

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075807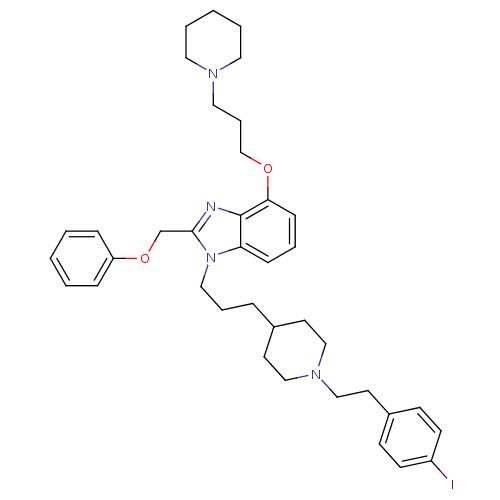 (1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075796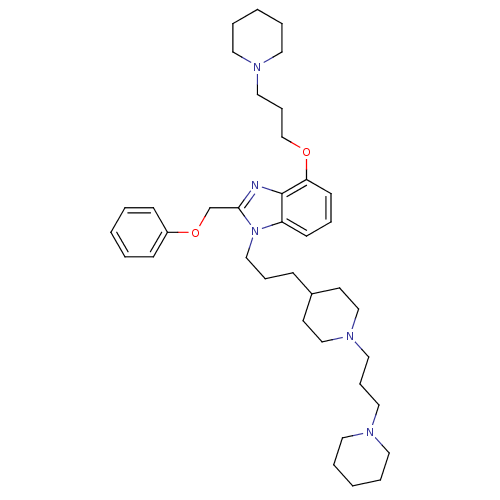 (2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075811 (3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075803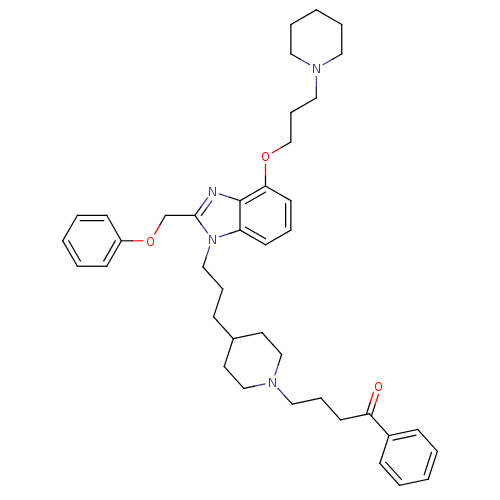 (4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186291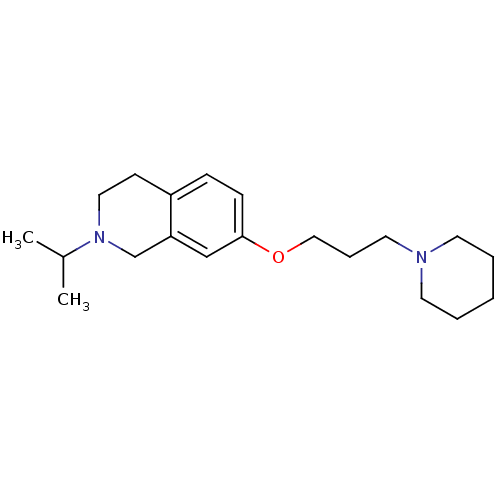 (2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075812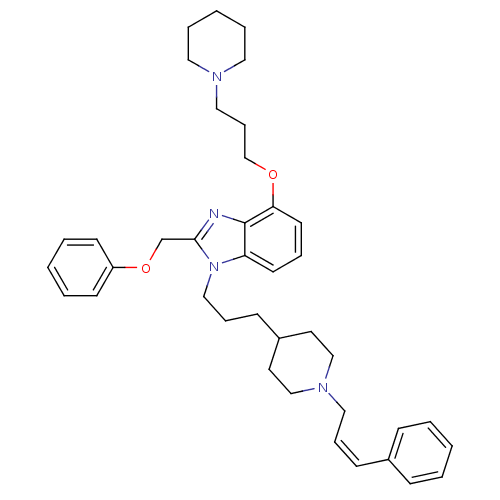 (2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075809 (1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186270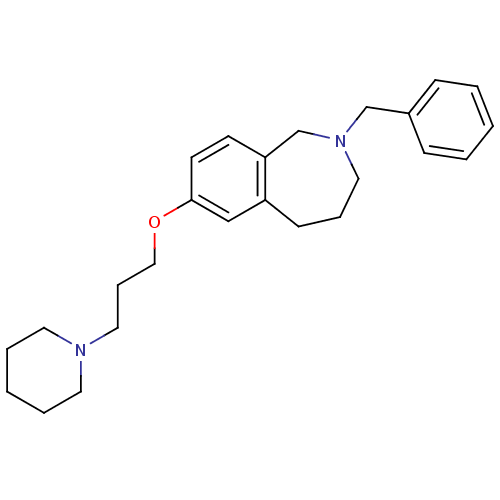 (2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186278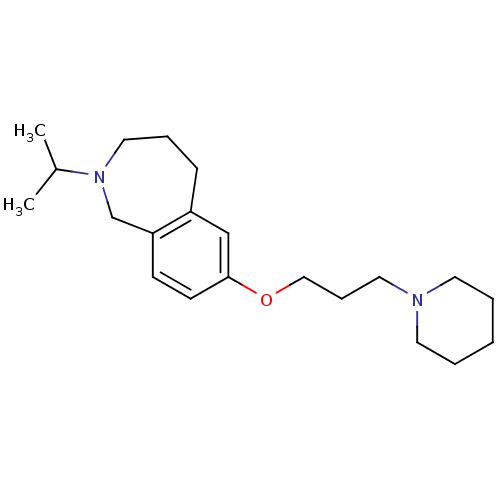 (2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186290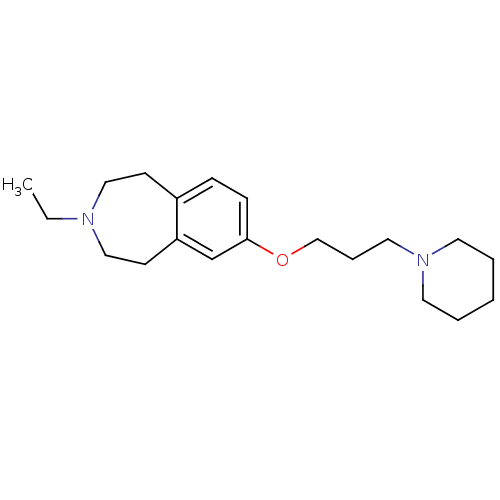 (3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075797 (1-{3-[1-(2-Cyclohexyl-ethyl)-piperidin-4-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075806 (1-{3-[1-(3-Methyl-butyl)-piperidin-4-yl]-propyl}-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075802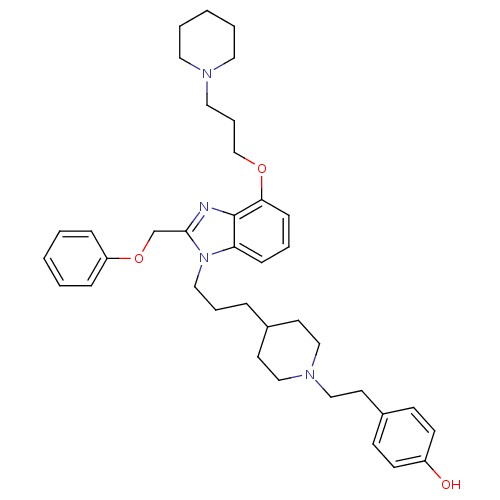 (4-[2-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186309 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186269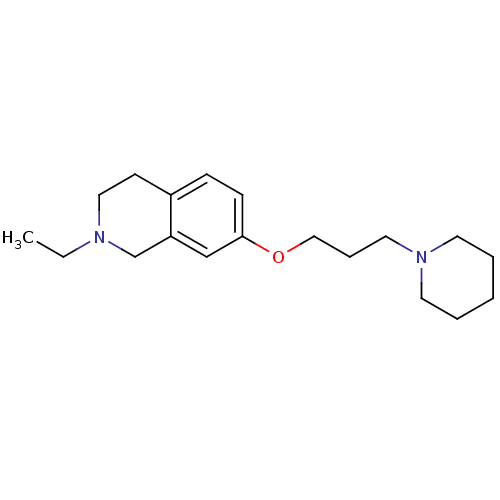 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186292 (2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075799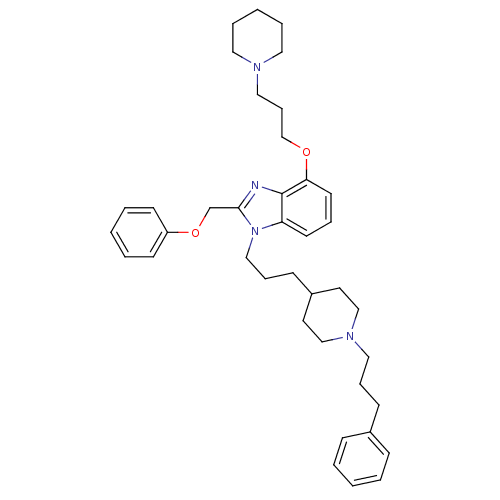 (2-Phenoxymethyl-1-{3-[1-(3-phenyl-propyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075798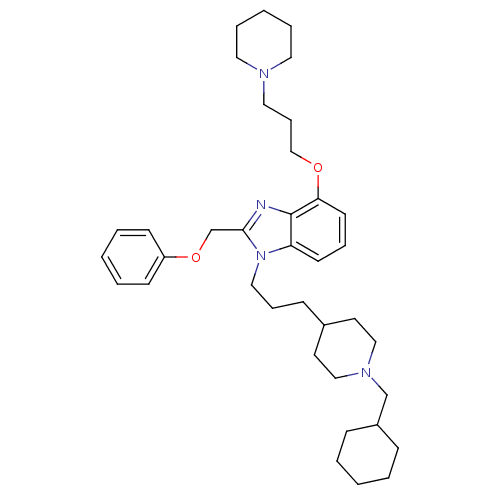 (1-[3-(1-Cyclohexylmethyl-piperidin-4-yl)-propyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186292 (2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186316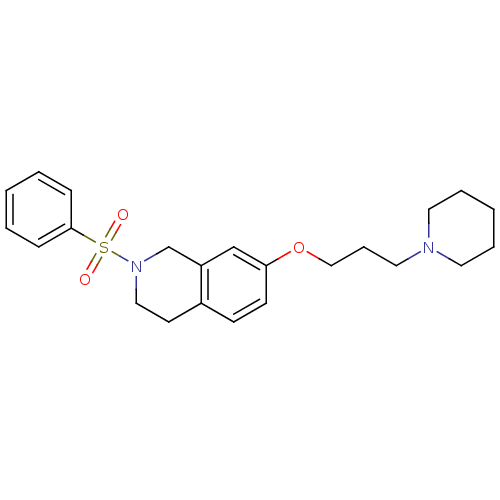 (2-(phenylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186306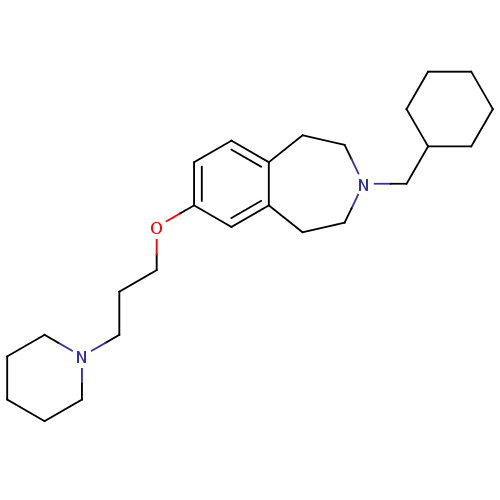 (3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186283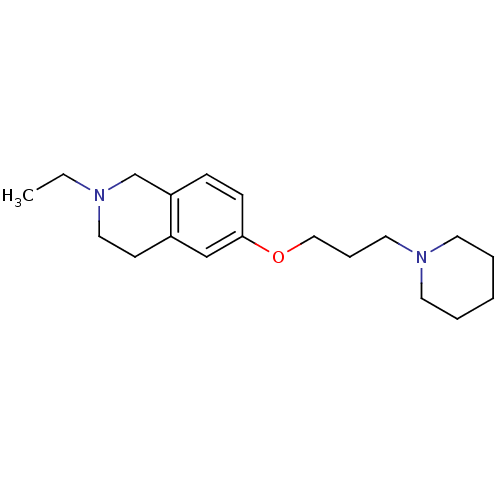 (2-ethyl-6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186309 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186293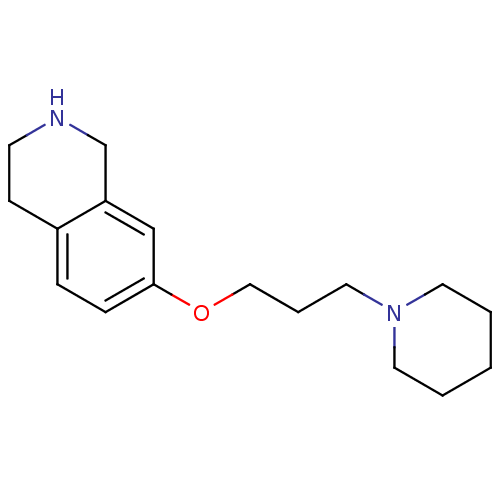 (7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydrois...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186269 (2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075795 (1-[3-(1-But-3-enyl-piperidin-4-yl)-propyl]-2-pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.707 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186311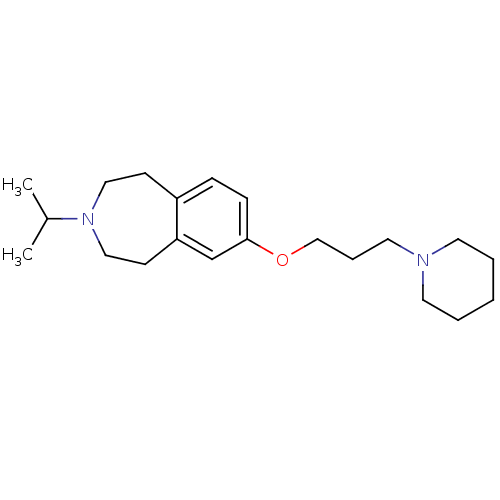 (3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075808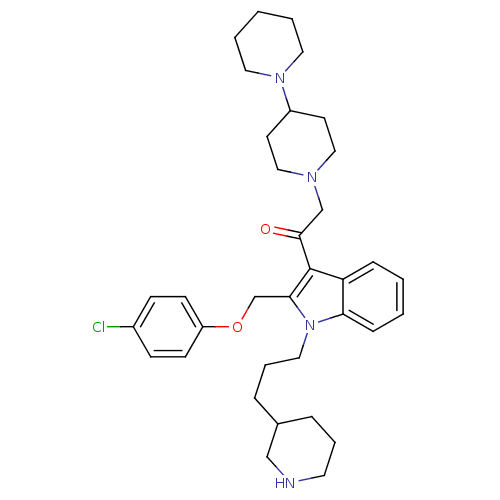 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060725 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060725 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186270 (2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075804 (1-[3-(1-Isobutyl-piperidin-4-yl)-propyl]-2-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186291 (2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186306 (3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186296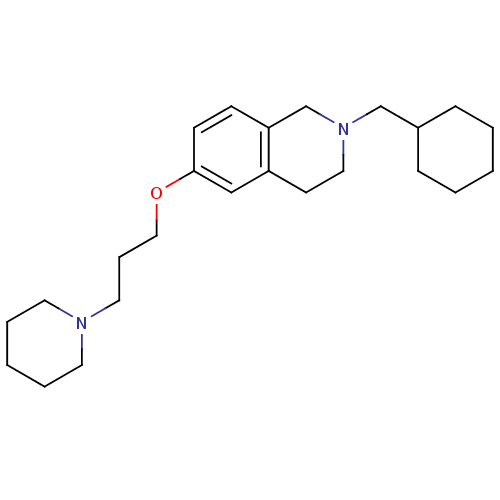 (2-(cyclohexylmethyl)-6-(3-(piperidin-1-yl)propoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186290 (3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186278 (2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060726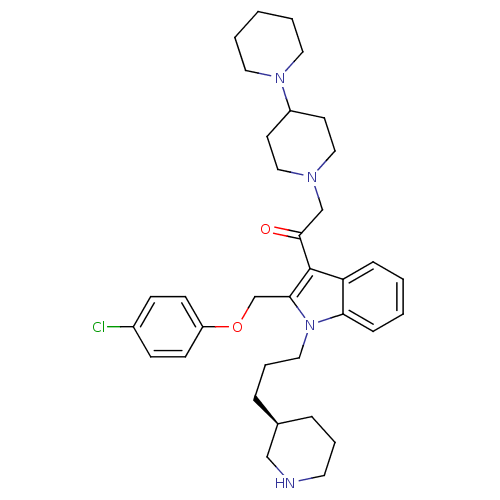 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50186311 (3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075810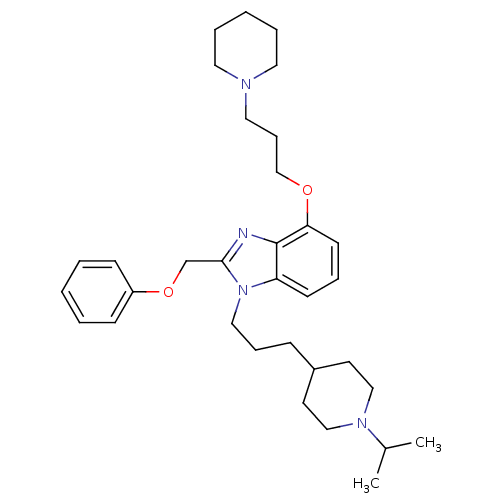 (1-[3-(1-Isopropyl-piperidin-4-yl)-propyl]-2-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075800 (1-[3-(1-Allyl-piperidin-4-yl)-propyl]-2-phenoxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075801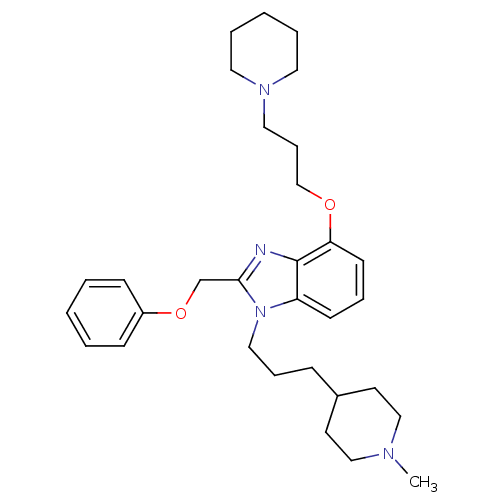 (1-[3-(1-Methyl-piperidin-4-yl)-propyl]-2-phenoxyme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468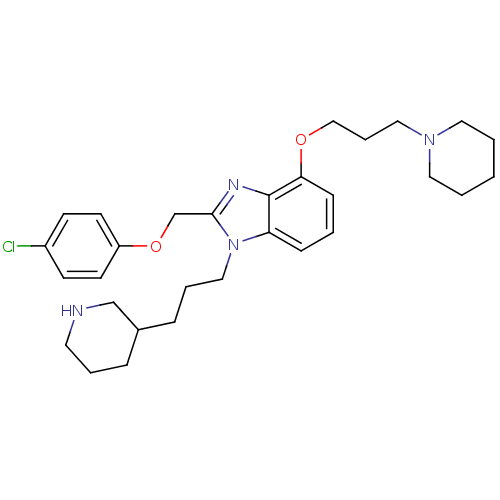 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060725 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability of the compound to reverse Neuropeptide Y receptor type 1-induced inhibition of forskolin-induced inhibition of forskolin-stimulated cAMP | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060724 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity of the compound at cloned Neuropeptide Y receptor type 1 expressed in AV-12 cells is evaluated. | Bioorg Med Chem Lett 8: 473-6 (1999) BindingDB Entry DOI: 10.7270/Q2GH9H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186281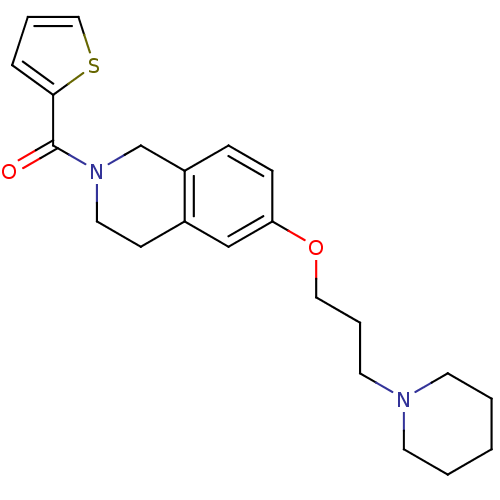 ((6-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50186295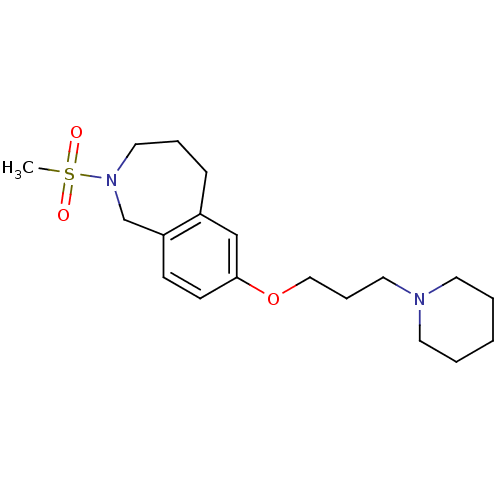 (2-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor | Bioorg Med Chem Lett 16: 3415-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.004 BindingDB Entry DOI: 10.7270/Q2WM1D07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50069414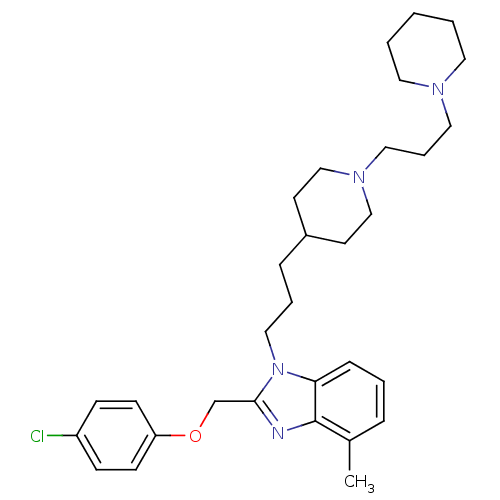 (2-(4-Chloro-phenoxymethyl)-4-methyl-1-{3-[1-(3-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 491 total ) | Next | Last >> |