 Found 3002 hits with Last Name = 'heng' and Initial = 'b'
Found 3002 hits with Last Name = 'heng' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM412060
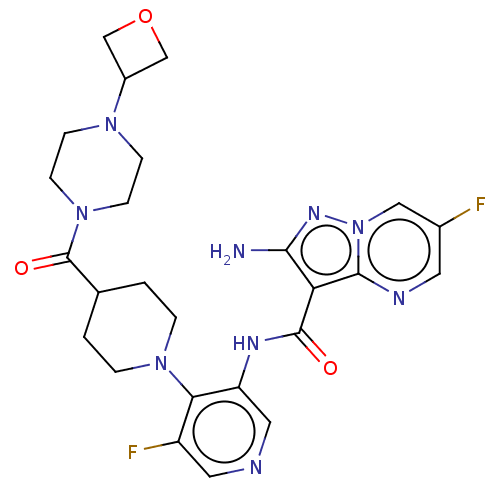
(2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)...)Show SMILES Nc1nn2cc(F)cnc2c1C(=O)Nc1cncc(F)c1N1CCC(CC1)C(=O)N1CCN(CC1)C1COC1 Show InChI InChI=1S/C25H29F2N9O3/c26-16-9-30-23-20(22(28)32-36(23)12-16)24(37)31-19-11-29-10-18(27)21(19)34-3-1-15(2-4-34)25(38)35-7-5-33(6-8-35)17-13-39-14-17/h9-12,15,17H,1-8,13-14H2,(H2,28,32)(H,31,37) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| <0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114109
BindingDB Entry DOI: 10.7270/Q2XK8KKJ |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126960
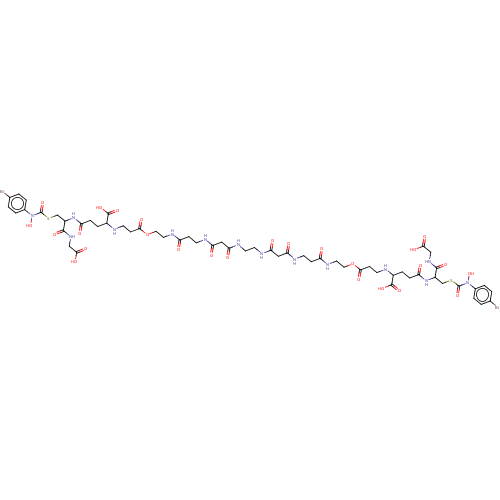
(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50081533
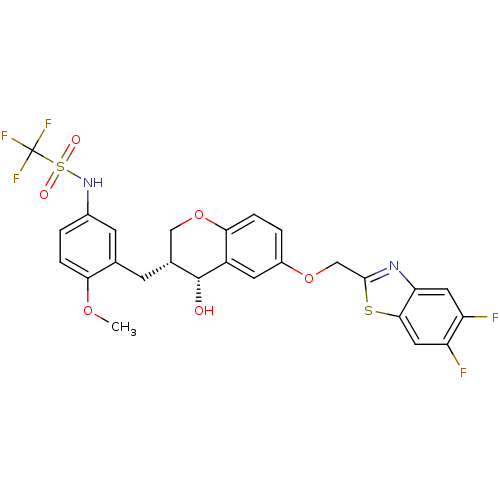
(CHEMBL96224 | CP-199331 | N-{3-[(3R,4R)-6-(5,6-Dif...)Show SMILES COc1ccc(NS(=O)(=O)C(F)(F)F)cc1C[C@@H]1COc2ccc(OCc3nc4cc(F)c(F)cc4s3)cc2[C@@H]1O Show InChI InChI=1S/C26H21F5N2O6S2/c1-37-21-4-2-15(33-41(35,36)26(29,30)31)7-13(21)6-14-11-39-22-5-3-16(8-17(22)25(14)34)38-12-24-32-20-9-18(27)19(28)10-23(20)40-24/h2-5,7-10,14,25,33-34H,6,11-12H2,1H3/t14-,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the binding of Cysteinyl leukotriene receptor 1 to guinea pig lung membranes |
Bioorg Med Chem Lett 9: 2773-8 (1999)
BindingDB Entry DOI: 10.7270/Q2MP52G3 |
More data for this
Ligand-Target Pair | |
Dipeptidase 1
(GUINEA PIG) | BDBM50292408
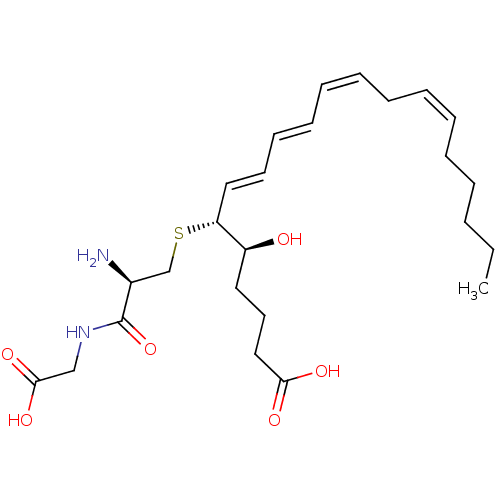
((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(=O)NCC(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C25H40N2O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22(21(28)15-14-17-23(29)30)34-19-20(26)25(33)27-18-24(31)32/h6-7,9-13,16,20-22,28H,2-5,8,14-15,17-19,26H2,1H3,(H,27,33)(H,29,30)(H,31,32)/b7-6-,10-9-,12-11+,16-13+/t20-,21-,22+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 258: 531-6 (1991)
BindingDB Entry DOI: 10.7270/Q2BK19TF |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 2
(GUINEA PIG) | BDBM50292408

((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(=O)NCC(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C25H40N2O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22(21(28)15-14-17-23(29)30)34-19-20(26)25(33)27-18-24(31)32/h6-7,9-13,16,20-22,28H,2-5,8,14-15,17-19,26H2,1H3,(H,27,33)(H,29,30)(H,31,32)/b7-6-,10-9-,12-11+,16-13+/t20-,21-,22+/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 258: 531-6 (1991)
BindingDB Entry DOI: 10.7270/Q2BK19TF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621
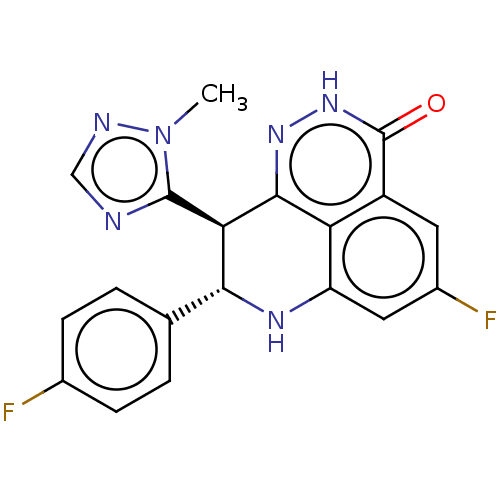
(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114109
BindingDB Entry DOI: 10.7270/Q2XK8KKJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50081532
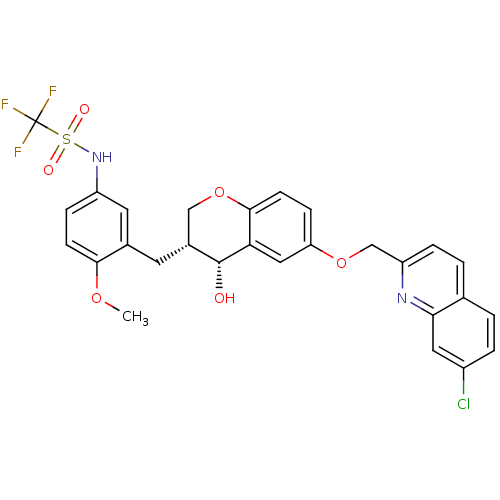
(CHEMBL96206 | CP-199330 | N-{3-[(3R,4R)-6-(7-Chlor...)Show SMILES COc1ccc(NS(=O)(=O)C(F)(F)F)cc1C[C@@H]1COc2ccc(OCc3ccc4ccc(Cl)cc4n3)cc2[C@@H]1O Show InChI InChI=1S/C28H24ClF3N2O6S/c1-38-25-8-6-20(34-41(36,37)28(30,31)32)11-17(25)10-18-14-40-26-9-7-22(13-23(26)27(18)35)39-15-21-5-3-16-2-4-19(29)12-24(16)33-21/h2-9,11-13,18,27,34-35H,10,14-15H2,1H3/t18-,27-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the binding of Cysteinyl leukotriene receptor 1 to guinea pig lung membranes |
Bioorg Med Chem Lett 9: 2773-8 (1999)
BindingDB Entry DOI: 10.7270/Q2MP52G3 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50070919
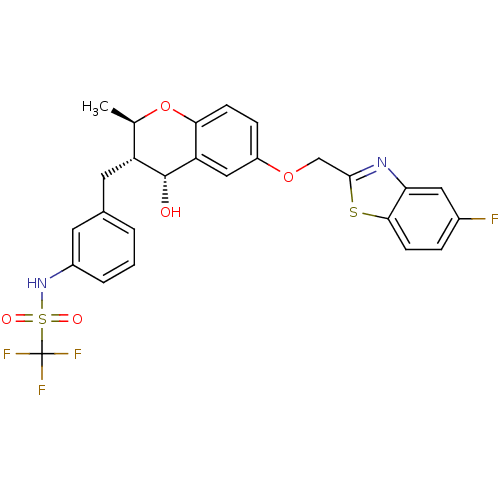
(C,C,C-Trifluoro-N-{3-[(2R,3S,4R)-6-(5-fluoro-benzo...)Show SMILES C[C@H]1Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@H](O)[C@@H]1Cc1cccc(NS(=O)(=O)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F4N2O5S2/c1-14-19(10-15-3-2-4-17(9-15)32-39(34,35)26(28,29)30)25(33)20-12-18(6-7-22(20)37-14)36-13-24-31-21-11-16(27)5-8-23(21)38-24/h2-9,11-12,14,19,25,32-33H,10,13H2,1H3/t14-,19-,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the binding of Cysteinyl leukotriene receptor 1 to guinea pig lung membranes |
Bioorg Med Chem Lett 9: 2773-8 (1999)
BindingDB Entry DOI: 10.7270/Q2MP52G3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526945
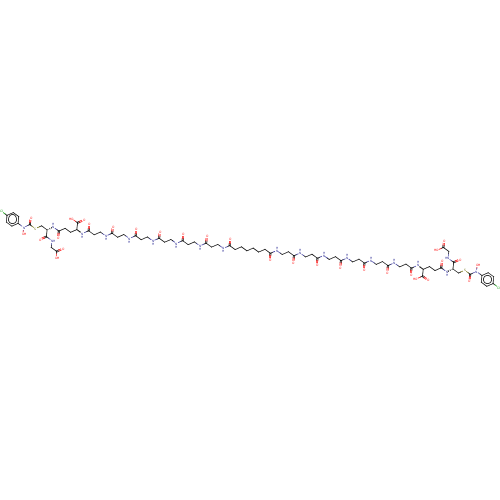
(CHEMBL4473806)Show SMILES ON(C(=O)SC[C@H](NC(=O)CC[C@H](NC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCCCCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C78H112Cl2N20O30S2/c79-47-7-11-49(12-8-47)99(129)77(127)131-45-53(73(121)93-43-71(117)118)97-67(113)17-15-51(75(123)124)95-69(115)29-41-91-65(111)27-39-89-63(109)25-37-87-61(107)23-35-85-59(105)21-33-83-57(103)19-31-81-55(101)5-3-1-2-4-6-56(102)82-32-20-58(104)84-34-22-60(106)86-36-24-62(108)88-38-26-64(110)90-40-28-66(112)92-42-30-70(116)96-52(76(125)126)16-18-68(114)98-54(74(122)94-44-72(119)120)46-132-78(128)100(130)50-13-9-48(80)10-14-50/h7-14,51-54,129-130H,1-6,15-46H2,(H,81,101)(H,82,102)(H,83,103)(H,84,104)(H,85,105)(H,86,106)(H,87,107)(H,88,108)(H,89,109)(H,90,110)(H,91,111)(H,92,112)(H,93,121)(H,94,122)(H,95,115)(H,96,116)(H,97,113)(H,98,114)(H,117,118)(H,119,120)(H,123,124)(H,125,126)/t51-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126961
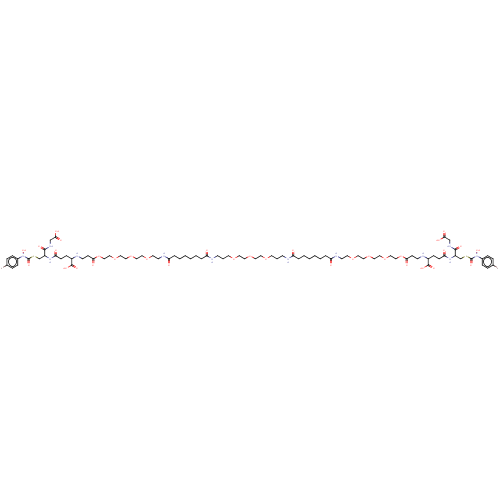
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526943
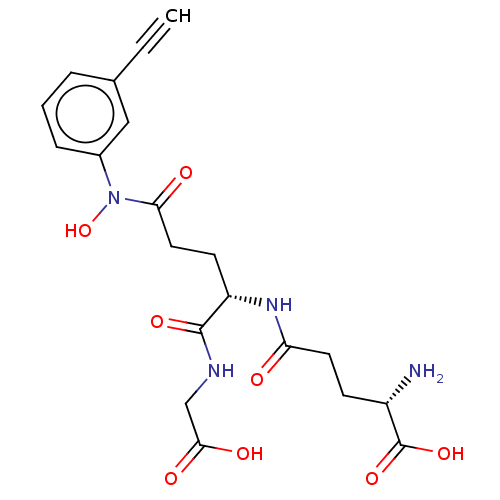
(CHEMBL4436073)Show SMILES N[C@@H](CCC(=O)N[C@@H](CCC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H24N4O8/c1-2-12-4-3-5-13(10-12)24(32)17(26)9-7-15(19(29)22-11-18(27)28)23-16(25)8-6-14(21)20(30)31/h1,3-5,10,14-15,32H,6-9,11,21H2,(H,22,29)(H,23,25)(H,27,28)(H,30,31)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114109
BindingDB Entry DOI: 10.7270/Q2XK8KKJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidase 1
(GUINEA PIG) | BDBM50297387
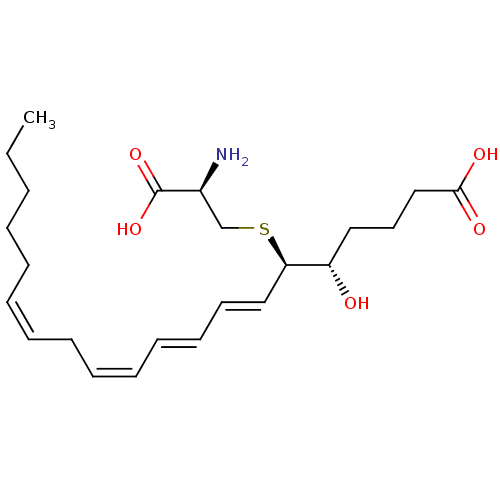
((5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyic...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C23H37NO5S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-21(30-18-19(24)23(28)29)20(25)15-14-17-22(26)27/h6-7,9-13,16,19-21,25H,2-5,8,14-15,17-18,24H2,1H3,(H,26,27)(H,28,29)/b7-6-,10-9-,12-11+,16-13+/t19-,20-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 258: 531-6 (1991)
BindingDB Entry DOI: 10.7270/Q2BK19TF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM493757
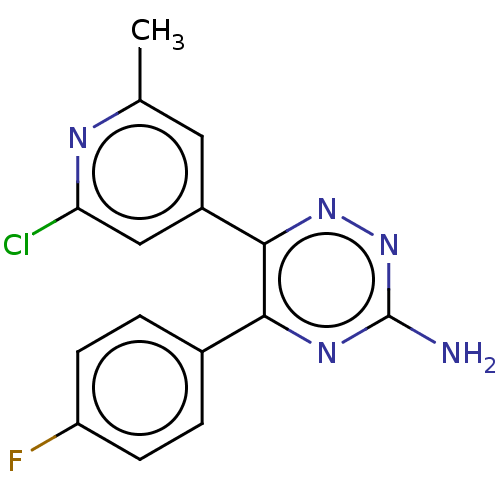
(US10988455, Example 1(xcv))Show InChI InChI=1S/C15H11ClFN5/c1-8-6-10(7-12(16)19-8)14-13(20-15(18)22-21-14)9-2-4-11(17)5-3-9/h2-7H,1H3,(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50073277
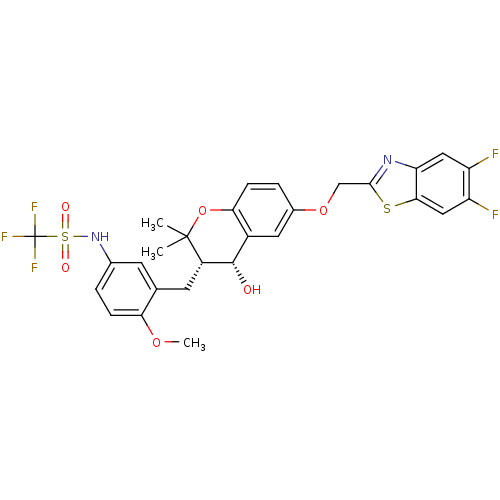
(CHEMBL332680 | N-{3-[(3S,4R)-6-(5,6-Difluoro-benzo...)Show SMILES COc1ccc(NS(=O)(=O)C(F)(F)F)cc1C[C@H]1[C@@H](O)c2cc(OCc3nc4cc(F)c(F)cc4s3)ccc2OC1(C)C Show InChI InChI=1S/C28H25F5N2O6S2/c1-27(2)18(9-14-8-15(4-6-22(14)39-3)35-43(37,38)28(31,32)33)26(36)17-10-16(5-7-23(17)41-27)40-13-25-34-21-11-19(29)20(30)12-24(21)42-25/h4-8,10-12,18,26,35-36H,9,13H2,1-3H3/t18-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50070919

(C,C,C-Trifluoro-N-{3-[(2R,3S,4R)-6-(5-fluoro-benzo...)Show SMILES C[C@H]1Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@H](O)[C@@H]1Cc1cccc(NS(=O)(=O)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F4N2O5S2/c1-14-19(10-15-3-2-4-17(9-15)32-39(34,35)26(28,29)30)25(33)20-12-18(6-7-22(20)37-14)36-13-24-31-21-11-16(27)5-8-23(21)38-24/h2-9,11-12,14,19,25,32-33H,10,13H2,1H3/t14-,19-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the binding of cysLT1 receptor to guinea pig lung membranes. |
Bioorg Med Chem Lett 9: 2773-8 (1999)
BindingDB Entry DOI: 10.7270/Q2MP52G3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50070919

(C,C,C-Trifluoro-N-{3-[(2R,3S,4R)-6-(5-fluoro-benzo...)Show SMILES C[C@H]1Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@H](O)[C@@H]1Cc1cccc(NS(=O)(=O)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F4N2O5S2/c1-14-19(10-15-3-2-4-17(9-15)32-39(34,35)26(28,29)30)25(33)20-12-18(6-7-22(20)37-14)36-13-24-31-21-11-16(27)5-8-23(21)38-24/h2-9,11-12,14,19,25,32-33H,10,13H2,1H3/t14-,19-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215874
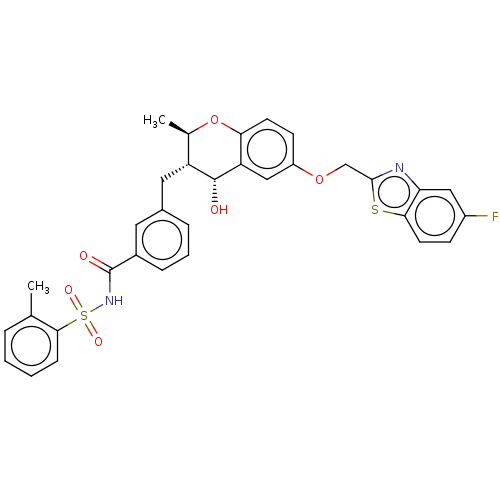
(CHEMBL52102)Show SMILES C[C@H]1Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@H](O)[C@@H]1Cc1cccc(c1)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C33H29FN2O6S2/c1-19-6-3-4-9-30(19)44(39,40)36-33(38)22-8-5-7-21(14-22)15-25-20(2)42-28-12-11-24(17-26(28)32(25)37)41-18-31-35-27-16-23(34)10-13-29(27)43-31/h3-14,16-17,20,25,32,37H,15,18H2,1-2H3,(H,36,38)/t20-,25-,32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM209932
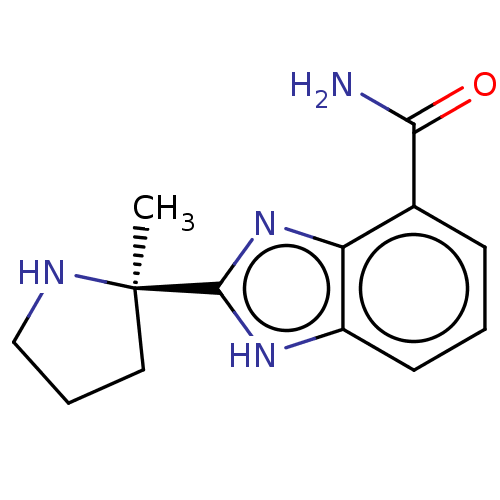
(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114109
BindingDB Entry DOI: 10.7270/Q2XK8KKJ |
More data for this
Ligand-Target Pair | |
Dipeptidase 1
(GUINEA PIG) | BDBM81801
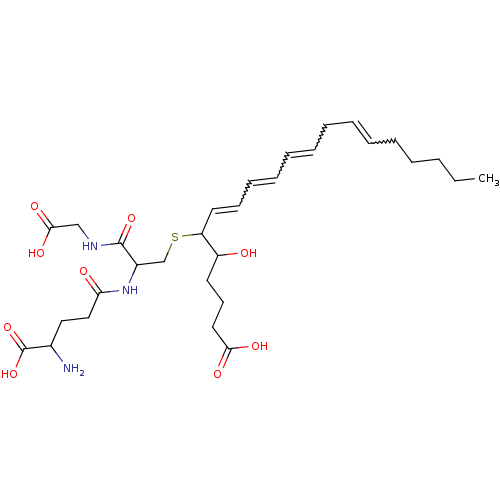
(CAS_5283121 | LTC4 | NSC_5283121)Show SMILES CCCCCC=CCC=CC=CC=CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(O)CCCC(O)=O |w:5.4,8.7,10.9,12.11| Show InChI InChI=1S/C30H47N3O9S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-25(24(34)15-14-17-27(36)37)43-21-23(29(40)32-20-28(38)39)33-26(35)19-18-22(31)30(41)42/h6-7,9-13,16,22-25,34H,2-5,8,14-15,17-21,31H2,1H3,(H,32,40)(H,33,35)(H,36,37)(H,38,39)(H,41,42) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 258: 531-6 (1991)
BindingDB Entry DOI: 10.7270/Q2BK19TF |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50073279
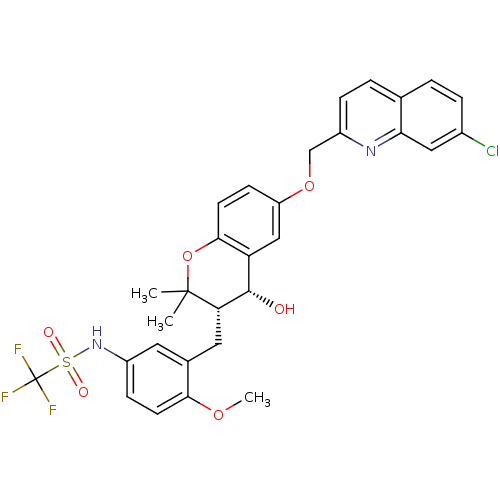
(CHEMBL119214 | N-{3-[(3S,4R)-6-(7-Chloro-quinolin-...)Show SMILES COc1ccc(NS(=O)(=O)C(F)(F)F)cc1C[C@H]1[C@@H](O)c2cc(OCc3ccc4ccc(Cl)cc4n3)ccc2OC1(C)C Show InChI InChI=1S/C30H28ClF3N2O6S/c1-29(2)24(13-18-12-20(8-10-26(18)40-3)36-43(38,39)30(32,33)34)28(37)23-15-22(9-11-27(23)42-29)41-16-21-7-5-17-4-6-19(31)14-25(17)35-21/h4-12,14-15,24,28,36-37H,13,16H2,1-3H3/t24-,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
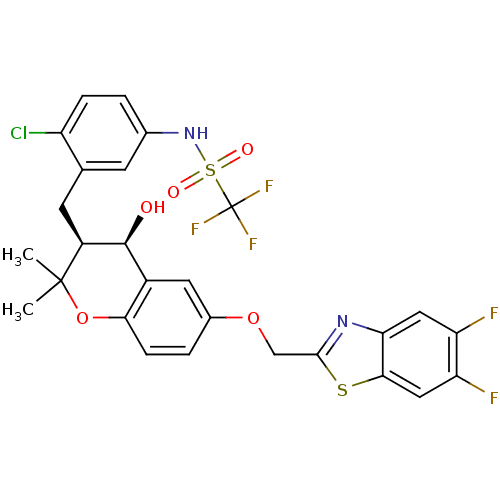
(GUINEA PIG) | BDBM50073276
(CHEMBL120200 | N-{4-Chloro-3-[(3S,4R)-6-(5,6-diflu...)Show SMILES CC1(C)Oc2ccc(OCc3nc4cc(F)c(F)cc4s3)cc2[C@H](O)[C@@H]1Cc1cc(NS(=O)(=O)C(F)(F)F)ccc1Cl Show InChI InChI=1S/C27H22ClF5N2O5S2/c1-26(2)17(8-13-7-14(3-5-18(13)28)35-42(37,38)27(31,32)33)25(36)16-9-15(4-6-22(16)40-26)39-12-24-34-21-10-19(29)20(30)11-23(21)41-24/h3-7,9-11,17,25,35-36H,8,12H2,1-2H3/t17-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
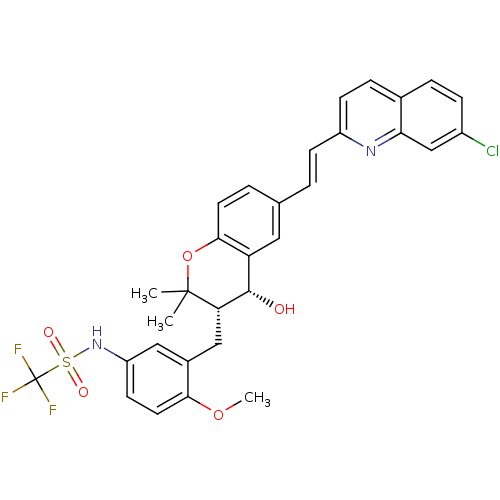
(GUINEA PIG) | BDBM50073275
(CHEMBL331884 | N-(3-{(3S,4R)-6-[(E)-2-(7-Chloro-qu...)Show SMILES COc1ccc(NS(=O)(=O)C(F)(F)F)cc1C[C@H]1[C@@H](O)c2cc(\C=C\c3ccc4ccc(Cl)cc4n3)ccc2OC1(C)C Show InChI InChI=1S/C31H28ClF3N2O5S/c1-30(2)25(16-20-15-23(11-13-27(20)41-3)37-43(39,40)31(33,34)35)29(38)24-14-18(5-12-28(24)42-30)4-9-22-10-7-19-6-8-21(32)17-26(19)36-22/h4-15,17,25,29,37-38H,16H2,1-3H3/b9-4+/t25-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 2
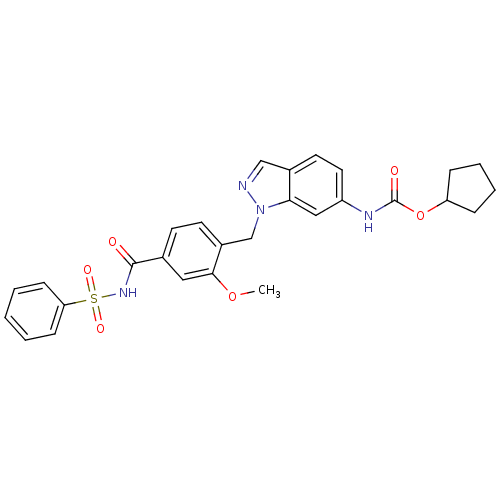
(GUINEA PIG) | BDBM50009075
(CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H28N4O6S/c1-37-26-15-19(27(33)31-39(35,36)24-9-3-2-4-10-24)11-12-21(26)18-32-25-16-22(14-13-20(25)17-29-32)30-28(34)38-23-7-5-6-8-23/h2-4,9-17,23H,5-8,18H2,1H3,(H,30,34)(H,31,33) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 6.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 258: 531-6 (1991)
BindingDB Entry DOI: 10.7270/Q2BK19TF |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526944
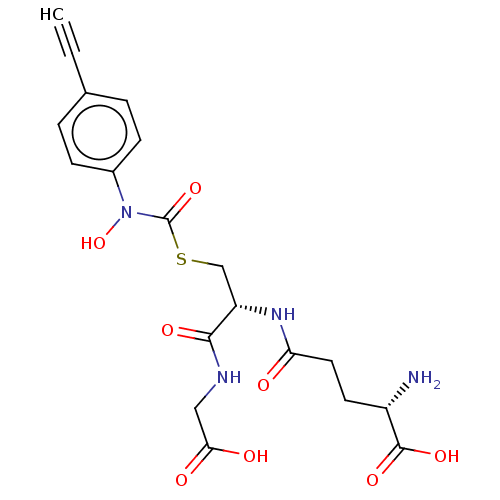
(CHEMBL4450158)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(cc1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H22N4O8S/c1-2-11-3-5-12(6-4-11)23(31)19(30)32-10-14(17(27)21-9-16(25)26)22-15(24)8-7-13(20)18(28)29/h1,3-6,13-14,31H,7-10,20H2,(H,21,27)(H,22,24)(H,25,26)(H,28,29)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50073278
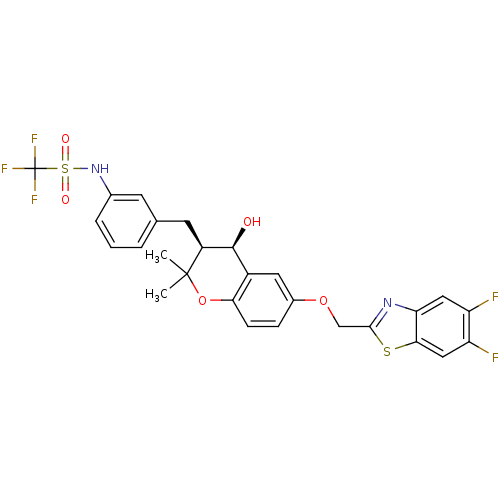
(CHEMBL331747 | N-{3-[(3S,4R)-6-(5,6-Difluoro-benzo...)Show SMILES CC1(C)Oc2ccc(OCc3nc4cc(F)c(F)cc4s3)cc2[C@H](O)[C@@H]1Cc1cccc(NS(=O)(=O)C(F)(F)F)c1 Show InChI InChI=1S/C27H23F5N2O5S2/c1-26(2)18(9-14-4-3-5-15(8-14)34-41(36,37)27(30,31)32)25(35)17-10-16(6-7-22(17)39-26)38-13-24-33-21-11-19(28)20(29)12-23(21)40-24/h3-8,10-12,18,25,34-35H,9,13H2,1-2H3/t18-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526942
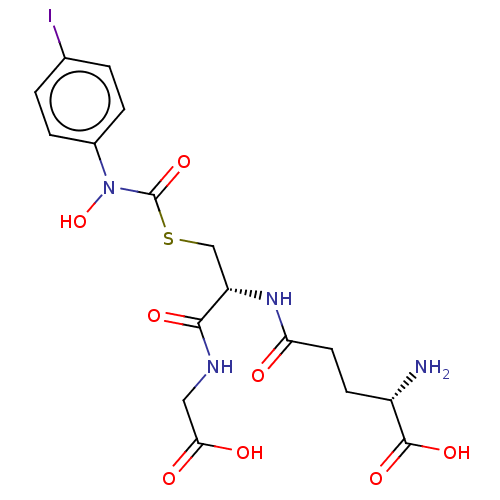
(CHEMBL4438930)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(I)cc1)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H21IN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50070921
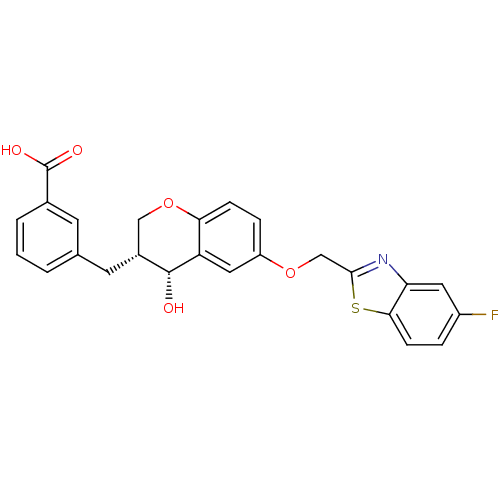
(3-[(3R,4R)-6-(5-Fluoro-benzothiazol-2-ylmethoxy)-4...)Show SMILES O[C@@H]1[C@H](Cc2cccc(c2)C(O)=O)COc2ccc(OCc3nc4cc(F)ccc4s3)cc12 Show InChI InChI=1S/C25H20FNO5S/c26-17-4-7-22-20(10-17)27-23(33-22)13-31-18-5-6-21-19(11-18)24(28)16(12-32-21)9-14-2-1-3-15(8-14)25(29)30/h1-8,10-11,16,24,28H,9,12-13H2,(H,29,30)/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 3577-82 (1999)
BindingDB Entry DOI: 10.7270/Q2833R5M |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826
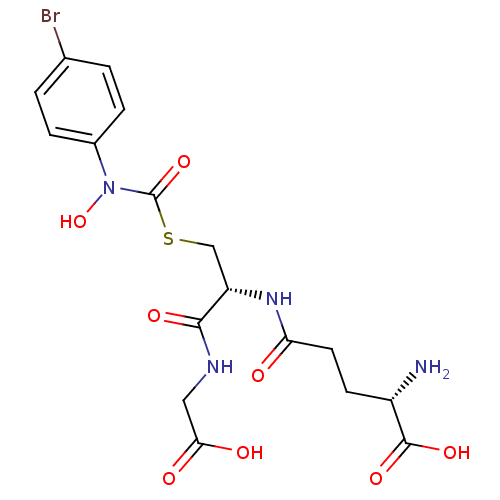
((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826

((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50070921

(3-[(3R,4R)-6-(5-Fluoro-benzothiazol-2-ylmethoxy)-4...)Show SMILES O[C@@H]1[C@H](Cc2cccc(c2)C(O)=O)COc2ccc(OCc3nc4cc(F)ccc4s3)cc12 Show InChI InChI=1S/C25H20FNO5S/c26-17-4-7-22-20(10-17)27-23(33-22)13-31-18-5-6-21-19(11-18)24(28)16(12-32-21)9-14-2-1-3-15(8-14)25(29)30/h1-8,10-11,16,24,28H,9,12-13H2,(H,29,30)/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM209932

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114109
BindingDB Entry DOI: 10.7270/Q2XK8KKJ |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526948
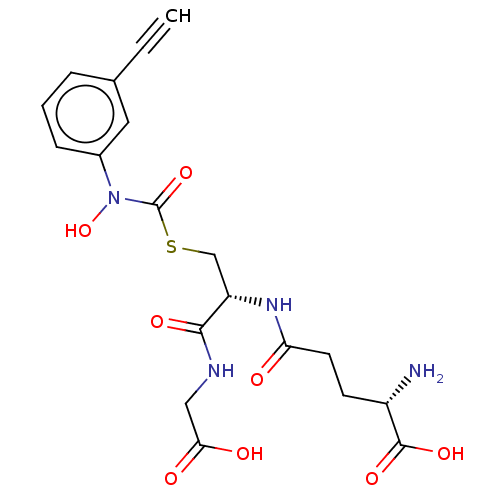
(CHEMBL4559486)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H22N4O8S/c1-2-11-4-3-5-12(8-11)23(31)19(30)32-10-14(17(27)21-9-16(25)26)22-15(24)7-6-13(20)18(28)29/h1,3-5,8,13-14,31H,6-7,9-10,20H2,(H,21,27)(H,22,24)(H,25,26)(H,28,29)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215871
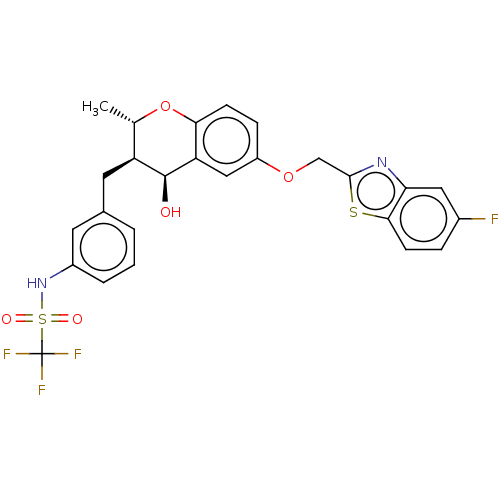
(CHEMBL415954)Show SMILES C[C@@H]1Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@@H](O)[C@H]1Cc1cccc(NS(=O)(=O)C(F)(F)F)c1 Show InChI InChI=1S/C26H22F4N2O5S2/c1-14-19(10-15-3-2-4-17(9-15)32-39(34,35)26(28,29)30)25(33)20-12-18(6-7-22(20)37-14)36-13-24-31-21-11-16(27)5-8-23(21)38-24/h2-9,11-12,14,19,25,32-33H,10,13H2,1H3/t14-,19-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215875

(CHEMBL299779)Show SMILES [H][C@]1(C)Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@]([H])(O)[C@@H]1Cc1cccc(c1)C(O)=O Show InChI InChI=1S/C26H22FNO5S/c1-14-19(10-15-3-2-4-16(9-15)26(30)31)25(29)20-12-18(6-7-22(20)33-14)32-13-24-28-21-11-17(27)5-8-23(21)34-24/h2-9,11-12,14,19,25,29H,10,13H2,1H3,(H,30,31)/t14-,19-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 2
(GUINEA PIG) | BDBM50297387

((5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyic...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C23H37NO5S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-21(30-18-19(24)23(28)29)20(25)15-14-17-22(26)27/h6-7,9-13,16,19-21,25H,2-5,8,14-15,17-18,24H2,1H3,(H,26,27)(H,28,29)/b7-6-,10-9-,12-11+,16-13+/t19-,20-,21+/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 258: 531-6 (1991)
BindingDB Entry DOI: 10.7270/Q2BK19TF |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824
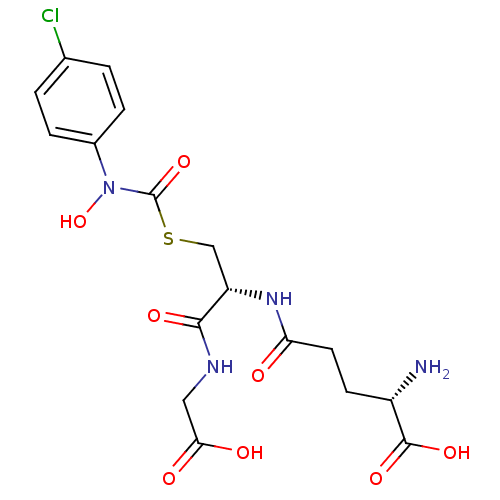
(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824

(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126957
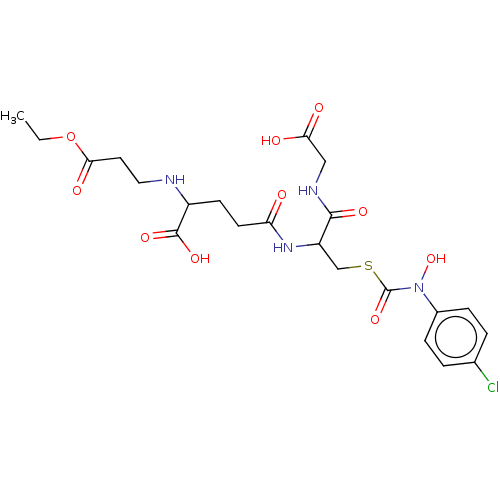
(CHEMBL3629119)Show SMILES CCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C22H29ClN4O10S/c1-2-37-19(31)9-10-24-15(21(33)34)7-8-17(28)26-16(20(32)25-11-18(29)30)12-38-22(35)27(36)14-5-3-13(23)4-6-14/h3-6,15-16,24,36H,2,7-12H2,1H3,(H,25,32)(H,26,28)(H,29,30)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126960

(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50215872
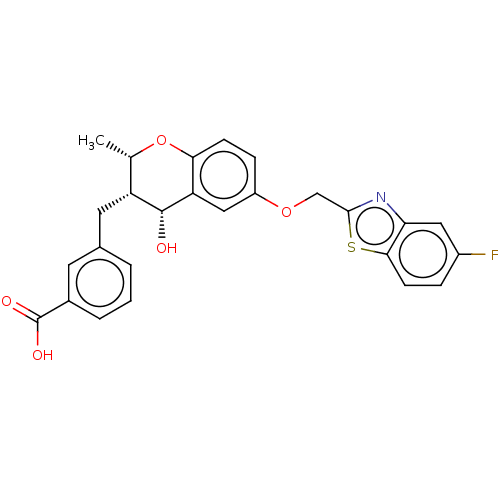
(CHEMBL50399)Show SMILES [H][C@@]1(C)Oc2ccc(OCc3nc4cc(F)ccc4s3)cc2[C@]([H])(O)[C@@H]1Cc1cccc(c1)C(O)=O Show InChI InChI=1S/C26H22FNO5S/c1-14-19(10-15-3-2-4-16(9-15)26(30)31)25(29)20-12-18(6-7-22(20)33-14)32-13-24-28-21-11-17(27)5-8-23(21)34-24/h2-9,11-12,14,19,25,29H,10,13H2,1H3,(H,30,31)/t14-,19+,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Ability to antagonize LTD4 receptors isolated from guinea pig lung membranes |
Bioorg Med Chem Lett 8: 1791-6 (1998)
BindingDB Entry DOI: 10.7270/Q2N3004B |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50594891
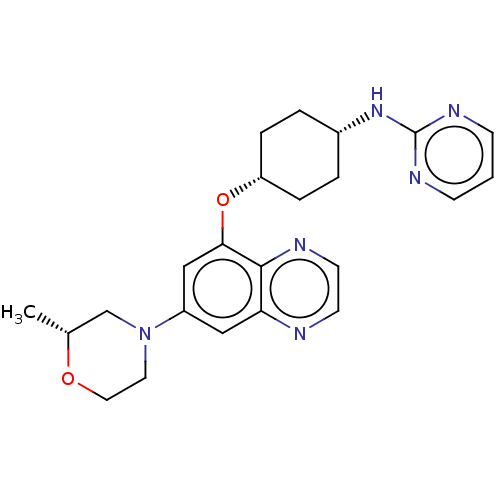
(CHEMBL5171087)Show SMILES C[C@@H]1CN(CCO1)c1cc(O[C@@H]2CC[C@@H](CC2)Nc2ncccn2)c2nccnc2c1 |r,wU:11.11,14.18,1.0,(6.01,-5,;4.68,-4.23,;4.68,-2.69,;3.35,-1.92,;2.01,-2.69,;2.01,-4.23,;3.35,-5,;3.35,-.38,;2.01,.38,;1.99,1.91,;.66,2.68,;-.67,1.91,;-2.01,2.68,;-3.34,1.91,;-3.34,.37,;-2.01,-.4,;-.67,.37,;-4.67,-.4,;-4.67,-1.94,;-3.34,-2.71,;-3.34,-4.24,;-4.68,-5.01,;-6,-4.25,;-6.01,-2.71,;3.33,2.7,;3.33,4.24,;4.66,5.01,;5.99,4.24,;5.99,2.7,;4.67,1.93,;4.67,.4,)| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114109
BindingDB Entry DOI: 10.7270/Q2XK8KKJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data