 Found 325 hits with Last Name = 'goyal' and Initial = 'v'
Found 325 hits with Last Name = 'goyal' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
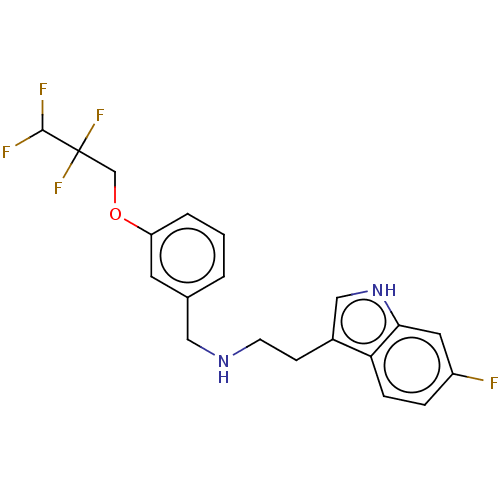
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT6 receptor (unknown origin) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236846
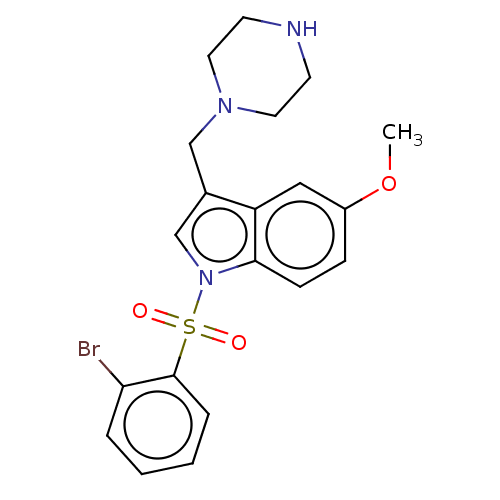
(CHEMBL4080401)Show SMILES O.O.CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCNCC3)c2c1)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C20H22BrN3O3S/c1-27-16-6-7-19-17(12-16)15(13-23-10-8-22-9-11-23)14-24(19)28(25,26)20-5-3-2-4-18(20)21/h2-7,12,14,22H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mammalian Geranylgeranyl transferase type I expressed in baculovirus |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044616
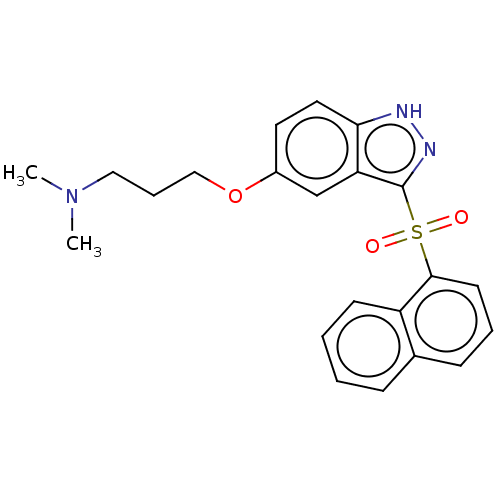
(Cerlapirdine | PF-05212365 | SAM-531 | WAY-262531)Show SMILES CN(C)CCCOc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H23N3O3S/c1-25(2)13-6-14-28-17-11-12-20-19(15-17)22(24-23-20)29(26,27)21-10-5-8-16-7-3-4-9-18(16)21/h3-5,7-12,15H,6,13-14H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration required for agonistic activity at Metabotropic glutamate receptor 2 |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236844
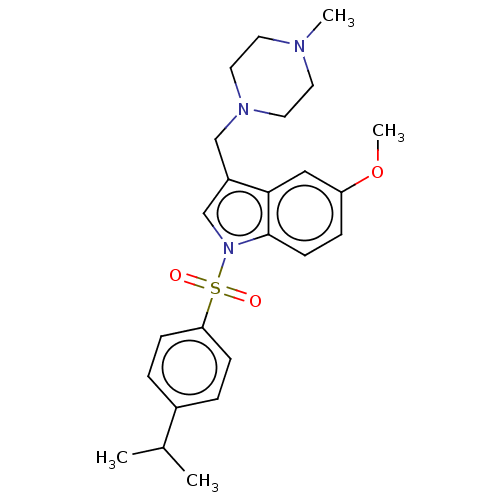
(CHEMBL4100484)Show SMILES CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C Show InChI InChI=1S/C24H31N3O3S/c1-18(2)19-5-8-22(9-6-19)31(28,29)27-17-20(16-26-13-11-25(3)12-14-26)23-15-21(30-4)7-10-24(23)27/h5-10,15,17-18H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236780
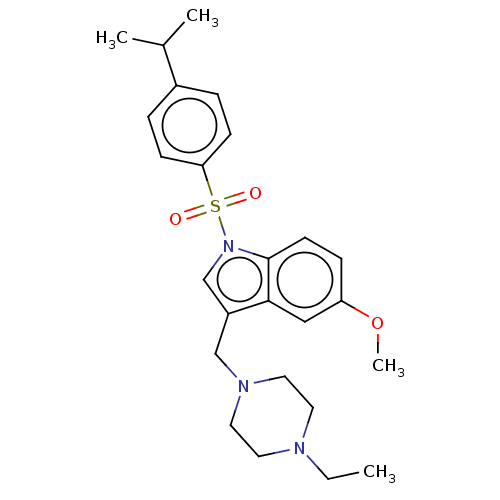
(CHEMBL4068232)Show SMILES CCN1CCN(Cc2cn(c3ccc(OC)cc23)S(=O)(=O)c2ccc(cc2)C(C)C)CC1 Show InChI InChI=1S/C25H33N3O3S/c1-5-26-12-14-27(15-13-26)17-21-18-28(25-11-8-22(31-4)16-24(21)25)32(29,30)23-9-6-20(7-10-23)19(2)3/h6-11,16,18-19H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236754
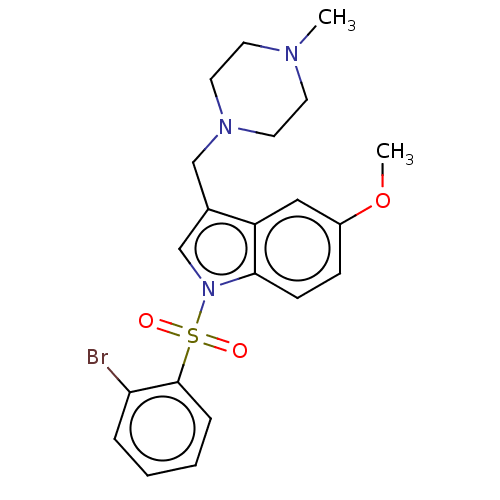
(CHEMBL4082473)Show SMILES O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C21H24BrN3O3S/c1-23-9-11-24(12-10-23)14-16-15-25(20-8-7-17(28-2)13-18(16)20)29(26,27)21-6-4-3-5-19(21)22/h3-8,13,15H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration required for partial agonistic activity at Metabotropic glutamate receptor 2; Partial agonist |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236756
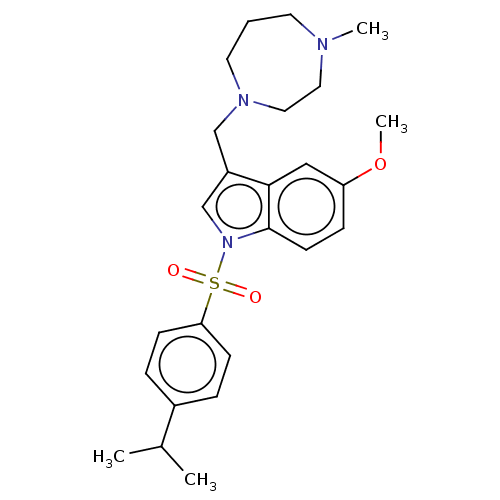
(CHEMBL4101284)Show SMILES Cl.Cl.COc1ccc2n(cc(CN3CCCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C Show InChI InChI=1S/C25H33N3O3S/c1-19(2)20-6-9-23(10-7-20)32(29,30)28-18-21(17-27-13-5-12-26(3)14-15-27)24-16-22(31-4)8-11-25(24)28/h6-11,16,18-19H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236773
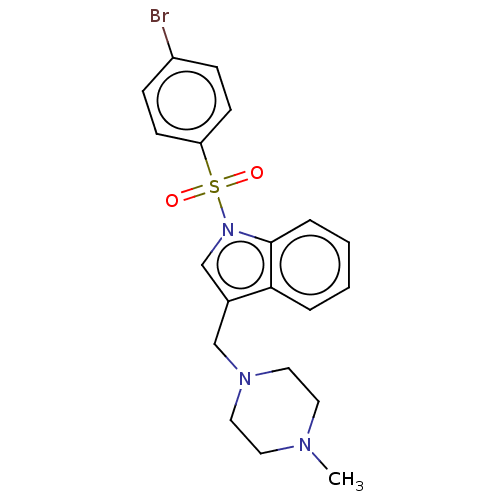
(CHEMBL4065437)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(Br)cc2)CC1 Show InChI InChI=1S/C20H22BrN3O2S/c1-22-10-12-23(13-11-22)14-16-15-24(20-5-3-2-4-19(16)20)27(25,26)18-8-6-17(21)7-9-18/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236832

(CHEMBL4093699)Show SMILES Cl.Cl.CC(C)c1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2cc(F)ccc12 Show InChI InChI=1S/C23H28FN3O2S/c1-17(2)18-4-7-21(8-5-18)30(28,29)27-16-19(15-26-12-10-25(3)11-13-26)22-14-20(24)6-9-23(22)27/h4-9,14,16-17H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236806

(CHEMBL4100411)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H25N3O3S/c1-22-10-12-23(13-11-22)15-17-16-24(21-9-8-18(27-2)14-20(17)21)28(25,26)19-6-4-3-5-7-19/h3-9,14,16H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mammalian Geranylgeranyl transferase type I expressed in baculovirus |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236831

(CHEMBL4077537)Show SMILES Cl.Cl.Brc1ccccc1S(=O)(=O)n1cc(CN2CCNCC2)c2ccccc12 Show InChI InChI=1S/C19H20BrN3O2S/c20-17-6-2-4-8-19(17)26(24,25)23-14-15(13-22-11-9-21-10-12-22)16-5-1-3-7-18(16)23/h1-8,14,21H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236836
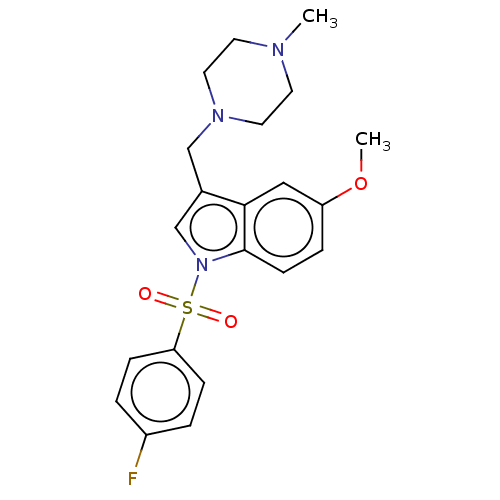
(CHEMBL4073586)Show SMILES CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C21H24FN3O3S/c1-23-9-11-24(12-10-23)14-16-15-25(21-8-5-18(28-2)13-20(16)21)29(26,27)19-6-3-17(22)4-7-19/h3-8,13,15H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236792
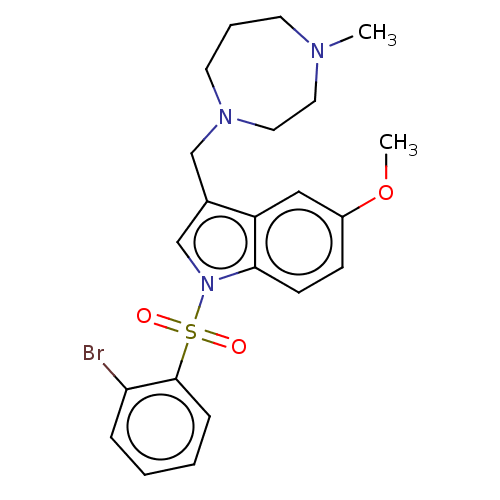
(CHEMBL4083289)Show SMILES COc1ccc2n(cc(CN3CCCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C22H26BrN3O3S/c1-24-10-5-11-25(13-12-24)15-17-16-26(21-9-8-18(29-2)14-19(17)21)30(27,28)22-7-4-3-6-20(22)23/h3-4,6-9,14,16H,5,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236843

(CHEMBL4105377)Show SMILES Cl.Cl.COc1ccc2n(cc(CN3CCNCC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H29N3O3S/c1-17(2)18-4-7-21(8-5-18)30(27,28)26-16-19(15-25-12-10-24-11-13-25)22-14-20(29-3)6-9-23(22)26/h4-9,14,16-17,24H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236777

(CHEMBL4097459)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C23H29N3O2S/c1-18(2)19-8-10-21(11-9-19)29(27,28)26-17-20(22-6-4-5-7-23(22)26)16-25-14-12-24(3)13-15-25/h4-11,17-18H,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236833
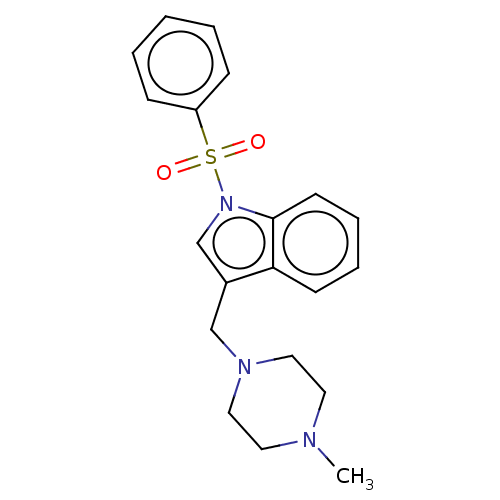
(CHEMBL4062687)Show SMILES Cl.Cl.CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccccc2)CC1 Show InChI InChI=1S/C20H23N3O2S/c1-21-11-13-22(14-12-21)15-17-16-23(20-10-6-5-9-19(17)20)26(24,25)18-7-3-2-4-8-18/h2-10,16H,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236821

(CHEMBL4075502)Show SMILES CCN1CCN(Cc2cn(c3ccc(OC)cc23)S(=O)(=O)c2ccccc2Br)CC1 Show InChI InChI=1S/C22H26BrN3O3S/c1-3-24-10-12-25(13-11-24)15-17-16-26(21-9-8-18(29-2)14-19(17)21)30(27,28)22-7-5-4-6-20(22)23/h4-9,14,16H,3,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236834
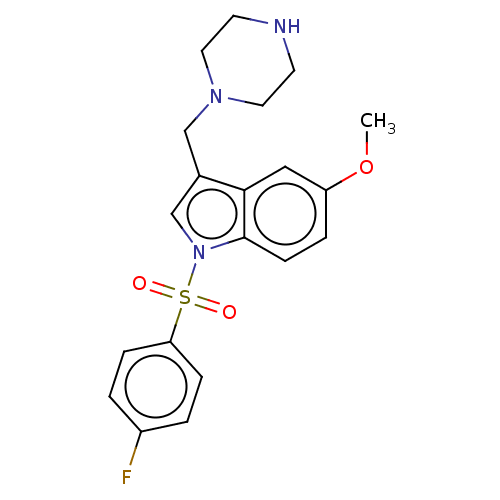
(CHEMBL4073450)Show SMILES CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCNCC3)c2c1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C20H22FN3O3S/c1-27-17-4-7-20-19(12-17)15(13-23-10-8-22-9-11-23)14-24(20)28(25,26)18-5-2-16(21)3-6-18/h2-7,12,14,22H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236803
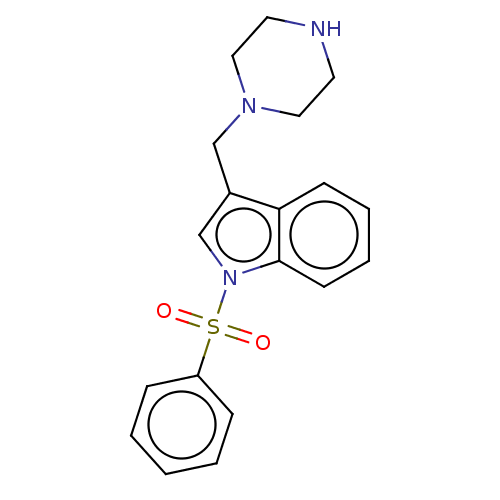
(CHEMBL4095054)Show InChI InChI=1S/C19H21N3O2S/c23-25(24,17-6-2-1-3-7-17)22-15-16(14-21-12-10-20-11-13-21)18-8-4-5-9-19(18)22/h1-9,15,20H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236839
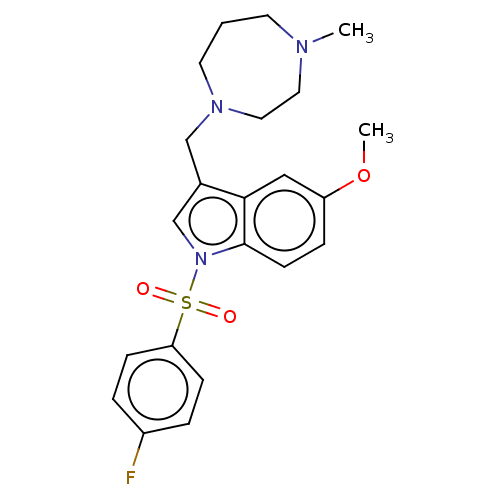
(CHEMBL4083893)Show SMILES Cl.Cl.COc1ccc2n(cc(CN3CCCN(C)CC3)c2c1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C22H26FN3O3S/c1-24-10-3-11-25(13-12-24)15-17-16-26(22-9-6-19(29-2)14-21(17)22)30(27,28)20-7-4-18(23)5-8-20/h4-9,14,16H,3,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236810
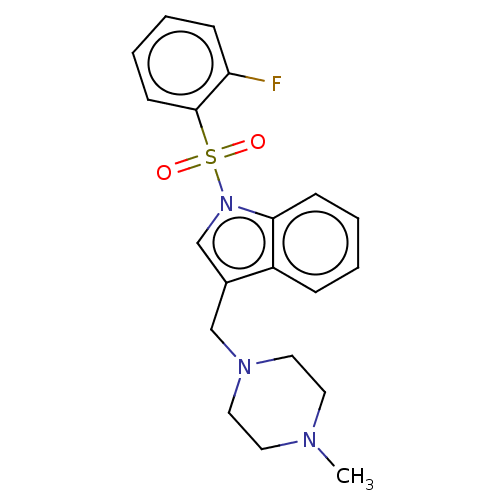
(CHEMBL4103281)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccccc2F)CC1 Show InChI InChI=1S/C20H22FN3O2S/c1-22-10-12-23(13-11-22)14-16-15-24(19-8-4-2-6-17(16)19)27(25,26)20-9-5-3-7-18(20)21/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236801
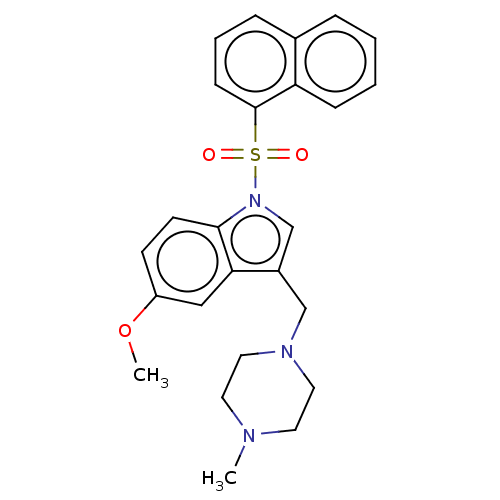
(CHEMBL4092681)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C25H27N3O3S/c1-26-12-14-27(15-13-26)17-20-18-28(24-11-10-21(31-2)16-23(20)24)32(29,30)25-9-5-7-19-6-3-4-8-22(19)25/h3-11,16,18H,12-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against fucosyltransferase (FucT V) in the presence of 1 mM fucosyl acceptor N-acetyllactosamine |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236793
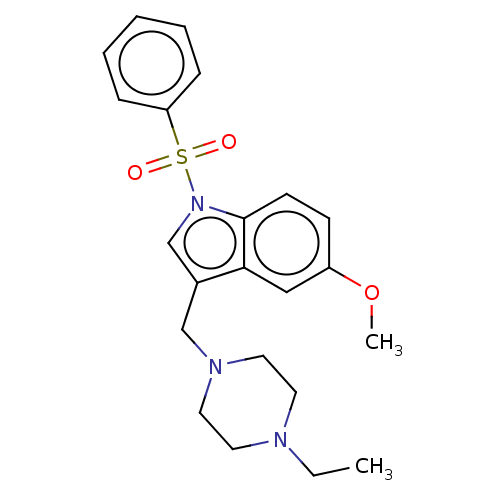
(CHEMBL4064478)Show SMILES CCN1CCN(Cc2cn(c3ccc(OC)cc23)S(=O)(=O)c2ccccc2)CC1 Show InChI InChI=1S/C22H27N3O3S/c1-3-23-11-13-24(14-12-23)16-18-17-25(22-10-9-19(28-2)15-21(18)22)29(26,27)20-7-5-4-6-8-20/h4-10,15,17H,3,11-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236812
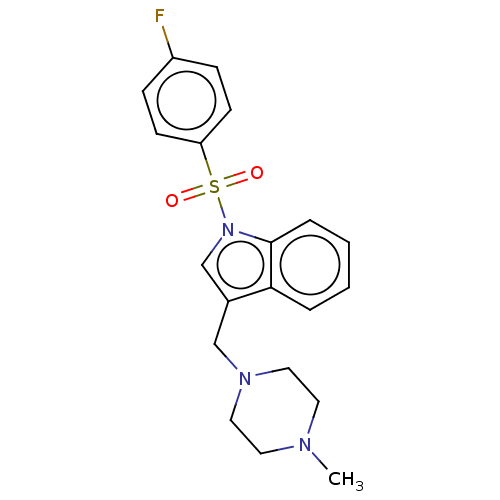
(CHEMBL4070284)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C20H22FN3O2S/c1-22-10-12-23(13-11-22)14-16-15-24(20-5-3-2-4-19(16)20)27(25,26)18-8-6-17(21)7-9-18/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236808
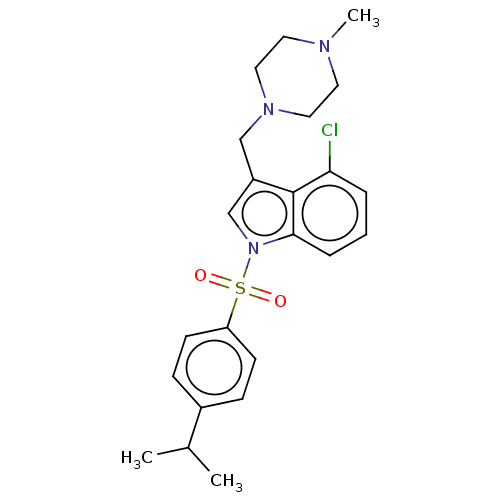
(CHEMBL4071816)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2c(Cl)cccc12 Show InChI InChI=1S/C23H28ClN3O2S/c1-17(2)18-7-9-20(10-8-18)30(28,29)27-16-19(15-26-13-11-25(3)12-14-26)23-21(24)5-4-6-22(23)27/h4-10,16-17H,11-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236788

(CHEMBL4085949)Show SMILES CN1CCN(Cc2cn(c3ccc(F)cc23)S(=O)(=O)c2ccc(Br)cc2)CC1 Show InChI InChI=1S/C20H21BrFN3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(20-7-4-17(22)12-19(15)20)28(26,27)18-5-2-16(21)3-6-18/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236829
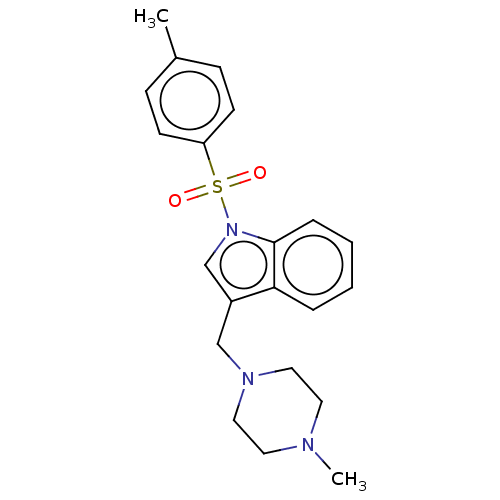
(CHEMBL4087957)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(C)cc2)CC1 Show InChI InChI=1S/C21H25N3O2S/c1-17-7-9-19(10-8-17)27(25,26)24-16-18(20-5-3-4-6-21(20)24)15-23-13-11-22(2)12-14-23/h3-10,16H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236770
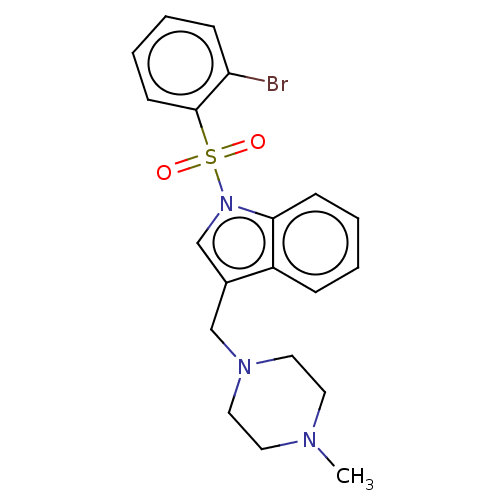
(CHEMBL4074058)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccccc2Br)CC1 Show InChI InChI=1S/C20H22BrN3O2S/c1-22-10-12-23(13-11-22)14-16-15-24(19-8-4-2-6-17(16)19)27(25,26)20-9-5-3-7-18(20)21/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236798
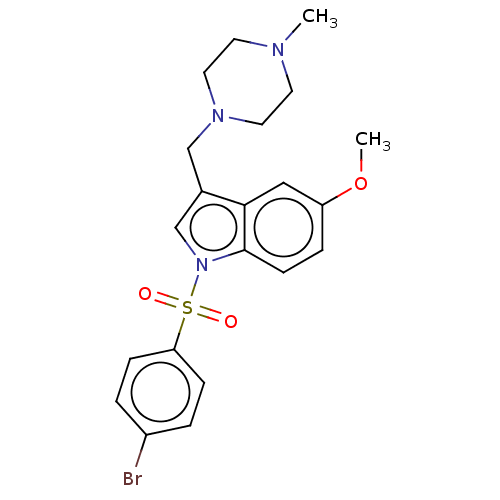
(CHEMBL4064995)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C21H24BrN3O3S/c1-23-9-11-24(12-10-23)14-16-15-25(21-8-5-18(28-2)13-20(16)21)29(26,27)19-6-3-17(22)4-7-19/h3-8,13,15H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236847
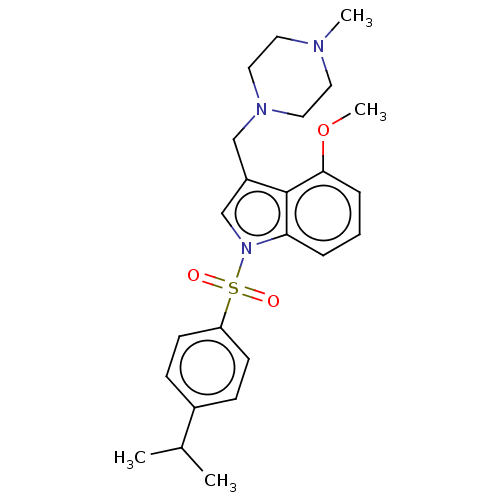
(CHEMBL4085003)Show SMILES Cl.Cl.COc1cccc2n(cc(CN3CCN(C)CC3)c12)S(=O)(=O)c1ccc(cc1)C(C)C Show InChI InChI=1S/C24H31N3O3S/c1-18(2)19-8-10-21(11-9-19)31(28,29)27-17-20(16-26-14-12-25(3)13-15-26)24-22(27)6-5-7-23(24)30-4/h5-11,17-18H,12-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236769
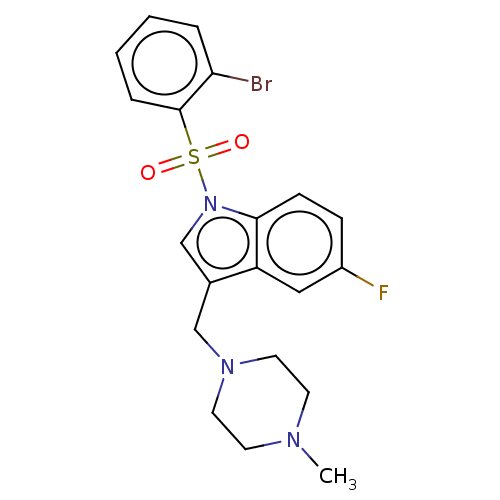
(CHEMBL4066125)Show SMILES CN1CCN(Cc2cn(c3ccc(F)cc23)S(=O)(=O)c2ccccc2Br)CC1 Show InChI InChI=1S/C20H21BrFN3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(19-7-6-16(22)12-17(15)19)28(26,27)20-5-3-2-4-18(20)21/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236851
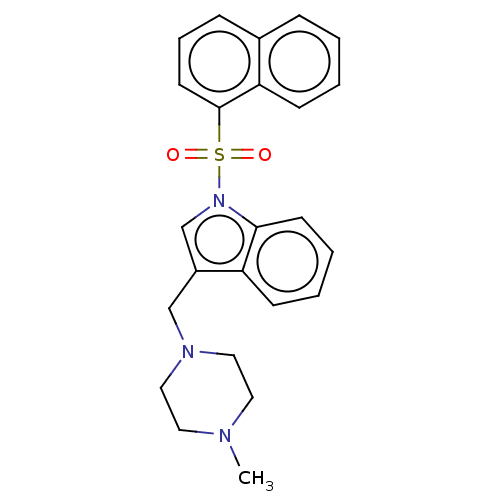
(CHEMBL4079859)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2cccc3ccccc23)CC1 Show InChI InChI=1S/C24H25N3O2S/c1-25-13-15-26(16-14-25)17-20-18-27(23-11-5-4-9-21(20)23)30(28,29)24-12-6-8-19-7-2-3-10-22(19)24/h2-12,18H,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration required for agonistic activity at Metabotropic glutamate receptor 2 |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236841
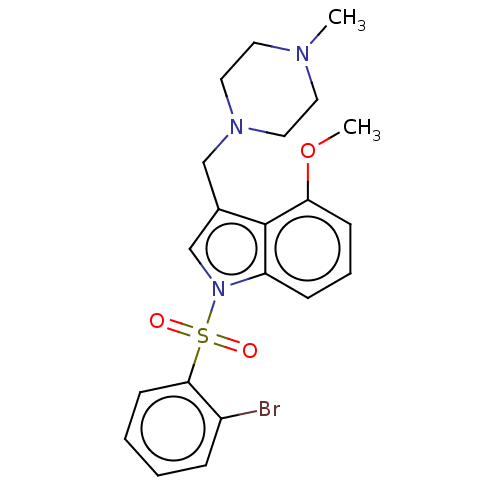
(CHEMBL4101418)Show SMILES Cl.Cl.COc1cccc2n(cc(CN3CCN(C)CC3)c12)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C21H24BrN3O3S/c1-23-10-12-24(13-11-23)14-16-15-25(18-7-5-8-19(28-2)21(16)18)29(26,27)20-9-4-3-6-17(20)22/h3-9,15H,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration for 50% protection of HIV-induced cytopathogenicity in MT-4 cells on the MTT assay |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236852
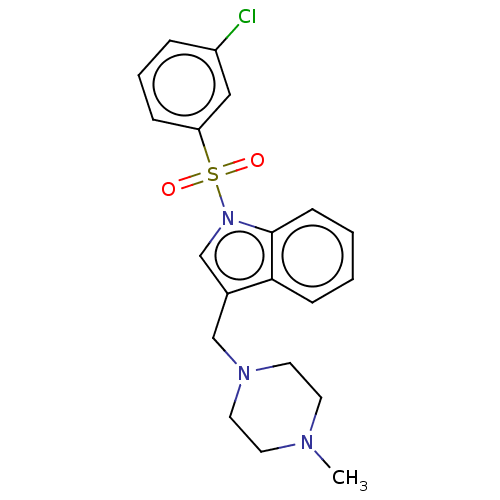
(CHEMBL4093110)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2cccc(Cl)c2)CC1 Show InChI InChI=1S/C20H22ClN3O2S/c1-22-9-11-23(12-10-22)14-16-15-24(20-8-3-2-7-19(16)20)27(25,26)18-6-4-5-17(21)13-18/h2-8,13,15H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration for 50% protection of HIV-induced cytopathogenicity in MT-4 cells on the MTT assay |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236776

(CHEMBL4091721)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H27N3O3S/c1-17-4-7-20(8-5-17)29(26,27)25-16-18(15-24-12-10-23(2)11-13-24)21-14-19(28-3)6-9-22(21)25/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236820
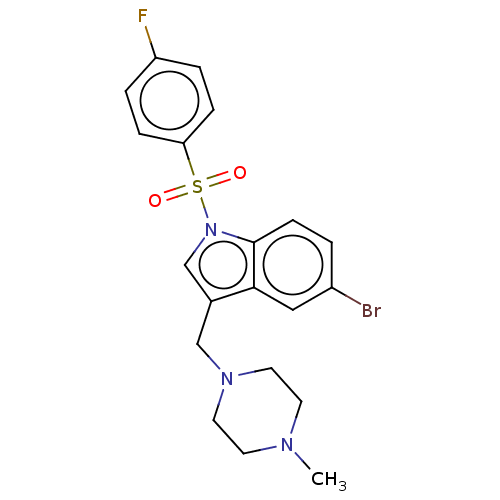
(CHEMBL4103877)Show SMILES CN1CCN(Cc2cn(c3ccc(Br)cc23)S(=O)(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C20H21BrFN3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(20-7-2-16(21)12-19(15)20)28(26,27)18-5-3-17(22)4-6-18/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236816
(CHEMBL4070850)Show SMILES COc1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2cc(OC)ccc12 Show InChI InChI=1S/C22H27N3O4S/c1-23-10-12-24(13-11-23)15-17-16-25(22-9-6-19(29-3)14-21(17)22)30(26,27)20-7-4-18(28-2)5-8-20/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236845

(CHEMBL4060415)Show SMILES CN1CCN(Cc2cn(c3ccc(Cl)cc23)S(=O)(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C20H21ClFN3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(20-7-2-16(21)12-19(15)20)28(26,27)18-5-3-17(22)4-6-18/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236819
(CHEMBL4074732)Show SMILES COc1cccc2n(cc(CN3CCN(C)CC3)c12)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C21H24FN3O3S/c1-23-10-12-24(13-11-23)14-16-15-25(19-4-3-5-20(28-2)21(16)19)29(26,27)18-8-6-17(22)7-9-18/h3-9,15H,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236813
(CHEMBL4090177)Show SMILES CN1CCN(Cc2cn(c3ccc(O)cc23)S(=O)(=O)c2ccccc2Br)CC1 Show InChI InChI=1S/C20H22BrN3O3S/c1-22-8-10-23(11-9-22)13-15-14-24(19-7-6-16(25)12-17(15)19)28(26,27)20-5-3-2-4-18(20)21/h2-7,12,14,25H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236800
(CHEMBL4100818)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2cccc(Br)c2)CC1 Show InChI InChI=1S/C20H22BrN3O2S/c1-22-9-11-23(12-10-22)14-16-15-24(20-8-3-2-7-19(16)20)27(25,26)18-6-4-5-17(21)13-18/h2-8,13,15H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Agonist efficacy as effective concentration to stimulate binding of [35S]GTP-gamma-S, by activation of human A3AR receptor |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236768
(CHEMBL4070785)Show SMILES CCN1CCN(Cc2cn(c3ccc(OC)cc23)S(=O)(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H26FN3O3S/c1-3-24-10-12-25(13-11-24)15-17-16-26(22-9-6-19(29-2)14-21(17)22)30(27,28)20-7-4-18(23)5-8-20/h4-9,14,16H,3,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration required for agonistic activity at Metabotropic glutamate receptor 2 |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236790
(CHEMBL4082319)Show SMILES CN1CCN(Cc2cn(c3cccc(Cl)c23)S(=O)(=O)c2ccccc2Br)CC1 Show InChI InChI=1S/C20H21BrClN3O2S/c1-23-9-11-24(12-10-23)13-15-14-25(18-7-4-6-17(22)20(15)18)28(26,27)19-8-3-2-5-16(19)21/h2-8,14H,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236799
(CHEMBL4095670)Show SMILES COc1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C21H25N3O3S/c1-22-11-13-23(14-12-22)15-17-16-24(21-6-4-3-5-20(17)21)28(25,26)19-9-7-18(27-2)8-10-19/h3-10,16H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236825
(CHEMBL4063362)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2cc(Br)ccc12 Show InChI InChI=1S/C23H28BrN3O2S/c1-17(2)18-4-7-21(8-5-18)30(28,29)27-16-19(15-26-12-10-25(3)11-13-26)22-14-20(24)6-9-23(22)27/h4-9,14,16-17H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236797

(CHEMBL4074234)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(Cl)cc2Cl)CC1 Show InChI InChI=1S/C20H21Cl2N3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(19-5-3-2-4-17(15)19)28(26,27)20-7-6-16(21)12-18(20)22/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236755
(CHEMBL4059662)Show SMILES COc1cccc(c1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C21H25N3O3S/c1-22-10-12-23(13-11-22)15-17-16-24(21-9-4-3-8-20(17)21)28(25,26)19-7-5-6-18(14-19)27-2/h3-9,14,16H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236849

(CHEMBL4090168)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(F)cc2Cl)CC1 Show InChI InChI=1S/C20H21ClFN3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(19-5-3-2-4-17(15)19)28(26,27)20-7-6-16(22)12-18(20)21/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Agonist activity in rat at mGlu2 receptor expressed in HEK293 cells |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236772
(CHEMBL4090395)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H21F2N3O2S/c1-23-8-10-24(11-9-23)13-15-14-25(19-5-3-2-4-17(15)19)28(26,27)20-7-6-16(21)12-18(20)22/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data