

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin I (Plasmodium falciparum) | BDBM50169100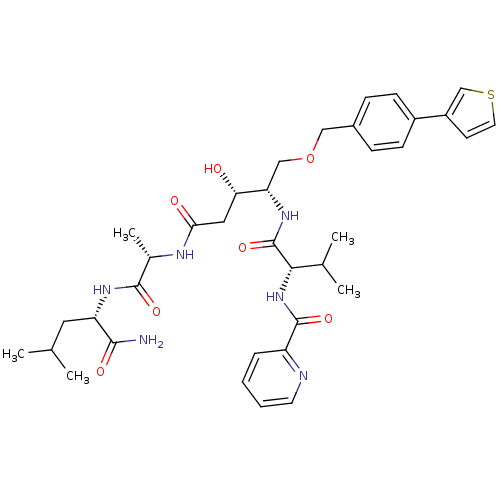 (CHEMBL191260 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169098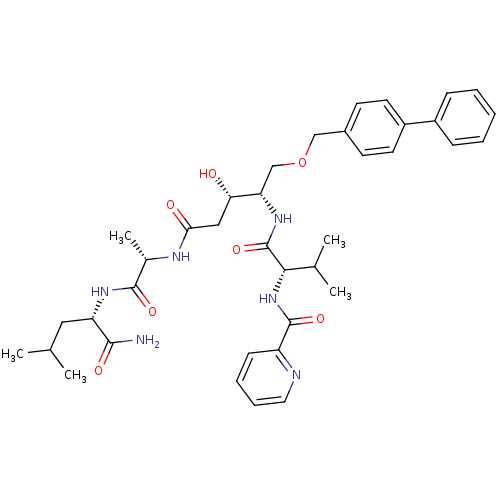 (CHEMBL264770 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169104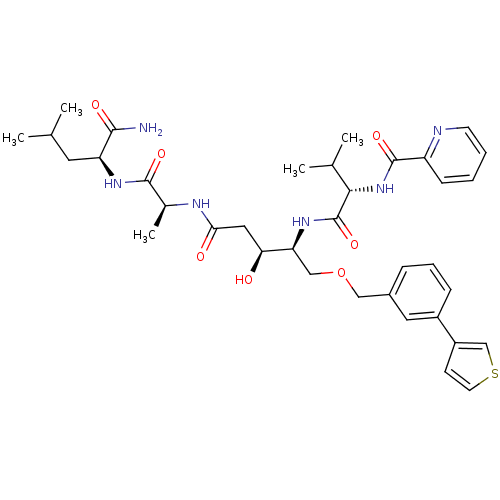 (CHEMBL371417 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169103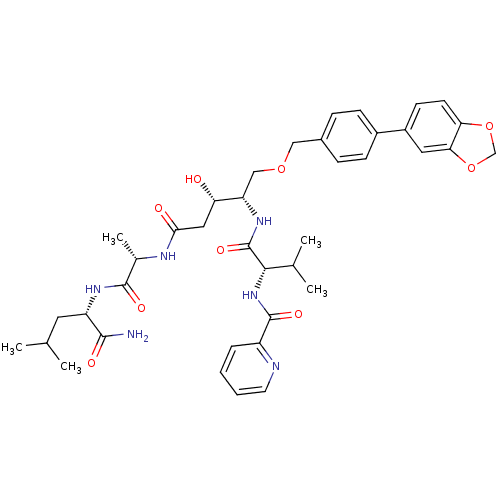 (CHEMBL191130 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8007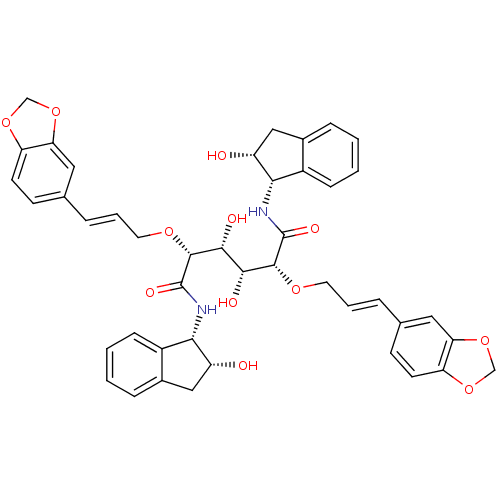 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(2H-1,3-benzodioxol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7974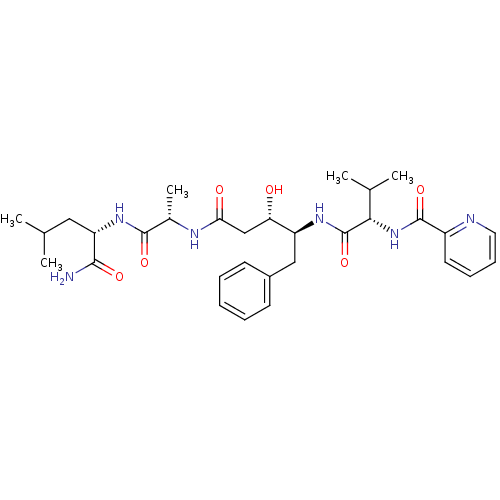 ((3S,4S)-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7974 ((3S,4S)-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | -52.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50169103 (CHEMBL191130 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the human Cathepsin D | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169106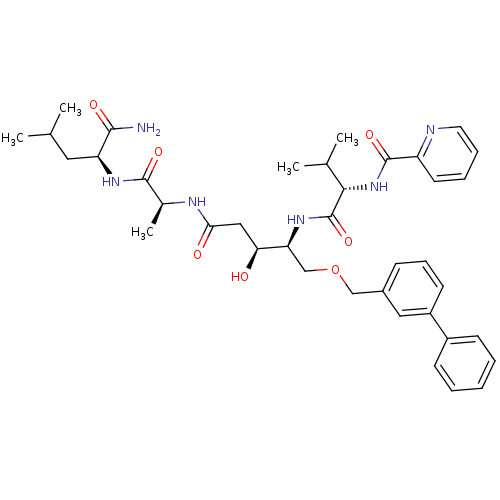 (CHEMBL363286 | Pyridine-2-carboxylic acid ((S)-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50333945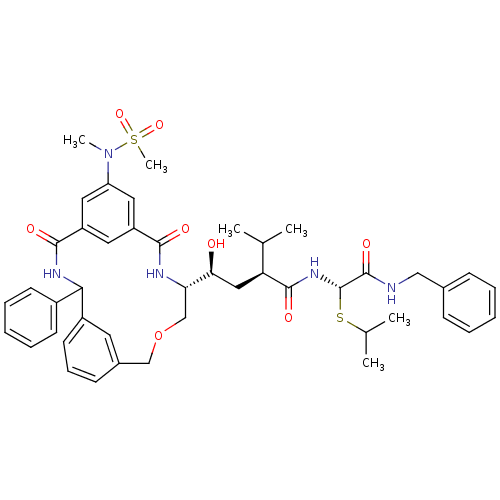 ((S)-N-((S)-Benzylcarbamoyl-isopropylsulfanyl-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 21: 358-62 (2010) Article DOI: 10.1016/j.bmcl.2010.10.140 BindingDB Entry DOI: 10.7270/Q2HM58RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8005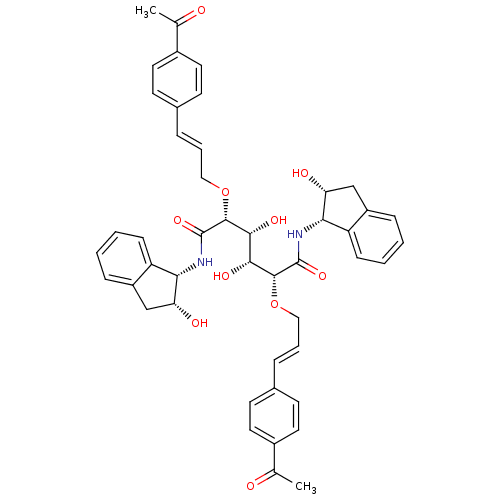 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-acetylphenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169098 (CHEMBL264770 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169109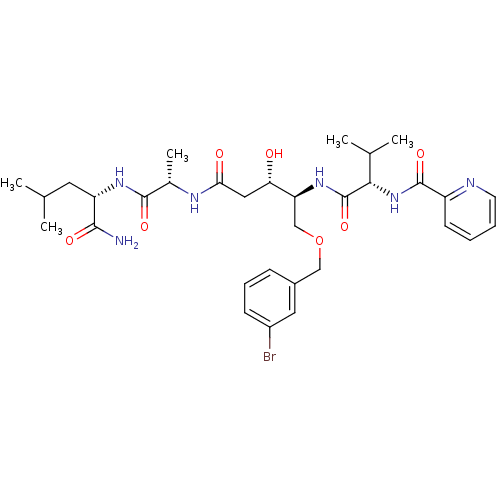 (CHEMBL190290 | Pyridine-2-carboxylic acid ((S)-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169105 (CHEMBL370086 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169100 (CHEMBL191260 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169103 (CHEMBL191130 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8008 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8002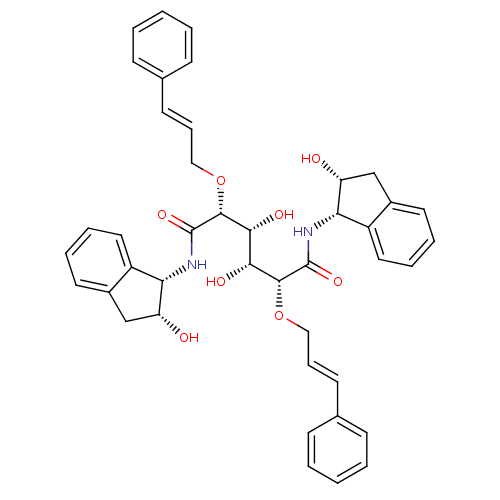 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50169104 (CHEMBL371417 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the human Cathepsin D | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169110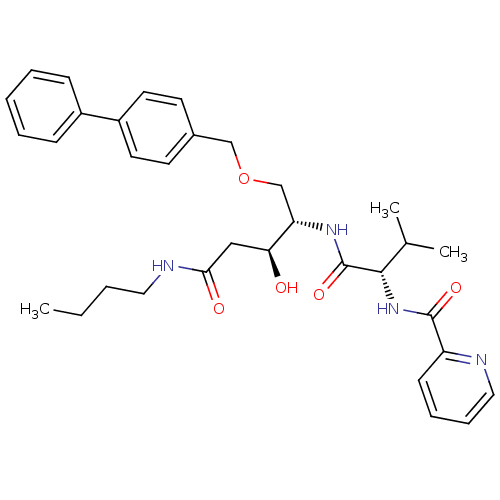 (CHEMBL364630 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169104 (CHEMBL371417 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8099 ((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-(4-phenylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Comb Chem 5: 456-64 (2003) Article DOI: 10.1021/cc0301014 BindingDB Entry DOI: 10.7270/Q27S7KZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169112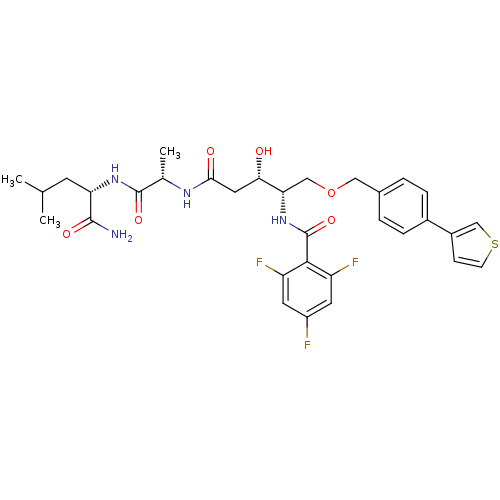 (CHEMBL371082 | N-[(1S,2S)-3-[(S)-1-((S)-1-Carbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8003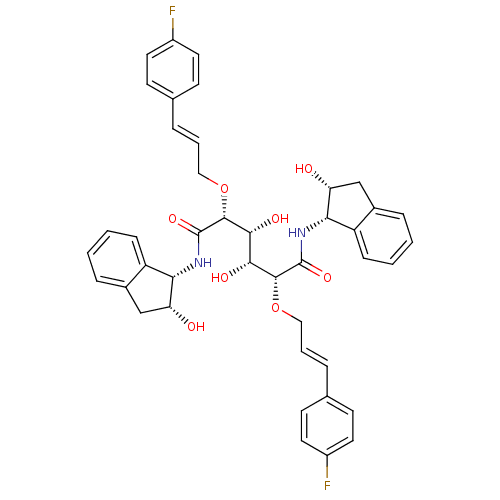 ((2R,3R,4R,5R)-2,5-bis({[(2E)-3-(4-fluorophenyl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50169098 (CHEMBL264770 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the human Cathepsin D | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8015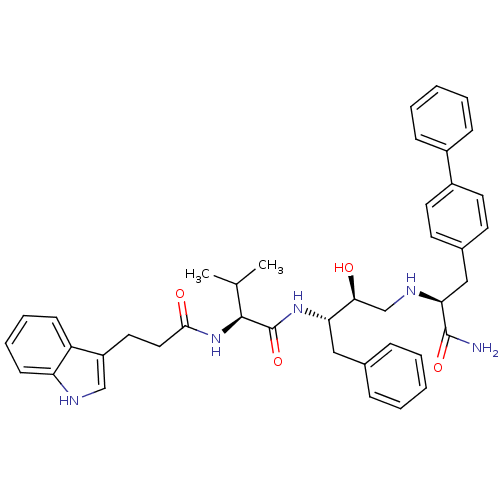 ((2S)-N-[(2S,3S)-4-{[(1S)-1-carbamoyl-2-(4-phenylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169113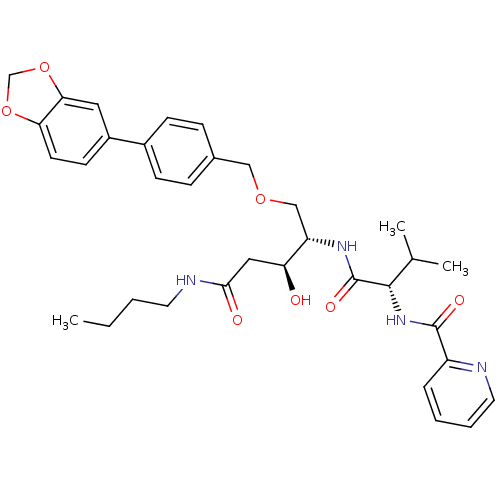 (CHEMBL371182 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50169100 (CHEMBL191260 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the human Cathepsin D | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50333943 (CHEMBL1644461 | N1-((2S,3S,5S)-5-((S)-1-(benzylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 21: 358-62 (2010) Article DOI: 10.1016/j.bmcl.2010.10.140 BindingDB Entry DOI: 10.7270/Q2HM58RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169102 (CHEMBL189976 | N-{(1S,2S)-1-(4-Benzo[1,3]dioxol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50333945 ((S)-N-((S)-Benzylcarbamoyl-isopropylsulfanyl-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of renin | Bioorg Med Chem Lett 21: 358-62 (2010) Article DOI: 10.1016/j.bmcl.2010.10.140 BindingDB Entry DOI: 10.7270/Q2HM58RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM7988 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM7988 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8011 ((2R,3R,4R,5R)-N1,N6-Bis[(1S,2R)-2-hydroxy-1-indany...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM7979 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-(cyclohexylme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50169106 (CHEMBL363286 | Pyridine-2-carboxylic acid ((S)-1-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the human Cathepsin D | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8111 ((2S)-N-[(2S,3S)-4-{[(1S)-2-[4-(1-benzofuran-2-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Comb Chem 5: 456-64 (2003) Article DOI: 10.1021/cc0301014 BindingDB Entry DOI: 10.7270/Q27S7KZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM7983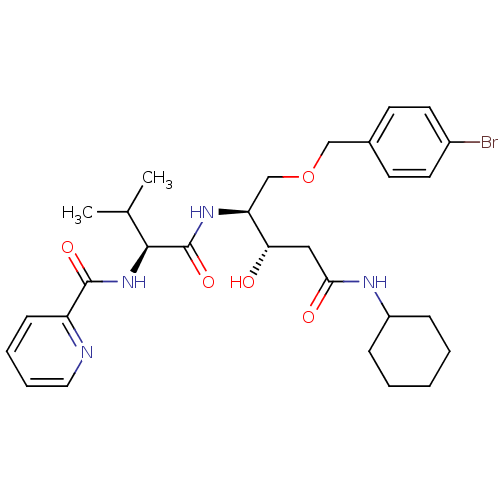 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-cyclohexyl-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM8019 (2-(3-chlorophenoxy)-N-[(2S,3S)-3-hydroxy-4-{N-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8010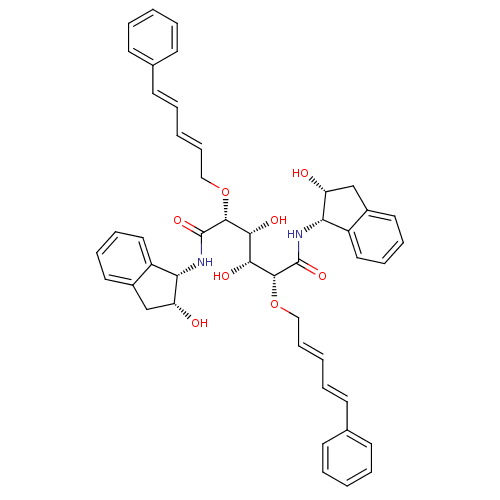 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8010 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 110-22 (2004) Article DOI: 10.1021/jm030933g BindingDB Entry DOI: 10.7270/Q2RV0KX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50169106 (CHEMBL363286 | Pyridine-2-carboxylic acid ((S)-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin II of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8084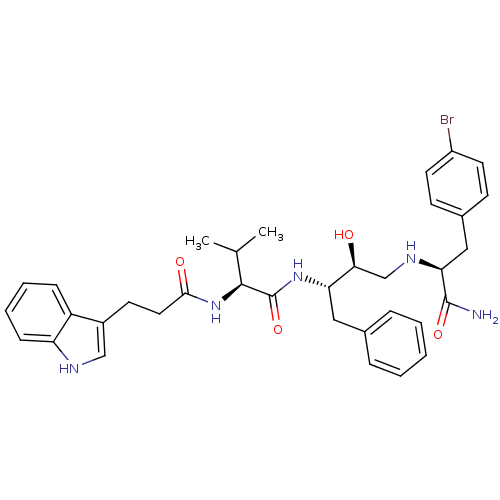 ((2S)-N-[(2S,3S)-4-{[(1S)-2-(4-bromophenyl)-1-carba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Comb Chem 5: 456-64 (2003) Article DOI: 10.1021/cc0301014 BindingDB Entry DOI: 10.7270/Q27S7KZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM8109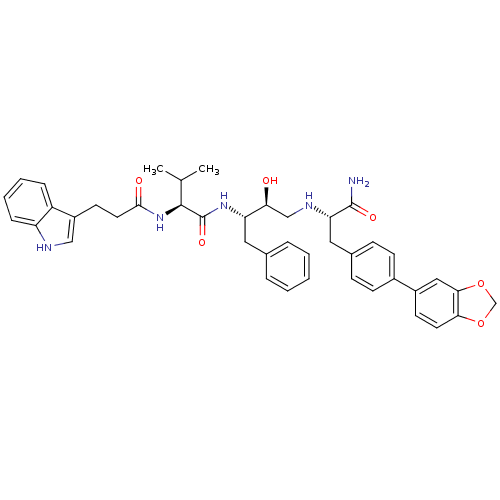 ((2S)-N-[(2S,3S)-4-{[(1S)-2-[4-(2H-1,3-benzodioxol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Comb Chem 5: 456-64 (2003) Article DOI: 10.1021/cc0301014 BindingDB Entry DOI: 10.7270/Q27S7KZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM7977 ((3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the human Cathepsin D | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1888 total ) | Next | Last >> |