

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073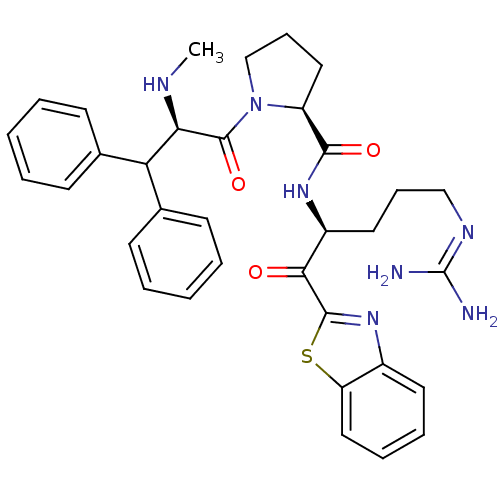 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065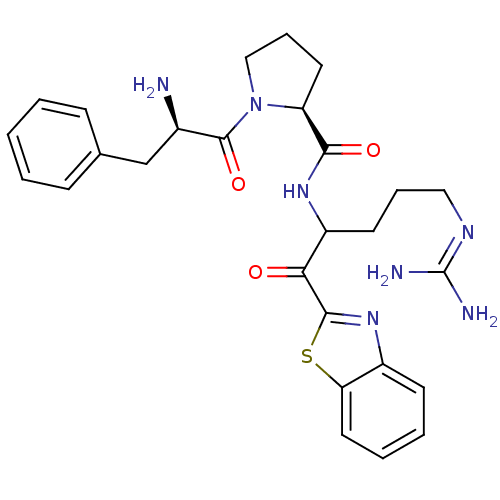 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127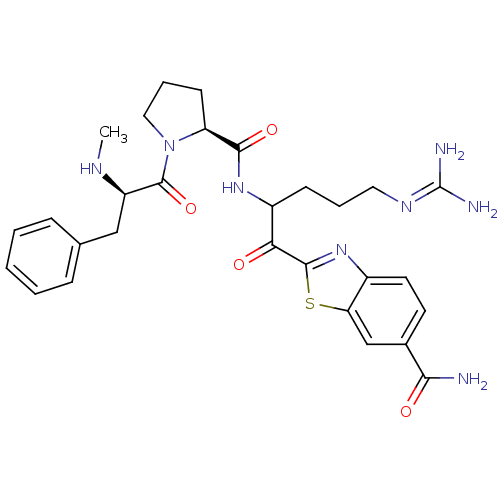 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14076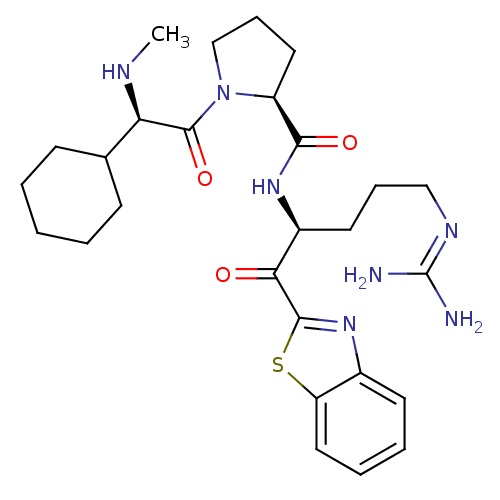 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504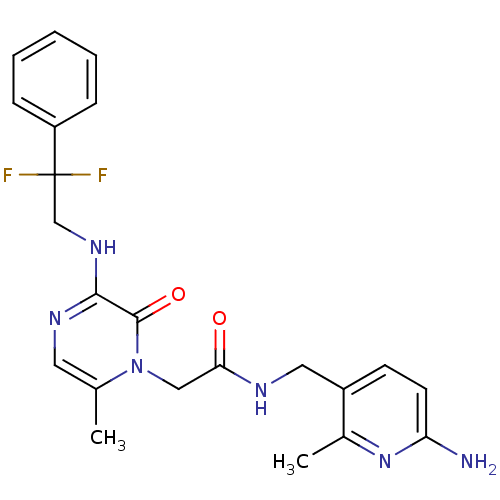 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14066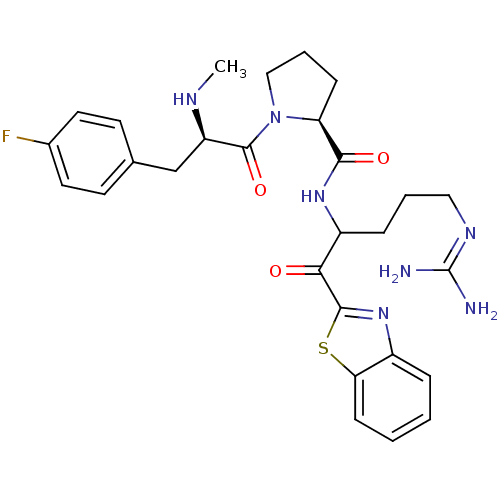 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -58.9 | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273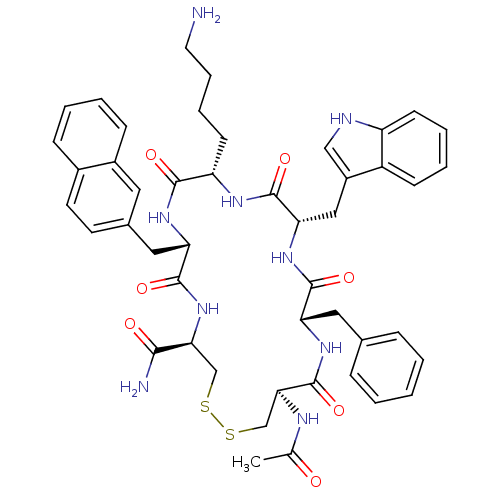 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14123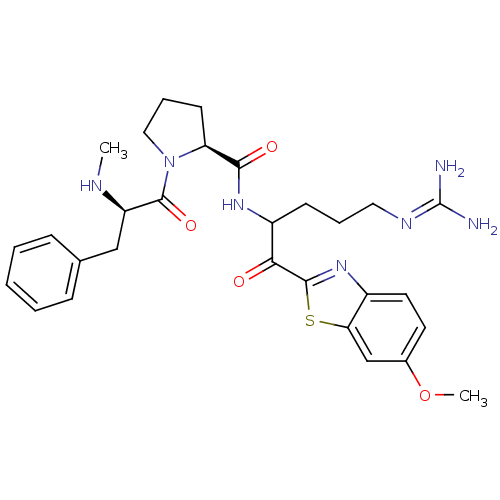 ((2S)-N-[5-carbamimidamido-1-(6-methoxy-1,3-benzoth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14068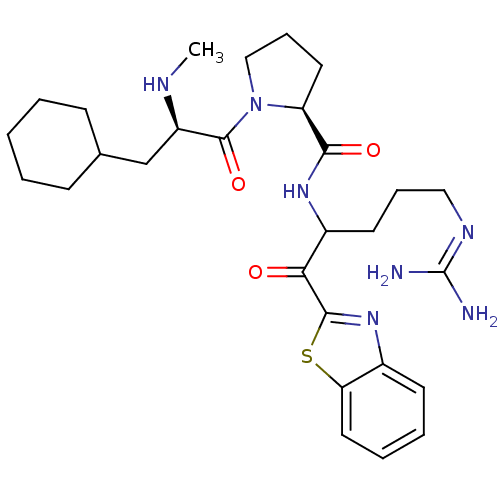 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -57.9 | 48 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14071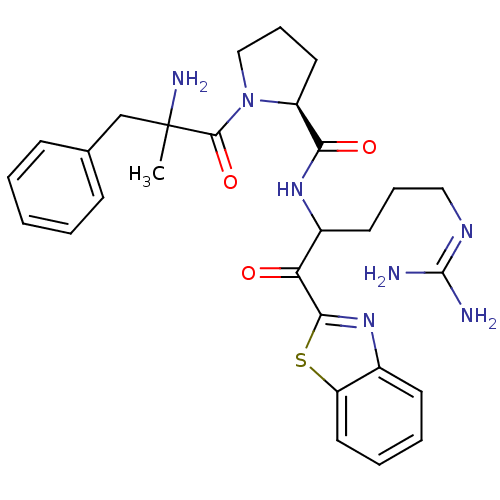 ((2S)-1-(2-amino-2-benzylpropanoyl)-N-[1-(1,3-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -57.6 | 3.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14063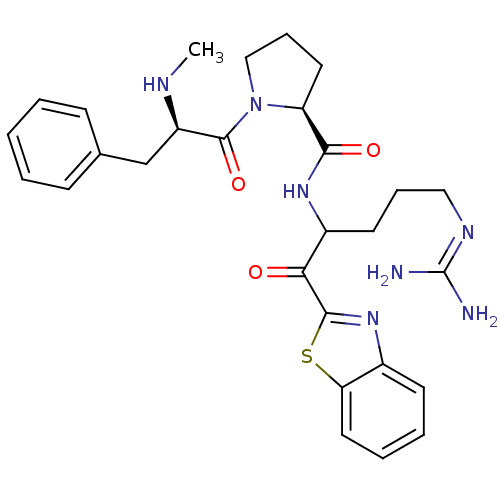 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -57.6 | 29 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14124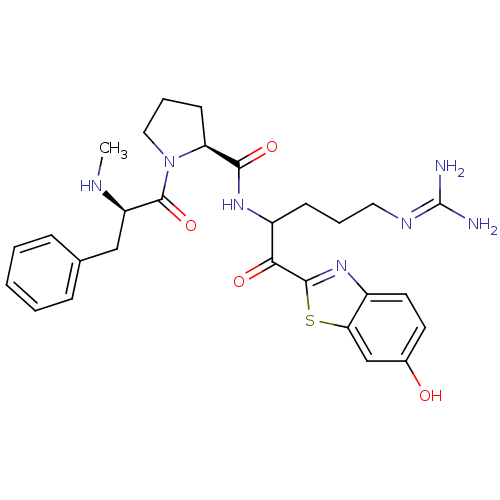 ((2S)-N-[5-carbamimidamido-1-(6-hydroxy-1,3-benzoth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14065 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14127 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14086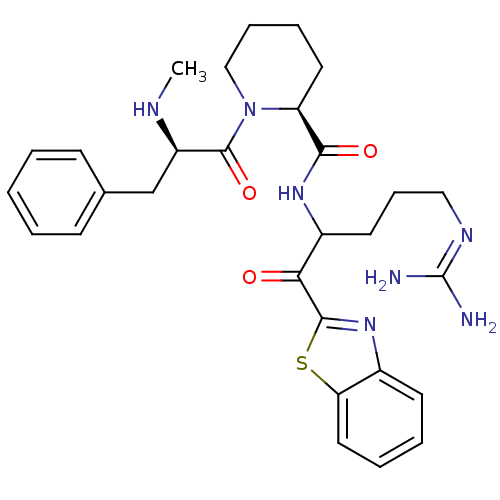 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14072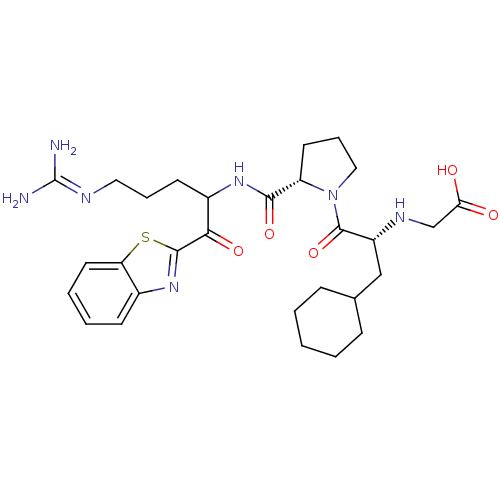 (2-ketobenzothiazole 12 | 2-{[(2R)-1-[(2S)-2-{[1-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.360 | -56.1 | 29 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14126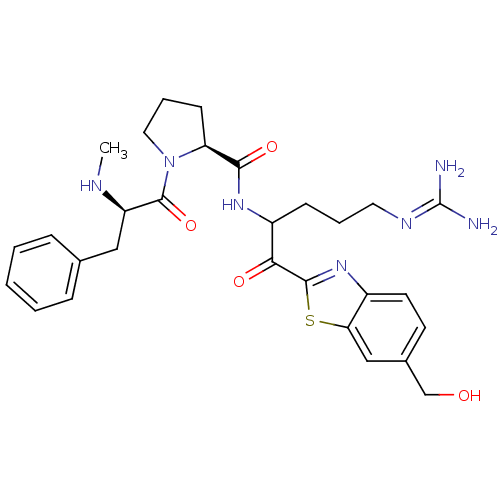 ((2S)-N-{5-carbamimidamido-1-[6-(hydroxymethyl)-1,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377618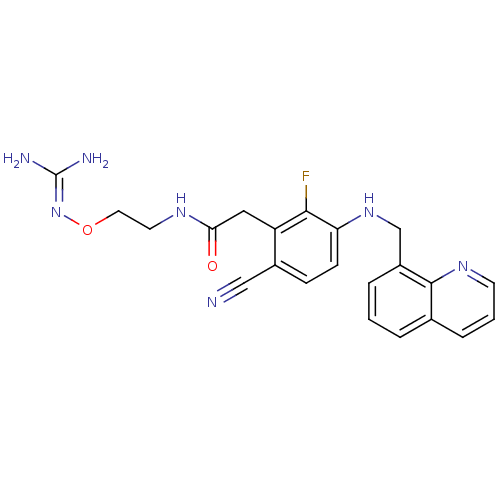 (CHEMBL254353) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14075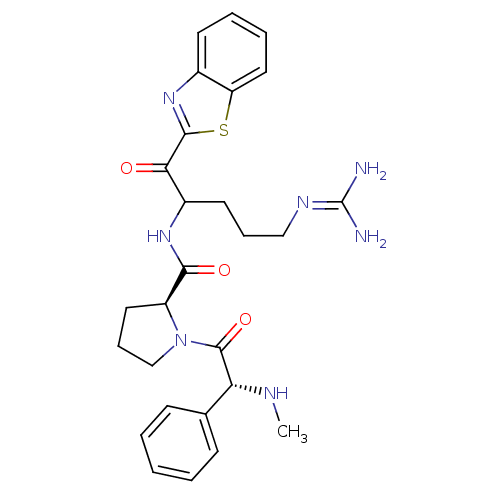 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.460 | -55.4 | 34 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377625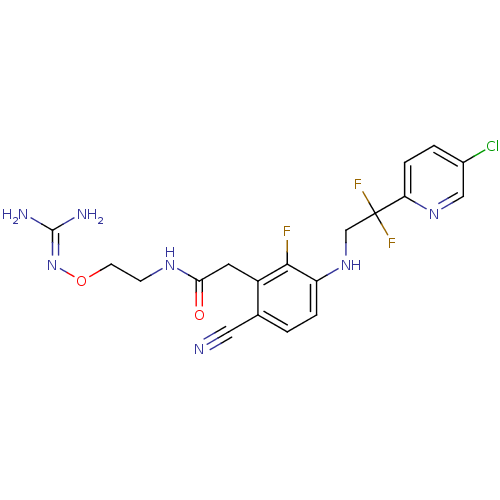 (CHEMBL254557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14122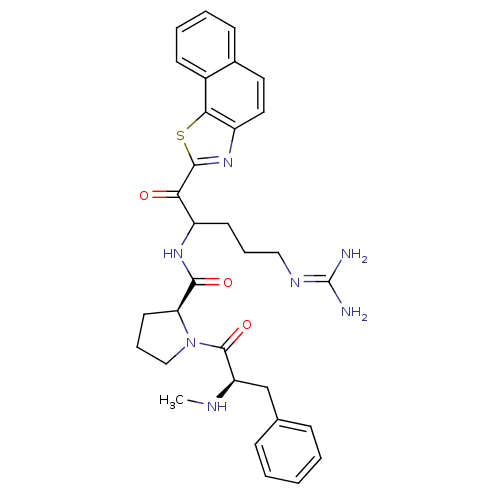 ((2S)-N-(5-carbamimidamido-1-oxo-1-{3-thia-5-azatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377615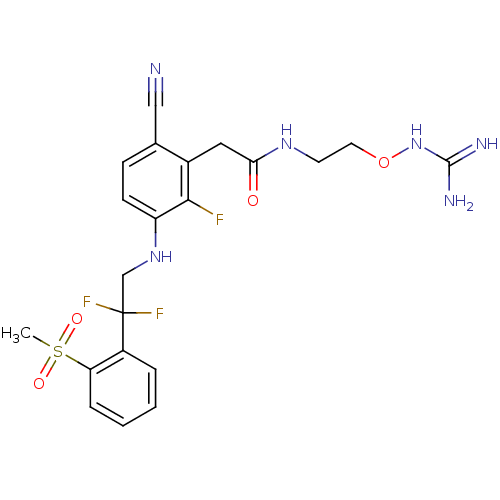 (CHEMBL254962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377623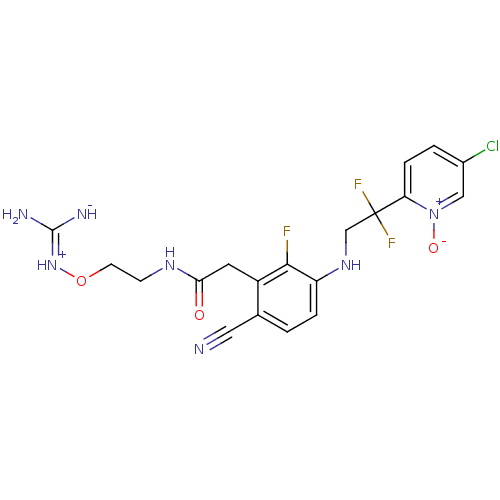 (CHEMBL254759) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377611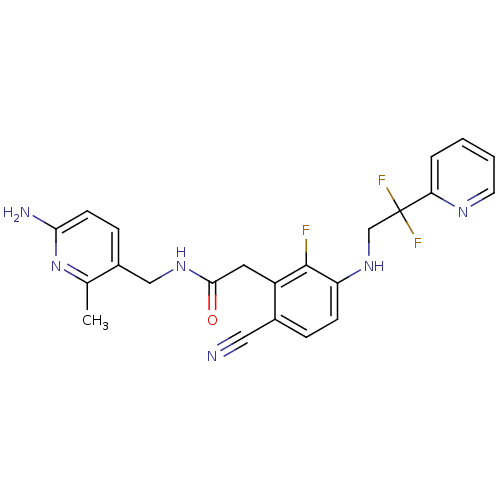 (CHEMBL258018) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14139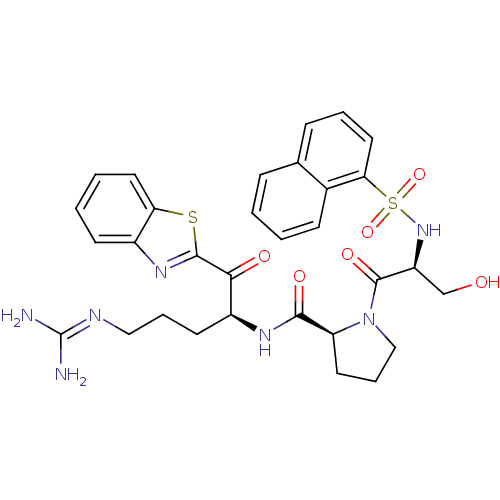 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.780 | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377619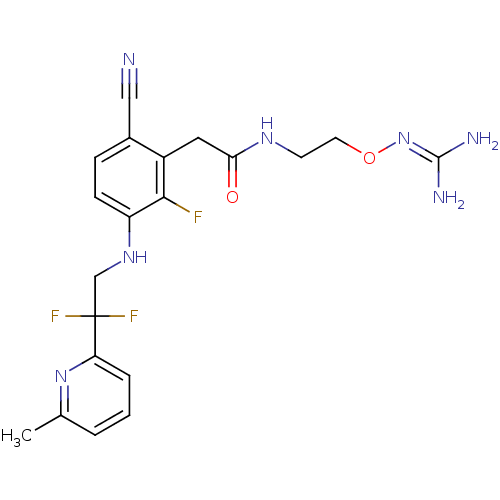 (CHEMBL402758) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14092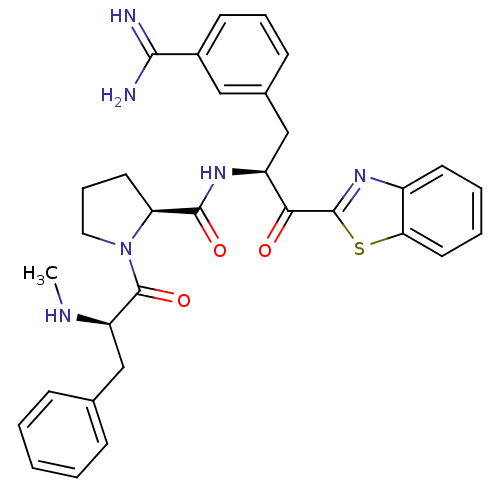 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-(3-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14074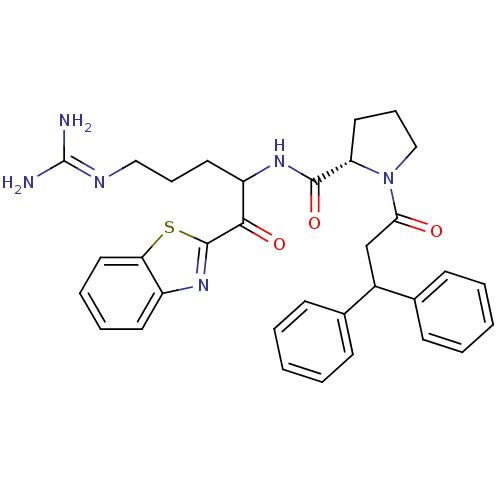 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -53.2 | 11 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377620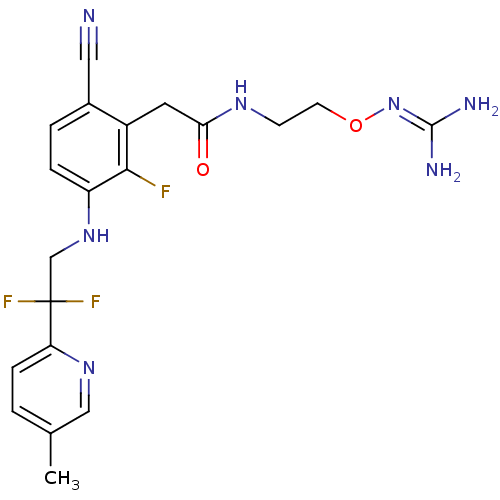 (CHEMBL254784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14076 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14117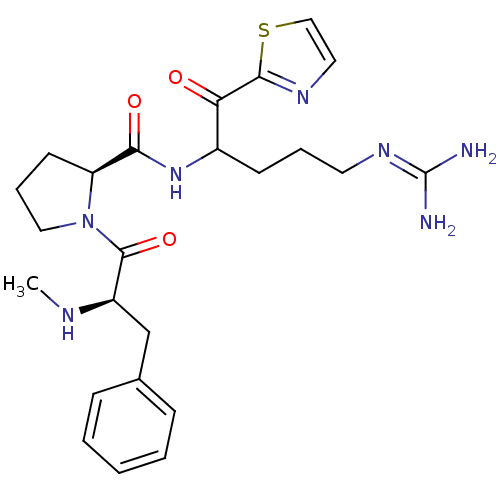 ((2S)-N-[5-carbamimidamido-1-oxo-1-(1,3-thiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377622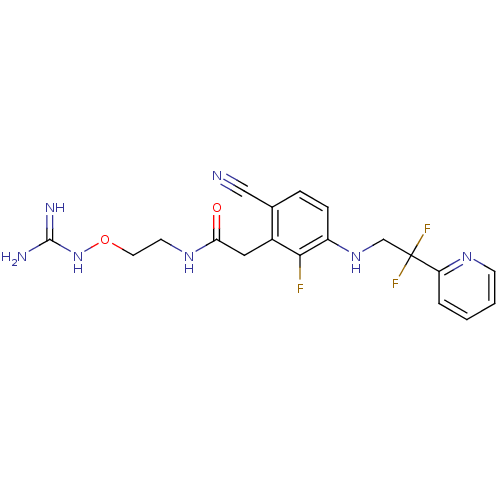 (CHEMBL257543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14128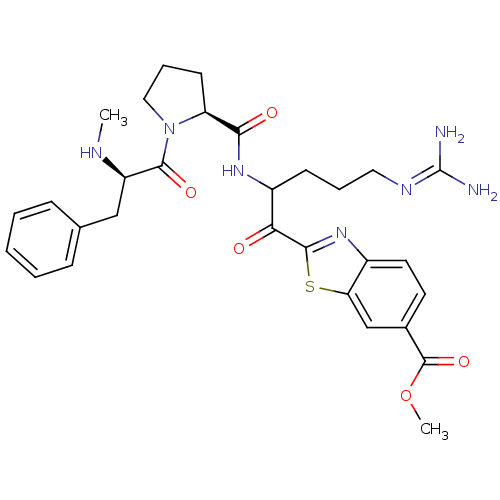 (2-ketobenzothiazole 68 | methyl 2-(5-carbamimidami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377614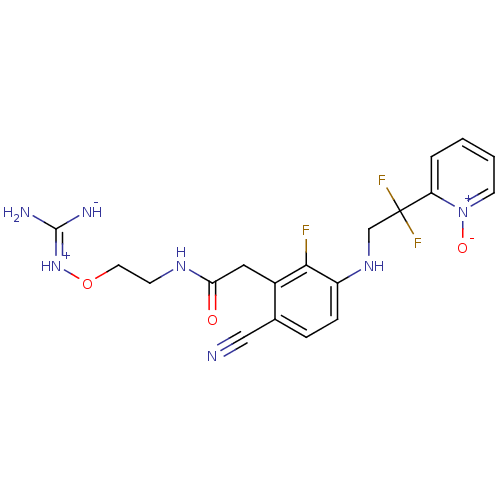 (CHEMBL401655) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14137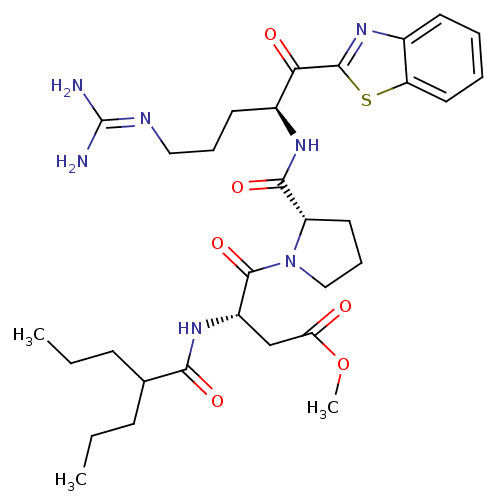 (2-ketobenzothiazole 75 | methyl (3S)-4-[(2S)-2-{[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14138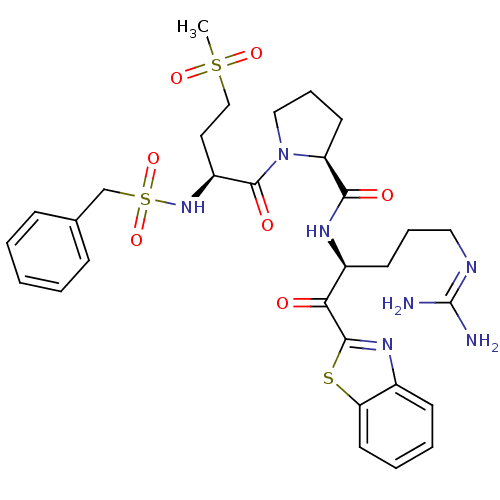 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14085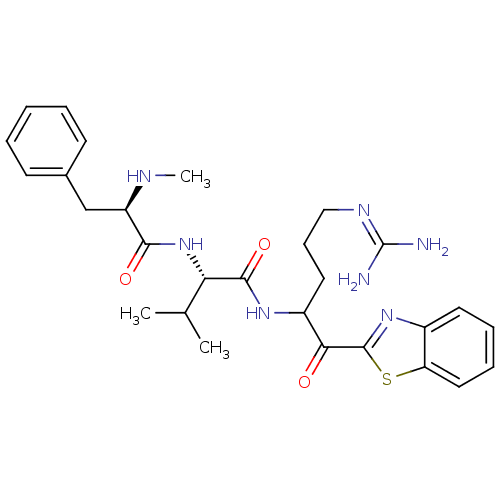 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14094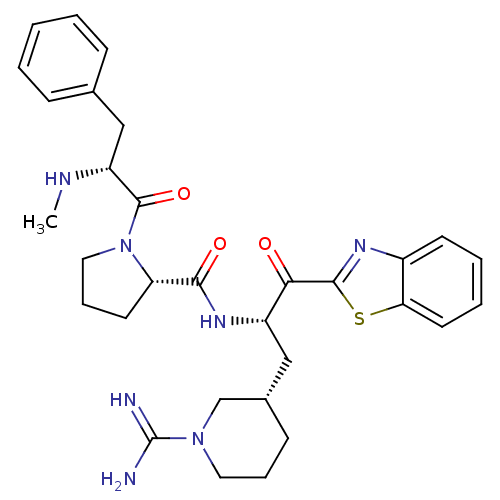 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-[(3S)-1-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377617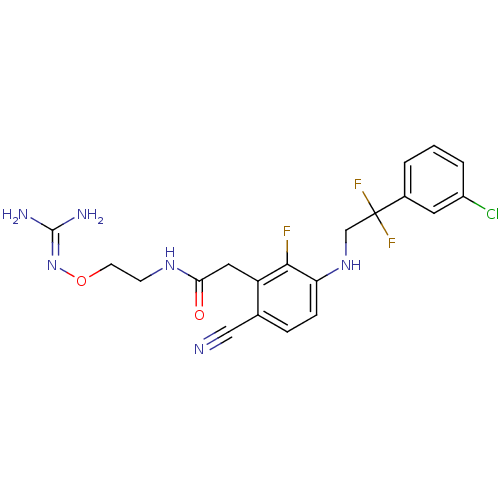 (CHEMBL403359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14086 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14092 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-3-(3-carbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14078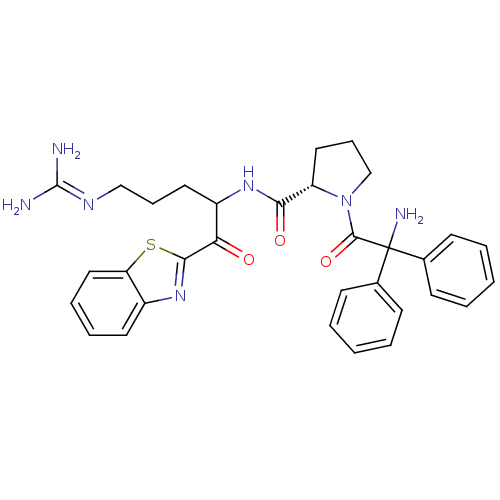 ((2S)-1-(2-amino-2,2-diphenylacetyl)-N-[1-(1,3-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50377624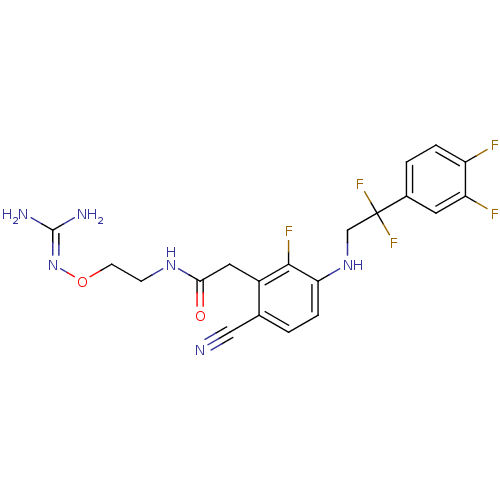 (CHEMBL403310) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14129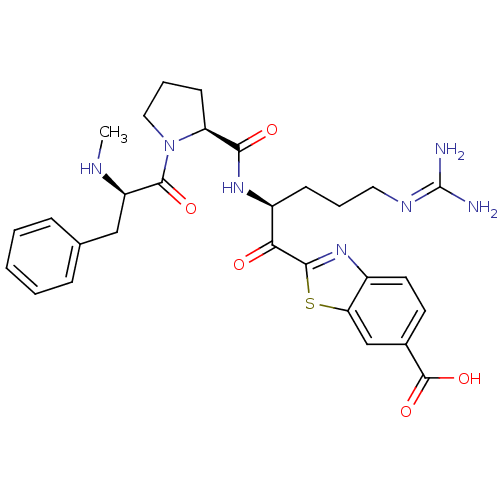 (2-[(2S)-5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14123 ((2S)-N-[5-carbamimidamido-1-(6-methoxy-1,3-benzoth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14079 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14078 ((2S)-1-(2-amino-2,2-diphenylacetyl)-N-[1-(1,3-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -51.5 | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14117 ((2S)-N-[5-carbamimidamido-1-oxo-1-(1,3-thiazol-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 466 total ) | Next | Last >> |