 Found 989 hits with Last Name = 'han' and Initial = 'ym'
Found 989 hits with Last Name = 'han' and Initial = 'ym' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185884
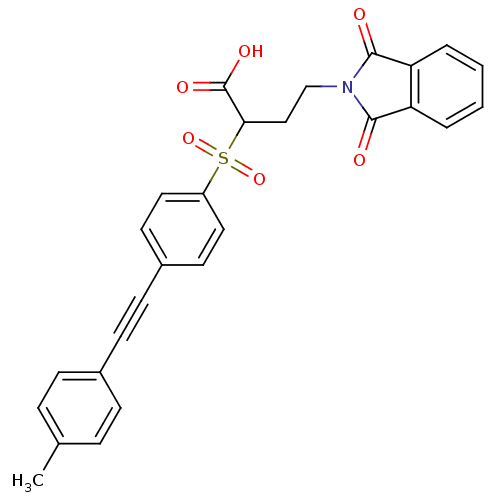
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50608318
(CHEMBL5279959) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185896

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-tr...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H18F3NO5S/c28-27(29,30)36-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)37-23(26(34)35)15-16-31-24(32)21-3-1-2-4-22(21)25(31)33/h1-4,7-14,23H,15-16H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185883
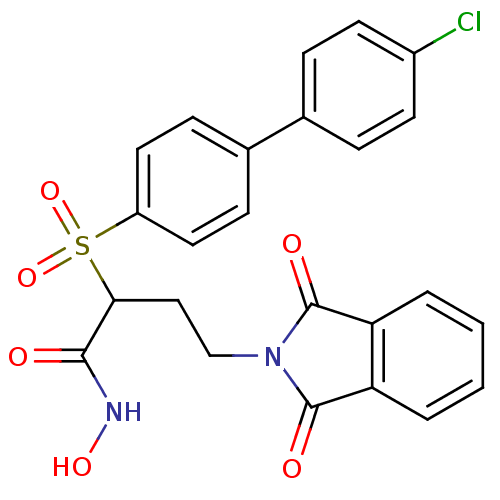
(2-(4'-chloro-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3...)Show SMILES ONC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19ClN2O6S/c25-17-9-5-15(6-10-17)16-7-11-18(12-8-16)34(32,33)21(22(28)26-31)13-14-27-23(29)19-3-1-2-4-20(19)24(27)30/h1-12,21,31H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185884

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185900

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S2/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185888
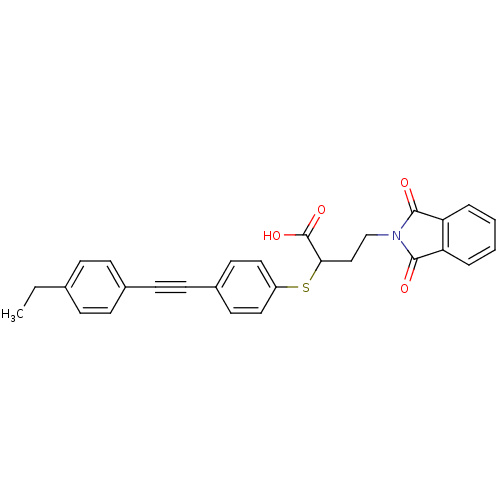
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-et...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C28H23NO4S/c1-2-19-7-9-20(10-8-19)11-12-21-13-15-22(16-14-21)34-25(28(32)33)17-18-29-26(30)23-5-3-4-6-24(23)27(29)31/h3-10,13-16,25H,2,17-18H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185875

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO7S/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374486
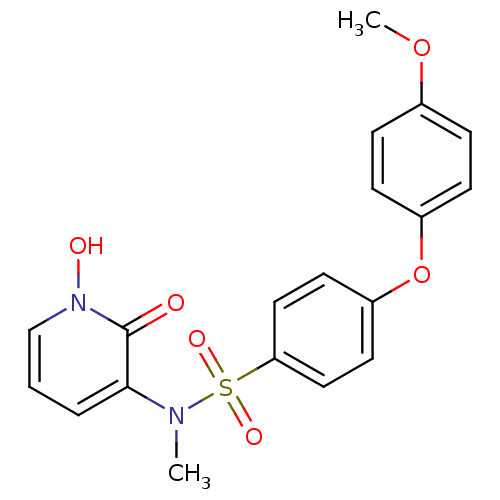
(CHEMBL408366)Show SMILES COc1ccc(Oc2ccc(cc2)S(=O)(=O)N(C)c2cccn(O)c2=O)cc1 Show InChI InChI=1S/C19H18N2O6S/c1-20(18-4-3-13-21(23)19(18)22)28(24,25)17-11-9-16(10-12-17)27-15-7-5-14(26-2)6-8-15/h3-13,23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185900

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S2/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185880

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-isop...)Show SMILES CC(C)c1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H25NO6S/c1-17(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)35(33,34)24(27(31)32)15-16-28-25(29)22-5-3-4-6-23(22)26(28)30/h3-14,17,24H,15-16H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 14: 111-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.10.010
BindingDB Entry DOI: 10.7270/Q2HQ3X4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 14: 111-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.10.010
BindingDB Entry DOI: 10.7270/Q2HQ3X4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185890
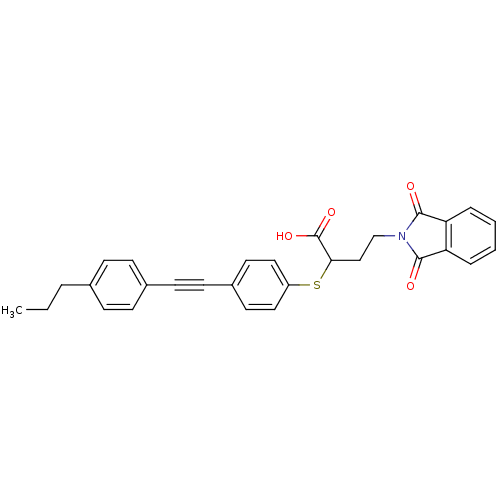
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-pr...)Show SMILES CCCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C29H25NO4S/c1-2-5-20-8-10-21(11-9-20)12-13-22-14-16-23(17-15-22)35-26(29(33)34)18-19-30-27(31)24-6-3-4-7-25(24)28(30)32/h3-4,6-11,14-17,26H,2,5,18-19H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM208271
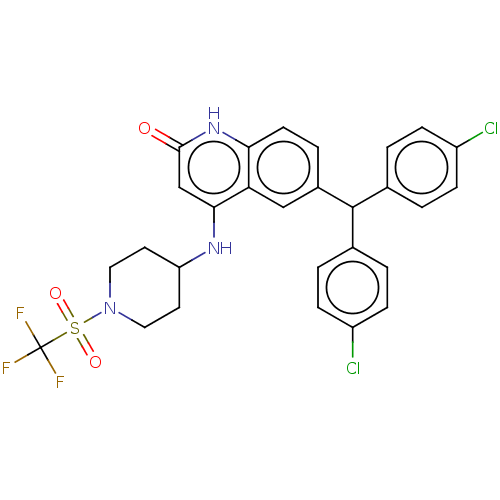
(US9266835, 44)Show SMILES FC(F)(F)S(=O)(=O)N1CCC(CC1)Nc1cc(=O)[nH]c2ccc(cc12)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H24Cl2F3N3O3S/c29-20-6-1-17(2-7-20)27(18-3-8-21(30)9-4-18)19-5-10-24-23(15-19)25(16-26(37)35-24)34-22-11-13-36(14-12-22)40(38,39)28(31,32)33/h1-10,15-16,22,27H,11-14H2,(H2,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO-K1 cell membranes |
J Med Chem 61: 10276-10298 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01467
BindingDB Entry DOI: 10.7270/Q2PR7ZPM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese National Center for Drug Screening
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC as substrate after 24 hrs by fluorescence assay |
ACS Med Chem Lett 5: 628-33 (2014)
Article DOI: 10.1021/ml400470s
BindingDB Entry DOI: 10.7270/Q2V40WRC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185877
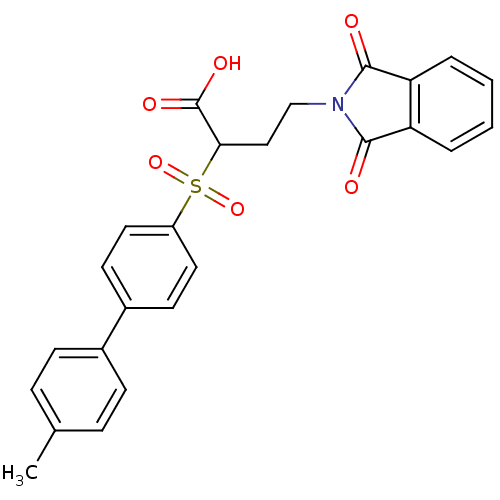
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S/c1-16-6-8-17(9-7-16)18-10-12-19(13-11-18)33(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185899
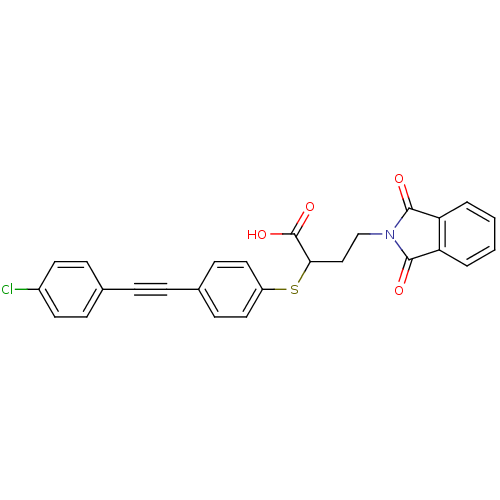
(2-[4-(4-chloro-phenylethynyl)-phenylsulfanyl]-4-(1...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(Cl)cc1 Show InChI InChI=1S/C26H18ClNO4S/c27-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)33-23(26(31)32)15-16-28-24(29)21-3-1-2-4-22(21)25(28)30/h1-4,7-14,23H,15-16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185896

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-tr...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H18F3NO5S/c28-27(29,30)36-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)37-23(26(34)35)15-16-31-24(32)21-3-1-2-4-22(21)25(31)33/h1-4,7-14,23H,15-16H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374494

(CHEMBL271736)Show SMILES CN1CCN(CCN(c2cccn(O)c2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C24H27ClN4O5S/c1-26-13-15-27(16-14-26)17-18-29(23-3-2-12-28(31)24(23)30)35(32,33)22-10-8-21(9-11-22)34-20-6-4-19(25)5-7-20/h2-12,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM208271

(US9266835, 44)Show SMILES FC(F)(F)S(=O)(=O)N1CCC(CC1)Nc1cc(=O)[nH]c2ccc(cc12)C(c1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H24Cl2F3N3O3S/c29-20-6-1-17(2-7-20)27(18-3-8-21(30)9-4-18)19-5-10-24-23(15-19)25(16-26(37)35-24)34-22-11-13-36(14-12-22)40(38,39)28(31,32)33/h1-10,15-16,22,27H,11-14H2,(H2,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rimonabant from human CB1 receptor expressed in CHO cell membranes after 60 mins by TopCount method |
J Med Chem 61: 10276-10298 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01467
BindingDB Entry DOI: 10.7270/Q2PR7ZPM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185888

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-et...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C28H23NO4S/c1-2-19-7-9-20(10-8-19)11-12-21-13-15-22(16-14-21)34-25(28(32)33)17-18-29-26(30)23-5-3-4-6-24(23)27(29)31/h3-10,13-16,25H,2,17-18H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185887
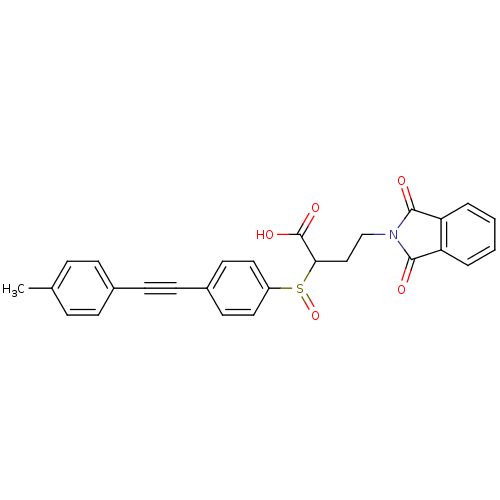
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO5S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)34(33)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50185884

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185883

(2-(4'-chloro-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3...)Show SMILES ONC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19ClN2O6S/c25-17-9-5-15(6-10-17)16-7-11-18(12-8-16)34(32,33)21(22(28)26-31)13-14-27-23(29)19-3-1-2-4-20(19)24(27)30/h1-12,21,31H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185887

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO5S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)34(33)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185880

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-isop...)Show SMILES CC(C)c1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H25NO6S/c1-17(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)35(33,34)24(27(31)32)15-16-28-25(29)22-5-3-4-6-23(22)26(28)30/h3-14,17,24H,15-16H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50514463

(CHEMBL4460552)Show SMILES C[C@H](NC(=O)c1nc(sc1C1CC1)-c1ncc(s1)-c1ccccc1)c1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C25H22N4O3S2/c1-14(15-7-11-18(12-8-15)22(30)29-32)27-23(31)20-21(17-9-10-17)34-25(28-20)24-26-13-19(33-24)16-5-3-2-4-6-16/h2-8,11-14,17,32H,9-10H2,1H3,(H,27,31)(H,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6/GST-tagged human HDAC6 expressed in baculovirus infected High5 insect cells using Boc-Lys(epsion-acetyl)-AMC as substr... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50374494

(CHEMBL271736)Show SMILES CN1CCN(CCN(c2cccn(O)c2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C24H27ClN4O5S/c1-26-13-15-27(16-14-26)17-18-29(23-3-2-12-28(31)24(23)30)35(32,33)22-10-8-21(9-11-22)34-20-6-4-19(25)5-7-20/h2-12,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185892
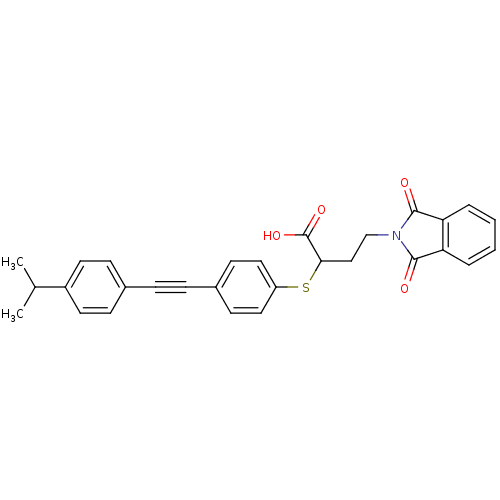
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-is...)Show SMILES CC(C)c1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C29H25NO4S/c1-19(2)22-13-9-20(10-14-22)7-8-21-11-15-23(16-12-21)35-26(29(33)34)17-18-30-27(31)24-5-3-4-6-25(24)28(30)32/h3-6,9-16,19,26H,17-18H2,1-2H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185895

(2-(4'-chloro-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18ClNO6S/c25-17-9-5-15(6-10-17)16-7-11-18(12-8-16)33(31,32)21(24(29)30)13-14-26-22(27)19-3-1-2-4-20(19)23(26)28/h1-12,21H,13-14H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374491
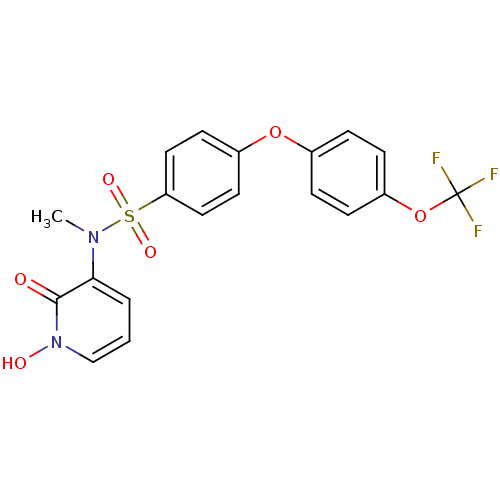
(CHEMBL271720)Show SMILES CN(c1cccn(O)c1=O)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C19H15F3N2O6S/c1-23(17-3-2-12-24(26)18(17)25)31(27,28)16-10-8-14(9-11-16)29-13-4-6-15(7-5-13)30-19(20,21)22/h2-12,26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185899

(2-[4-(4-chloro-phenylethynyl)-phenylsulfanyl]-4-(1...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(Cl)cc1 Show InChI InChI=1S/C26H18ClNO4S/c27-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)33-23(26(31)32)15-16-28-24(29)21-3-1-2-4-22(21)25(28)30/h1-4,7-14,23H,15-16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50514463

(CHEMBL4460552)Show SMILES C[C@H](NC(=O)c1nc(sc1C1CC1)-c1ncc(s1)-c1ccccc1)c1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C25H22N4O3S2/c1-14(15-7-11-18(12-8-15)22(30)29-32)27-23(31)20-21(17-9-10-17)34-25(28-20)24-26-13-19(33-24)16-5-3-2-4-6-16/h2-8,11-14,17,32H,9-10H2,1H3,(H,27,31)(H,29,30)/t14-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6/GST-tagged HDAC10 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC ... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50420961

(CHEMBL2088096)Show SMILES COc1cc(CCc2cc(Nc3ccnc(NCc4cc(C)no4)n3)[nH]n2)cc(OC)c1 Show InChI InChI=1S/C22H25N7O3/c1-14-8-19(32-29-14)13-24-22-23-7-6-20(26-22)25-21-11-16(27-28-21)5-4-15-9-17(30-2)12-18(10-15)31-3/h6-12H,4-5,13H2,1-3H3,(H3,23,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50514461
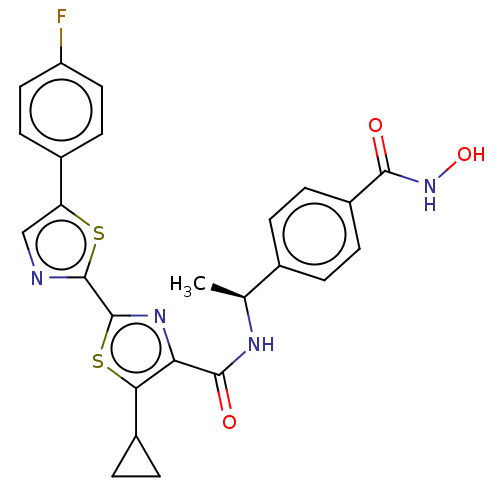
(CHEMBL4443998)Show SMILES C[C@H](NC(=O)c1nc(sc1C1CC1)-c1ncc(s1)-c1ccc(F)cc1)c1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C25H21FN4O3S2/c1-13(14-2-6-17(7-3-14)22(31)30-33)28-23(32)20-21(16-4-5-16)35-25(29-20)24-27-12-19(34-24)15-8-10-18(26)11-9-15/h2-3,6-13,16,33H,4-5H2,1H3,(H,28,32)(H,30,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6/GST-tagged human HDAC6 expressed in baculovirus infected High5 insect cells using Boc-Lys(epsion-acetyl)-AMC as substr... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185898
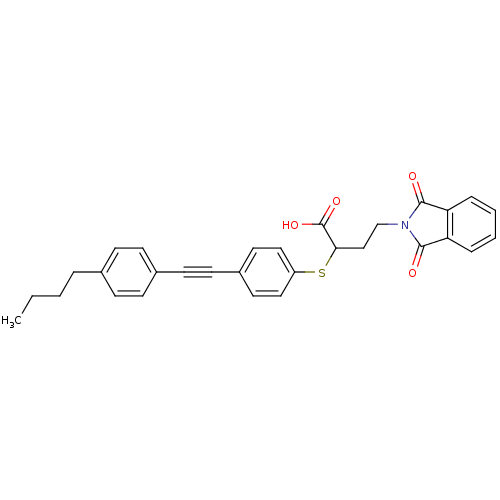
(2-[4-(4-butyl-phenylethynyl)-phenylsulfanyl]-4-(1,...)Show SMILES CCCCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C30H27NO4S/c1-2-3-6-21-9-11-22(12-10-21)13-14-23-15-17-24(18-16-23)36-27(30(34)35)19-20-31-28(32)25-7-4-5-8-26(25)29(31)33/h4-5,7-12,15-18,27H,2-3,6,19-20H2,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374195
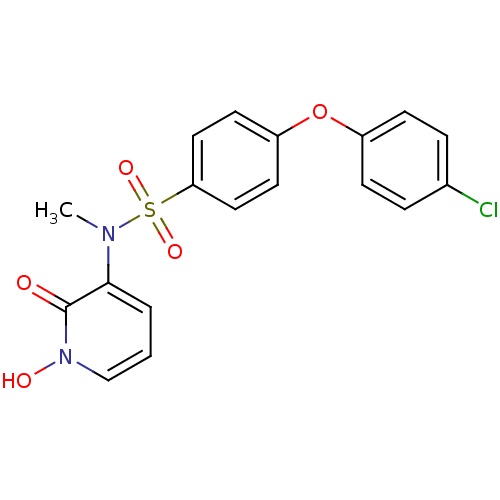
(CHEMBL257088)Show SMILES CN(c1cccn(O)c1=O)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H15ClN2O5S/c1-20(17-3-2-12-21(23)18(17)22)27(24,25)16-10-8-15(9-11-16)26-14-6-4-13(19)5-7-14/h2-12,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374195

(CHEMBL257088)Show SMILES CN(c1cccn(O)c1=O)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H15ClN2O5S/c1-20(17-3-2-12-21(23)18(17)22)27(24,25)16-10-8-15(9-11-16)26-14-6-4-13(19)5-7-14/h2-12,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 405-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.049
BindingDB Entry DOI: 10.7270/Q2T154HP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50514471
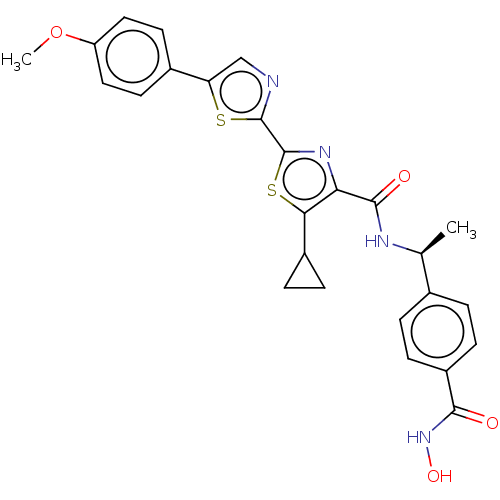
(CHEMBL4450360)Show SMILES COc1ccc(cc1)-c1cnc(s1)-c1nc(C(=O)N[C@@H](C)c2ccc(cc2)C(=O)NO)c(s1)C1CC1 |r| Show InChI InChI=1S/C26H24N4O4S2/c1-14(15-3-7-18(8-4-15)23(31)30-33)28-24(32)21-22(17-5-6-17)36-26(29-21)25-27-13-20(35-25)16-9-11-19(34-2)12-10-16/h3-4,7-14,17,33H,5-6H2,1-2H3,(H,28,32)(H,30,31)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6/GST-tagged human HDAC6 expressed in baculovirus infected High5 insect cells using Boc-Lys(epsion-acetyl)-AMC as substr... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50514461

(CHEMBL4443998)Show SMILES C[C@H](NC(=O)c1nc(sc1C1CC1)-c1ncc(s1)-c1ccc(F)cc1)c1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C25H21FN4O3S2/c1-13(14-2-6-17(7-3-14)22(31)30-33)28-23(32)20-21(16-4-5-16)35-25(29-20)24-27-12-19(34-24)15-8-10-18(26)11-9-15/h2-3,6-13,16,33H,4-5H2,1H3,(H,28,32)(H,30,31)/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6/GST-tagged human HDAC3 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC a... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50374195

(CHEMBL257088)Show SMILES CN(c1cccn(O)c1=O)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C18H15ClN2O5S/c1-20(17-3-2-12-21(23)18(17)22)27(24,25)16-10-8-15(9-11-16)26-14-6-4-13(19)5-7-14/h2-12,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50514461

(CHEMBL4443998)Show SMILES C[C@H](NC(=O)c1nc(sc1C1CC1)-c1ncc(s1)-c1ccc(F)cc1)c1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C25H21FN4O3S2/c1-13(14-2-6-17(7-3-14)22(31)30-33)28-23(32)20-21(16-4-5-16)35-25(29-20)24-27-12-19(34-24)15-8-10-18(26)11-9-15/h2-3,6-13,16,33H,4-5H2,1H3,(H,28,32)(H,30,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6/GST-tagged human HDAC1 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC a... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50232833

(CHEMBL4076610)Show SMILES CCN1CCN(CC1)c1ccc(cc1)C(=O)Nc1n[nH]c2cc(ccc12)-c1cccc(OC)c1F Show InChI InChI=1S/C27H28FN5O2/c1-3-32-13-15-33(16-14-32)20-10-7-18(8-11-20)27(34)29-26-22-12-9-19(17-23(22)30-31-26)21-5-4-6-24(35-2)25(21)28/h4-12,17H,3,13-16H2,1-2H3,(H2,29,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50463715

(CHEMBL4243470)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2cc(C)nc3noc(N)c23)c1 Show InChI InChI=1S/C21H19N5O2/c1-12-4-3-5-16(10-12)25-21(27)24-15-8-6-14(7-9-15)17-11-13(2)23-20-18(17)19(22)28-26-20/h3-11H,22H2,1-2H3,(H2,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human KDR using biotin labeled substrate after 1 hr by HTFR assay |
Bioorg Med Chem 26: 4735-4744 (2018)
Article DOI: 10.1016/j.bmc.2018.08.013
BindingDB Entry DOI: 10.7270/Q208680Z |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185878
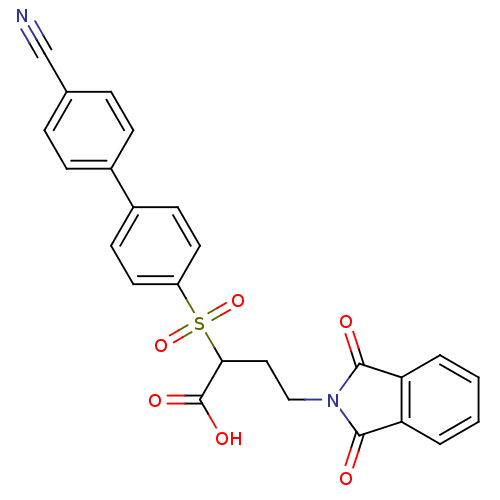
(2-(4'-cyano-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3-...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C25H18N2O6S/c26-15-16-5-7-17(8-6-16)18-9-11-19(12-10-18)34(32,33)22(25(30)31)13-14-27-23(28)20-3-1-2-4-21(20)24(27)29/h1-12,22H,13-14H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data