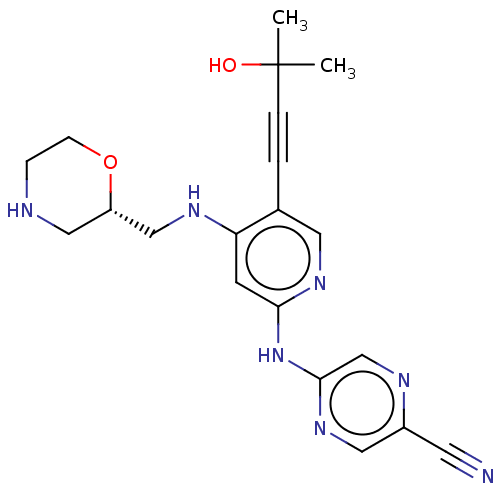
(1137478-46-6 | US11787792, Compound Y-153 | US9663...)Show SMILES CC(C)(O)C#Cc1cnc(Nc2cnc(cn2)C#N)cc1NC[C@@H]1CNCCO1 |r| Show InChI InChI=1S/C20H23N7O2/c1-20(2,28)4-3-14-9-25-18(27-19-13-23-15(8-21)10-26-19)7-17(14)24-12-16-11-22-5-6-29-16/h7,9-10,13,16,22,28H,5-6,11-12H2,1-2H3,(H2,24,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01938
BindingDB Entry DOI: 10.7270/Q2MG7T5V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data