 Found 14963 hits with Last Name = 'hua' and Initial = 'l'
Found 14963 hits with Last Name = 'hua' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146490
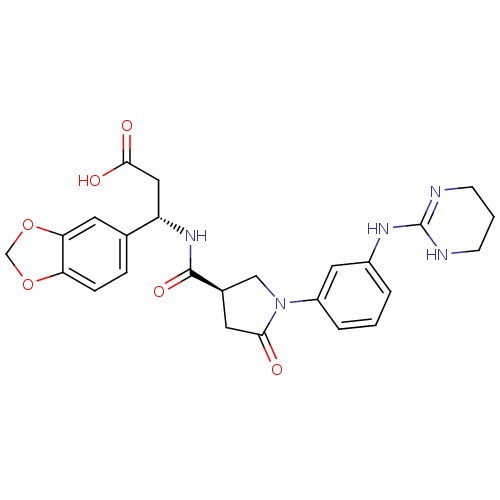
(3-(S)-Benzo[1,3]dioxol-5-yl-3-({(R)-5-oxo-1-[3-(1,...)Show SMILES OC(=O)C[C@H](NC(=O)[C@H]1CN(C(=O)C1)c1cccc(NC2=NCCCN2)c1)c1ccc2OCOc2c1 |t:21| Show InChI InChI=1S/C25H27N5O6/c31-22-10-16(13-30(22)18-4-1-3-17(11-18)28-25-26-7-2-8-27-25)24(34)29-19(12-23(32)33)15-5-6-20-21(9-15)36-14-35-20/h1,3-6,9,11,16,19H,2,7-8,10,12-14H2,(H,29,34)(H,32,33)(H2,26,27,28)/t16-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards vitronectin receptor (AlphaV-beta3 integrin). |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50273370
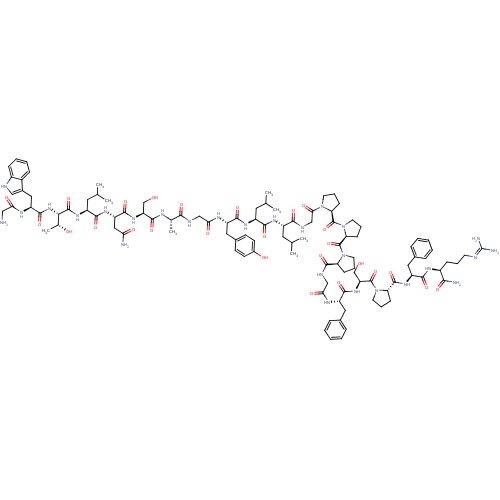
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85070
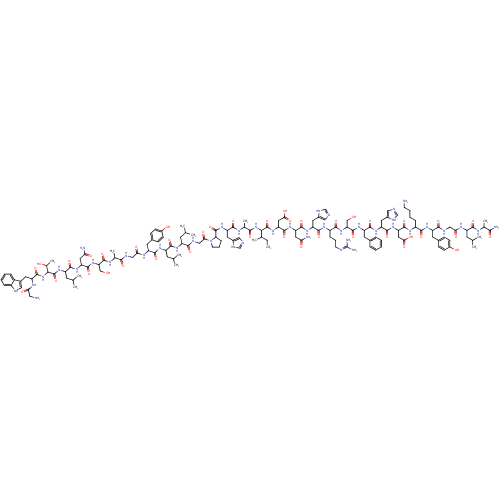
(Galanin, Porcine)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(C)C(N)=O |(22.15,12.84,;20.71,12.32,;20.44,10.8,;21.61,9.81,;18.99,10.28,;18.72,8.76,;19.9,7.77,;21.34,8.3,;19.63,6.25,;18.18,5.73,;20.8,5.26,;20.53,3.75,;19.09,3.22,;21.71,2.75,;21.44,1.24,;19.99,.71,;18.72,1.58,;17.5,.63,;18.03,-.82,;19.57,-.77,;23.16,3.28,;24.34,2.29,;24.07,.77,;25.79,2.81,;26.21,4.29,;27.75,4.34,;28.28,2.89,;27.06,1.95,;27.11,.41,;25.81,-.41,;28.47,-.32,;28.52,-1.86,;29.88,-2.58,;31.19,-1.77,;29.93,-4.12,;31.29,-4.85,;32.6,-4.03,;33.96,-4.76,;32.55,-2.49,;28.62,-4.93,;28.68,-6.47,;30.2,-6.69,;27.73,-7.69,;26.22,-8,;25.19,-6.86,;23.68,-7.17,;25.67,-5.39,;28.3,-9.11,;27.35,-10.33,;25.82,-10.11,;27.92,-11.76,;26.97,-12.97,;25.45,-12.75,;24.87,-11.32,;23.35,-11.1,;22.4,-12.32,;20.88,-12.1,;22.97,-13.75,;24.5,-13.96,;29.45,-11.97,;30.02,-13.4,;29.07,-14.61,;31.55,-13.62,;32.12,-15.05,;33.65,-15.26,;34.6,-14.05,;34.22,-16.69,;33.27,-17.9,;35.75,-16.91,;36.32,-18.34,;35.37,-19.55,;37.85,-18.56,;38.8,-17.34,;38.22,-15.91,;38.42,-19.98,;39.94,-20.2,;40.89,-18.99,;40.52,-21.63,;42.04,-21.85,;42.99,-20.64,;44.52,-20.85,;42.42,-19.21,;39.57,-22.84,;40.14,-24.27,;41.65,-23.95,;39.66,-25.74,;38.36,-26.55,;37,-25.82,;35.69,-26.64,;36.95,-24.28,;40.69,-26.88,;40.21,-28.35,;38.71,-28.66,;41.24,-29.49,;40.76,-30.96,;41.79,-32.1,;43.3,-31.78,;41.31,-33.57,;39.81,-33.88,;39.33,-35.35,;40.23,-36.59,;39.33,-37.83,;37.87,-37.36,;36.54,-38.13,;35.2,-37.36,;35.2,-35.82,;36.54,-35.05,;37.87,-35.82,;42.34,-34.71,;43.85,-34.39,;44.33,-32.93,;44.88,-35.54,;46.39,-35.22,;42.75,-29.17,;43.78,-30.32,;43.23,-27.71,;17.81,11.27,;16.36,10.75,;18.08,12.79,;16.9,13.78,;15.45,13.25,;14.28,14.25,;12.83,13.72,;14.55,15.76,;17.17,15.3,;15.99,16.29,;18.62,15.82,;18.89,17.34,;17.37,17.61,;16.85,19.05,;15.33,19.32,;17.84,20.23,;20.34,17.86,;21.52,16.87,;21.33,19.04,;22.85,18.77,;23.37,17.32,;24.89,17.05,;26,18.12,;27.36,17.39,;27.09,15.88,;25.56,15.66,;23.84,19.95,;25.35,19.68,;23.31,21.39,;24.31,22.57,;25.82,22.3,;26.81,23.48,;28.33,23.21,;29.32,24.39,;30.84,24.12,;31.83,25.3,;31.36,22.67,;23.78,24.02,;22.27,24.29,;24.77,25.2,;24.25,26.65,;22.73,26.92,;22.21,28.36,;25.24,27.82,;26.76,27.55,;24.72,29.27,;25.71,30.45,;27.23,30.18,;28.22,31.36,;29.73,31.09,;30.73,32.27,;30.2,33.71,;28.69,33.98,;27.69,32.81,;25.19,31.9,;26.18,33.08,;23.67,32.17,;23.14,33.62,;24.14,34.79,;23.61,36.24,;22.13,36.67,;22.08,38.21,;23.53,38.73,;24.48,37.52,;21.63,33.89,;21.1,35.33,;20.64,32.71,;19.12,32.98,;18.6,34.43,;17.42,35.42,;17.69,36.93,;15.97,34.89,;18.13,31.8,;18.65,30.35,;16.61,32.07,;15.62,30.89,;16.14,29.44,;15.15,28.27,;15.68,26.82,;14.69,25.64,;15.21,24.19,;14.1,31.16,;13.58,32.61,;13.11,29.98,;11.6,30.25,;11.07,31.7,;9.56,31.97,;9.03,33.42,;7.52,33.69,;6.52,32.51,;5.01,32.78,;7.05,31.06,;8.56,30.79,;10.6,29.08,;9.09,29.35,;11.13,27.63,;10.14,26.45,;8.62,26.72,;8.1,28.17,;7.63,25.54,;6.11,25.81,;5.59,27.26,;4.07,27.53,;3.55,28.98,;3.08,26.35,;5.12,24.63,;3.6,24.9,;5.64,23.19,;4.65,22.01,;3.14,22.28,;5.18,20.56,;4.19,19.38,;6.69,20.29,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85073
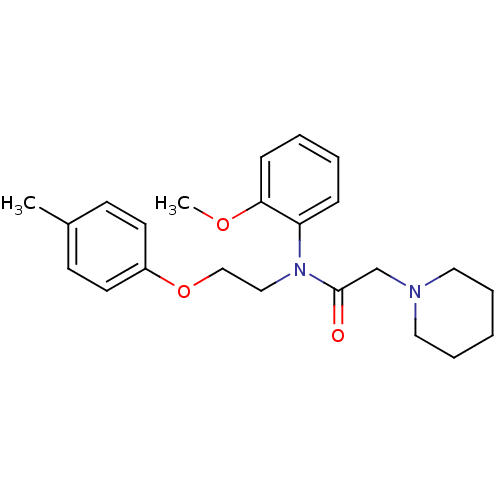
(CAS_3043476 | Galantide (M15) | NSC_3043476)Show InChI InChI=1S/C23H30N2O3/c1-19-10-12-20(13-11-19)28-17-16-25(21-8-4-5-9-22(21)27-2)23(26)18-24-14-6-3-7-15-24/h4-5,8-13H,3,6-7,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85168

(M32)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:111.115,131.138,95.103,84.88,68.80,52.59,20.28,4.4,2.2,145.150,161.166,162.169,179.184,188.193,199.204,wD:115.131,103.111,89.95,60.67,41.42,30.39,8.17,137.142,153.158,154.160,168.173,(13.69,11.51,;13.72,9.97,;12.46,9.14,;11.11,9.89,;12.54,7.63,;11.23,6.8,;9.89,7.48,;9.85,9.02,;8.62,6.65,;8.66,5.14,;10.01,4.43,;10.12,2.93,;11.51,2.18,;12.74,3.05,;14.16,2.37,;12.66,4.59,;11.27,5.26,;7.28,7.36,;5.97,6.57,;5.97,4.98,;4.67,7.32,;4.63,8.82,;5.93,9.65,;7.36,9.02,;8.39,10.12,;7.71,11.47,;6.13,11.19,;3.36,6.53,;2.02,7.16,;2.02,8.62,;.63,6.37,;.59,4.82,;1.94,3.99,;1.94,2.45,;3.24,1.66,;3.2,.08,;4.55,-.67,;1.86,-.67,;-.71,7.2,;-2.06,6.49,;-3.56,7.12,;-2.06,5.02,;-.75,4.11,;-1.19,2.65,;-2.69,2.61,;-3.16,4.03,;-4.63,4.43,;-4.98,5.93,;-5.77,3.36,;-7.24,3.84,;-8.27,2.73,;-7.91,1.19,;-9.73,3.08,;-10.12,4.59,;-9.02,5.7,;-9.41,7.2,;-7.51,5.26,;-10.84,2.06,;-12.34,2.37,;-12.66,3.8,;-13.41,1.27,;-13.05,-.2,;-11.59,-.59,;-10.48,.51,;-11.15,-2.06,;-14.87,1.7,;-15.98,.67,;-15.62,-.87,;-17.56,1.07,;-17.92,2.61,;-16.85,3.64,;-15.35,3.28,;-14.24,4.39,;-14.6,5.85,;-13.49,6.96,;-16.1,6.29,;-17.24,5.22,;-18.59,0,;-20.05,.44,;-20.45,1.9,;-21.12,-.67,;-22.54,-.28,;-23.65,-1.34,;-23.26,-2.81,;-25.19,-.99,;-25.55,.55,;-26.26,-2.02,;-27.73,-1.62,;-28.08,-.16,;-28.83,-2.69,;-28.44,-4.15,;-26.97,-4.63,;-30.3,-2.25,;-31.44,-3.28,;-31.09,-4.71,;-32.95,-2.81,;-33.3,-1.34,;-32.19,-.28,;-30.73,-.71,;-32.55,1.23,;-34.05,-3.92,;-35.56,-3.56,;-35.87,-2.14,;-36.63,-4.71,;-36.27,-6.13,;-34.8,-6.57,;-33.7,-5.5,;-34.41,-8.03,;-38.09,-4.23,;-39.23,-5.3,;-38.84,-6.76,;-40.7,-4.87,;-41.85,-5.93,;-43.27,-5.54,;-43.66,-4.03,;-44.46,-6.6,;-44.18,-8.11,;-42.64,-8.54,;-41.45,-7.59,;-40.11,-8.39,;-40.54,-9.93,;-39.71,-11.27,;-40.46,-12.66,;-41.96,-12.66,;-42.87,-11.35,;-42.12,-10.05,;-45.92,-6.13,;-47.07,-7.2,;-46.71,-8.66,;-48.57,-6.76,;-49.68,-7.83,;-41.05,-3.36,;-42.6,-2.89,;-39.91,-2.29,;13.88,6.88,;13.88,5.42,;15.19,7.67,;16.53,6.88,;16.53,5.38,;17.92,4.67,;19.18,5.46,;17.96,3.12,;17.84,7.71,;17.84,9.18,;19.18,7.04,;20.45,7.75,;20.49,9.25,;21.79,10.09,;21.83,11.59,;23.14,9.25,;21.79,7.04,;21.79,5.46,;23.14,7.75,;24.44,7,;24.48,5.5,;23.18,4.71,;25.79,4.75,;25.83,3.24,;25.71,7.75,;25.71,9.29,;27.01,6.92,;28.32,7.71,;28.32,9.25,;27.01,10.05,;29.66,10.01,;29.66,6.96,;29.66,5.46,;30.97,7.67,;32.31,6.88,;32.27,5.38,;33.62,4.59,;33.62,3.08,;34.88,2.37,;34.92,.83,;36.27,.04,;33.62,0,;33.66,7.63,;33.66,9.1,;35,6.88,;36.27,7.67,;36.27,9.18,;37.53,9.97,;37.53,11.47,;38.8,12.26,;36.19,12.26,;37.61,6.92,;37.61,5.38,;38.88,7.75,;40.18,7.04,;40.26,5.5,;41.61,4.71,;41.69,3.16,;42.99,2.49,;43.07,.95,;44.46,.16,;41.77,.04,;41.53,7.87,;41.49,9.25,;42.91,7.24,;44.22,7.99,;44.22,9.57,;45.56,10.32,;45.6,11.87,;46.99,12.66,;48.33,11.83,;49.68,12.58,;48.29,10.32,;46.91,9.53,;45.52,7.24,;46.79,8.07,;45.52,5.66,)| Show InChI InChI=1S/C136H209N41O34/c1-16-70(11)108(130(208)171-99(58-104(140)186)123(201)166-93(51-69(9)10)125(203)174-109(71(12)17-2)131(209)176-111(74(15)180)132(210)162-87(28-22-46-151-136(146)147)115(193)160-88(42-43-102(138)184)118(196)159-85(26-20-44-149-134(142)143)116(194)163-89(112(141)190)52-75-30-36-80(181)37-31-75)173-126(204)95(54-77-34-40-82(183)41-35-77)167-122(200)97(56-79-61-148-65-155-79)168-117(195)86(27-21-45-150-135(144)145)161-129(207)101-29-23-47-177(101)107(189)63-154-114(192)90(48-66(3)4)164-119(197)91(49-67(5)6)165-121(199)94(53-76-32-38-81(182)39-33-76)158-106(188)62-153-113(191)72(13)156-128(206)100(64-178)172-124(202)98(57-103(139)185)169-120(198)92(50-68(7)8)170-133(211)110(73(14)179)175-127(205)96(157-105(187)59-137)55-78-60-152-84-25-19-18-24-83(78)84/h18-19,24-25,30-41,60-61,65-74,85-101,108-111,152,178-183H,16-17,20-23,26-29,42-59,62-64,137H2,1-15H3,(H2,138,184)(H2,139,185)(H2,140,186)(H2,141,190)(H,148,155)(H,153,191)(H,154,192)(H,156,206)(H,157,187)(H,158,188)(H,159,196)(H,160,193)(H,161,207)(H,162,210)(H,163,194)(H,164,197)(H,165,199)(H,166,201)(H,167,200)(H,168,195)(H,169,198)(H,170,211)(H,171,208)(H,172,202)(H,173,204)(H,174,203)(H,175,205)(H,176,209)(H4,142,143,149)(H4,144,145,150)(H4,146,147,151)/t70-,71-,72-,73+,74+,85+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,108-,109-,110-,111-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85200
(Galanin (1-19), rat | Galanin rat)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C92H135N27O26/c1-12-46(8)75(91(144)115-66(33-74(128)129)87(140)112-64(31-69(94)123)85(138)108-57(77(96)130)29-52-36-97-41-102-52)117-79(132)48(10)105-81(134)63(30-53-37-98-42-103-53)113-90(143)68-18-15-23-119(68)73(127)39-101-80(133)58(24-43(2)3)109-82(135)59(25-44(4)5)110-84(137)61(27-50-19-21-54(122)22-20-50)107-72(126)38-100-78(131)47(9)104-89(142)67(40-120)116-86(139)65(32-70(95)124)111-83(136)60(26-45(6)7)114-92(145)76(49(11)121)118-88(141)62(106-71(125)34-93)28-51-35-99-56-17-14-13-16-55(51)56/h13-14,16-17,19-22,35-37,41-49,57-68,75-76,99,120-122H,12,15,18,23-34,38-40,93H2,1-11H3,(H2,94,123)(H2,95,124)(H2,96,130)(H,97,102)(H,98,103)(H,100,131)(H,101,133)(H,104,142)(H,105,134)(H,106,125)(H,107,126)(H,108,138)(H,109,135)(H,110,137)(H,111,136)(H,112,140)(H,113,143)(H,114,145)(H,115,144)(H,116,139)(H,117,132)(H,118,141)(H,128,129) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 25: 5299-305 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.045
BindingDB Entry DOI: 10.7270/Q26W9CW2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50293451

(1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...)Show SMILES C[N+]1(CCC(=O)Nc2ccc-3c(c2)C(=O)c2cccc4ccnc-3c24)CCCCC1 Show InChI InChI=1S/C25H25N3O2/c1-28(13-3-2-4-14-28)15-11-22(29)27-18-8-9-19-21(16-18)25(30)20-7-5-6-17-10-12-26-24(19)23(17)20/h5-10,12,16H,2-4,11,13-15H2,1H3/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by LB plot |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127506
BindingDB Entry DOI: 10.7270/Q2CR5Z20 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85072
(Galanin, Human)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O |wU:212.220,131.139,139.152,71.79,166.175,182.187,54.57,211.217,20.25,37.39,112.114,97.99,155.160,wD:202.210,102.111,123.131,93.95,81.87,174.183,160.167,60.68,186.203,4.4,12.16,43.51,29.33,218.224,(13.66,18.44,;13.66,16.9,;12.32,16.13,;14.99,16.13,;14.99,14.59,;16.32,13.82,;17.86,14.59,;17.86,16.13,;19.2,13.82,;20.53,14.59,;22.07,13.82,;22.07,12.28,;23.4,14.59,;23.4,16.13,;22.07,16.9,;22.07,18.44,;20.74,16.13,;24.74,13.82,;26.28,14.59,;26.28,16.13,;27.61,13.82,;27.61,12.28,;28.95,11.51,;28.95,9.97,;30.28,9.2,;31.61,9.97,;28.95,14.59,;30.49,13.82,;30.49,12.28,;31.82,14.59,;31.82,16.13,;33.15,16.9,;33.15,18.44,;34.49,16.13,;33.15,13.82,;32.43,3.03,;32.43,1.49,;31.1,3.8,;31.1,5.34,;32.43,6.11,;29.76,3.03,;28.22,3.8,;28.22,5.34,;26.89,3.03,;26.89,1.49,;28.22,.72,;28.22,-.82,;29.56,-1.59,;30.89,-.82,;30.89,.72,;29.56,1.49,;25.56,3.8,;24.02,3.03,;24.02,1.49,;22.68,3.8,;22.68,5.34,;24.02,6.11,;21.35,3.03,;19.81,3.8,;19.81,5.34,;18.47,3.03,;18.47,1.49,;19.81,.72,;19.81,-.82,;21.14,-1.59,;22.48,-.82,;23.81,-1.59,;22.48,.72,;17.14,3.8,;15.6,3.03,;15.6,1.49,;14.27,3.8,;14.27,5.34,;15.6,6.11,;17.01,5.49,;18.04,6.63,;17.27,7.97,;15.76,7.65,;12.93,3.03,;11.39,3.8,;11.39,5.34,;10.06,3.03,;10.06,1.49,;11.39,.72,;11.39,-.82,;12.73,1.49,;8.73,3.8,;7.19,3.03,;7.19,1.49,;5.85,3.8,;4.52,3.03,;5.24,-7.75,;5.24,-9.29,;6.57,-6.98,;7.91,-7.75,;9.45,-6.98,;9.45,-5.44,;10.78,-7.75,;10.78,-9.29,;12.12,-6.98,;13.66,-7.75,;13.66,-9.29,;14.99,-6.98,;14.99,-5.44,;13.66,-4.67,;12.25,-5.29,;11.22,-4.15,;11.99,-2.81,;13.49,-3.13,;16.32,-7.75,;17.66,-6.98,;17.66,-5.44,;18.99,-7.75,;19.15,-9.28,;20.66,-9.6,;21.43,-8.26,;20.4,-7.12,;20.72,-5.61,;19.57,-4.58,;22.18,-5.14,;23.33,-6.17,;24.79,-5.69,;25.11,-4.19,;25.94,-6.72,;27.4,-6.25,;27.72,-4.74,;29.19,-4.26,;26.58,-3.71,;25.62,-8.23,;26.76,-9.26,;28.22,-8.78,;26.44,-10.77,;24.98,-11.24,;24.65,-12.75,;23.19,-13.22,;25.8,-13.78,;27.58,-11.8,;29.05,-11.32,;29.37,-9.81,;30.19,-12.35,;29.87,-13.86,;31.02,-14.89,;30.7,-16.39,;31.84,-17.42,;33.31,-16.95,;34.45,-17.98,;33.63,-15.44,;32.48,-14.41,;31.66,-11.87,;31.98,-10.37,;30.83,-9.34,;33.44,-9.89,;33.76,-8.39,;35.23,-7.91,;35.55,-6.4,;36.37,-8.94,;36.05,-10.45,;37.84,-8.46,;38.98,-9.49,;38.66,-11,;40.45,-9.02,;40.77,-7.51,;42.23,-7.04,;41.59,-10.05,;43.06,-9.57,;43.38,-8.07,;44.2,-10.6,;43.88,-12.11,;45.02,-13.14,;44.7,-14.65,;46.49,-12.66,;45.66,-10.13,;45.98,-8.62,;44.84,-7.59,;47.45,-8.15,;47.77,-6.64,;46.62,-5.61,;46.94,-4.1,;45.16,-6.08,;48.59,-9.18,;50.06,-8.7,;50.38,-7.19,;51.2,-9.73,;52.67,-9.25,;53.81,-10.29,;53.49,-11.79,;55.28,-9.81,;55.6,-8.3,;57.06,-7.83,;58.3,-8.73,;59.54,-7.83,;59.07,-6.37,;59.85,-5.04,;59.08,-3.71,;57.54,-3.7,;56.77,-5.04,;57.53,-6.37,;56.42,-10.84,;56.1,-12.35,;54.64,-12.82,;57.25,-13.38,;56.93,-14.88,;50.88,-11.24,;49.42,-11.71,;52.03,-12.27,;6.57,-5.44,;5.24,-4.67,;7.91,-4.67,;13.66,13.82,;13.66,12.28,;12.12,14.59,;10.78,13.82,;10.78,12.28,;12.12,11.51,;9.45,11.51,;9.45,14.59,;9.45,16.13,;7.91,13.82,;6.57,14.59,;6.57,16.13,;5.24,16.9,;5.24,13.82,;3.91,14.59,;5.24,12.28,)| Show InChI InChI=1S/C139H210N42O43/c1-65(2)38-84(165-121(206)86(40-67(5)6)166-123(208)88(43-75-31-33-79(188)34-32-75)161-106(193)55-151-114(199)70(11)157-131(216)97(59-182)175-127(212)95(49-104(144)191)170-122(207)87(41-68(7)8)173-136(221)112(72(13)186)180-130(215)90(159-105(192)51-141)44-76-52-150-81-27-19-18-26-80(76)81)116(201)154-58-109(196)181-37-23-30-101(181)134(219)172-91(45-77-53-147-63-155-77)120(205)158-71(12)115(200)178-111(69(9)10)135(220)153-57-108(195)162-94(48-103(143)190)126(211)168-92(46-78-54-148-64-156-78)125(210)164-83(29-22-36-149-139(145)146)119(204)174-98(60-183)132(217)167-89(42-74-24-16-15-17-25-74)124(209)176-99(61-184)133(218)171-96(50-110(197)198)128(213)163-82(28-20-21-35-140)118(203)169-93(47-102(142)189)117(202)152-56-107(194)160-85(39-66(3)4)129(214)179-113(73(14)187)137(222)177-100(62-185)138(223)224/h15-19,24-27,31-34,52-54,63-73,82-101,111-113,150,182-188H,20-23,28-30,35-51,55-62,140-141H2,1-14H3,(H2,142,189)(H2,143,190)(H2,144,191)(H,147,155)(H,148,156)(H,151,199)(H,152,202)(H,153,220)(H,154,201)(H,157,216)(H,158,205)(H,159,192)(H,160,194)(H,161,193)(H,162,195)(H,163,213)(H,164,210)(H,165,206)(H,166,208)(H,167,217)(H,168,211)(H,169,203)(H,170,207)(H,171,218)(H,172,219)(H,173,221)(H,174,204)(H,175,212)(H,176,209)(H,177,222)(H,178,200)(H,179,214)(H,180,215)(H,197,198)(H,223,224)(H4,145,146,149)/t70-,71-,72+,73+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,111-,112-,113-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor [1078-1345,H1094R,L1272V]
(Homo sapiens (Human)) | BDBM24466
(1-(2-hydroxy-2-methylpropyl)-N-{5-[(7-methoxyquino...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CC(C)(C)O)n(-c5ccccc5)c4=O)nc3)ccnc2c1 Show InChI InChI=1S/C30H29N5O5/c1-19-27(29(37)35(20-8-6-5-7-9-20)34(19)18-30(2,3)38)28(36)33-26-13-11-22(17-32-26)40-25-14-15-31-24-16-21(39-4)10-12-23(24)25/h5-17,38H,18H2,1-4H3,(H,32,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146492
(3-Benzo[1,3]dioxol-5-yl-3-({1-[2-fluoro-5-(1,4,5,6...)Show SMILES OC(=O)CC(NC(=O)C1CN(C(=O)C1)c1cc(NC2=NCCCN2)ccc1F)c1ccc2OCOc2c1 |t:19| Show InChI InChI=1S/C25H26FN5O6/c26-17-4-3-16(29-25-27-6-1-7-28-25)10-19(17)31-12-15(9-22(31)32)24(35)30-18(11-23(33)34)14-2-5-20-21(8-14)37-13-36-20/h2-5,8,10,15,18H,1,6-7,9,11-13H2,(H,30,35)(H,33,34)(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards vitronectin receptor (AlphaV-beta3 integrin). |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(Homo sapiens (Human)) | BDBM85068
(Gal(1-13)-Std I)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)Cc1ccccc1 |wU:79.83,114.118,96.99,139.144,4.4,16.25,164.176,51.56,37.40,63.76,110.115,126.132,92.96,wD:130.135,148.153,8.12,172.184,180.200,194.204,43.48,59.60,32.33,(17.09,11.7,;17.76,10.31,;16.89,9.03,;19.29,10.2,;19.97,8.81,;19.1,7.54,;19.78,6.15,;21.31,6.04,;18.91,4.88,;19.59,3.49,;21.12,3.38,;21.79,2,;21.99,4.66,;17.38,4.99,;16.51,3.71,;17.19,2.33,;14.98,3.82,;14.3,5.21,;12.77,5.32,;12.1,6.7,;10.56,6.81,;9.7,5.54,;8.16,5.65,;10.37,4.15,;11.91,4.04,;14.11,2.55,;14.79,1.16,;16.32,1.05,;13.92,-.11,;14.6,-1.5,;13.73,-2.77,;12.2,-2.66,;14.41,-4.16,;15.94,-4.27,;13.54,-5.43,;14.21,-6.82,;15.75,-6.93,;13.35,-8.09,;11.82,-7.98,;10.95,-9.26,;14.02,-9.48,;13.16,-10.75,;11.62,-10.64,;13.83,-12.14,;15.37,-12.25,;16.04,-13.64,;17.58,-13.75,;15.18,-14.91,;12.97,-13.42,;11.43,-13.31,;10.76,-11.92,;10.57,-14.58,;9.03,-14.47,;8.36,-13.09,;6.83,-12.98,;9.22,-11.81,;11.24,-15.97,;10.38,-17.24,;8.84,-17.13,;11.05,-18.63,;10.19,-19.9,;10.86,-21.29,;12.4,-21.4,;10,-22.56,;8.46,-22.45,;7.6,-23.73,;8.12,-25.17,;6.92,-26.11,;5.64,-25.26,;4.15,-25.63,;3.08,-24.52,;3.5,-23.04,;5,-22.67,;6.07,-23.78,;10.67,-23.95,;12.21,-24.06,;13.07,-22.78,;12.88,-25.44,;14.42,-25.55,;12.59,-18.74,;13.45,-17.46,;13.26,-20.12,;21.5,8.7,;22.18,7.32,;22.37,9.98,;23.9,9.87,;24.77,11.14,;26.3,11.03,;24.09,12.53,;22.58,12.8,;22.37,14.33,;23.75,15,;24.82,13.89,;26.34,14.1,;27.29,12.89,;26.92,15.53,;28.45,15.74,;29.4,14.52,;28.82,13.1,;29.76,11.88,;29.18,10.45,;30.13,9.24,;29.55,7.81,;31.65,9.45,;29.03,17.17,;30.55,17.38,;28.08,18.38,;26.54,18.33,;26.02,19.78,;27.23,20.72,;28.51,19.86,;29.96,20.38,;30.23,21.9,;31.14,19.39,;32.58,19.92,;32.85,21.43,;31.68,22.43,;31.95,23.94,;30.77,24.93,;31.04,26.45,;33.76,18.93,;33.49,17.41,;35.21,19.45,;35.64,20.93,;37.18,20.98,;37.7,19.53,;36.48,18.59,;36.54,17.05,;35.23,16.23,;37.89,16.32,;37.95,14.78,;36.64,13.97,;35.28,14.69,;33.97,13.88,;32.61,14.61,;34.02,12.34,;39.3,14.06,;40.61,14.87,;39.36,12.52,;40.71,11.79,;42.02,12.61,;41.97,14.15,;43.28,14.96,;43.23,16.5,;44.64,14.23,;40.76,10.25,;39.46,9.44,;42.12,9.53,;42.17,7.99,;43.53,7.26,;44.84,8.08,;44.95,9.6,;46.43,9.98,;47.25,8.68,;48.76,8.41,;49.29,6.97,;48.3,5.79,;46.79,6.05,;46.26,7.5,;40.87,7.17,;39.51,7.9,;40.92,5.64,;39.61,4.82,;38.25,5.55,;39.66,3.28,;38.35,2.47,;37,3.19,;35.69,2.38,;36.94,4.73,;41.02,2.56,;41.07,1.02,;39.56,.7,;42.1,-.13,;43.62,-.35,;44.57,.87,;46.1,.65,;44,2.3,;41.62,-1.59,;42.65,-2.74,;44.16,-2.42,;42.17,-4.2,;40.66,-4.52,;40.19,-5.98,;41.08,-7.22,;40.19,-8.46,;38.73,-7.99,;37.4,-8.76,;36.06,-7.99,;36.06,-6.45,;37.4,-5.68,;38.73,-6.45,;43.2,-5.35,;44.71,-5.03,;45.19,-3.57,;45.74,-6.18,;45.26,-7.64,;47.24,-5.86,;48.27,-7.01,;47.79,-8.47,;48.82,-9.62,;50.33,-9.3,;50.81,-7.83,;49.78,-6.69,)| Show InChI InChI=1S/C138H199N35O30/c1-72(2)54-96(161-122(188)97(55-73(3)4)162-125(191)101(60-80-41-43-84(176)44-42-80)155-114(181)69-151-117(183)77(11)153-131(197)106(71-174)168-127(193)105(64-112(144)179)166-124(190)99(57-75(7)8)167-135(201)116(78(12)175)169-128(194)102(154-113(180)65-140)61-81-66-148-89-33-19-16-30-85(81)89)119(185)152-70-115(182)171-51-25-38-107(171)132(198)159-95(37-24-50-147-138(145)146)137(203)173-53-27-40-109(173)134(200)158-94(36-22-23-49-139)136(202)172-52-26-39-108(172)133(199)157-93(46-48-111(143)178)120(186)156-92(45-47-110(142)177)121(187)165-104(63-83-68-150-91-35-21-18-32-87(83)91)130(196)170-129(195)100(58-76(9)10)164-123(189)98(56-74(5)6)163-126(192)103(62-82-67-149-90-34-20-17-31-86(82)90)160-118(184)88(141)59-79-28-14-13-15-29-79/h13-21,28-35,41-44,66-68,72-78,88,92-109,116,148-150,174-176H,22-27,36-40,45-65,69-71,139-141H2,1-12H3,(H2,142,177)(H2,143,178)(H2,144,179)(H,151,183)(H,152,185)(H,153,197)(H,154,180)(H,155,181)(H,156,186)(H,157,199)(H,158,200)(H,159,198)(H,160,184)(H,161,188)(H,162,191)(H,163,192)(H,164,189)(H,165,187)(H,166,190)(H,167,201)(H,168,193)(H,169,194)(H4,145,146,147)(H,170,195,196)/t77-,78+,88-,92-,93-,94-,95+,96-,97-,98-,99-,100-,101-,102-,103+,104+,105-,106-,107-,108-,109-,116-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146497
(3-Benzo[1,3]dioxol-5-yl-3-({5-oxo-1-[3-(1,4,5,6-te...)Show SMILES OC(=O)CC(NC(=O)C1CN(C(=O)C1)c1cccc(NC2=NCCCN2)c1)c1ccc2OCOc2c1 |t:21| Show InChI InChI=1S/C25H27N5O6/c31-22-10-16(13-30(22)18-4-1-3-17(11-18)28-25-26-7-2-8-27-25)24(34)29-19(12-23(32)33)15-5-6-20-21(9-15)36-14-35-20/h1,3-6,9,11,16,19H,2,7-8,10,12-14H2,(H,29,34)(H,32,33)(H2,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Affinity for alphaIIb-beta3 integrin |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50108858

(CHEMBL347459 | {1-[2-Oxo-1-phenethyl-3-(3-phenyl-p...)Show SMILES O=C(N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C(=O)CSCCCc1ccccc1)OCc1cccnc1 Show InChI InChI=1S/C36H39N3O4S/c40-34(27-44-23-11-19-28-12-4-1-5-13-28)32(21-20-29-14-6-2-7-15-29)38-35(41)33(24-30-16-8-3-9-17-30)39-36(42)43-26-31-18-10-22-37-25-31/h1-10,12-18,22,25,32-33H,11,19-21,23-24,26-27H2,(H,38,41)(H,39,42)/t32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50108858

(CHEMBL347459 | {1-[2-Oxo-1-phenethyl-3-(3-phenyl-p...)Show SMILES O=C(N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)C(=O)CSCCCc1ccccc1)OCc1cccnc1 Show InChI InChI=1S/C36H39N3O4S/c40-34(27-44-23-11-19-28-12-4-1-5-13-28)32(21-20-29-14-6-2-7-15-29)38-35(41)33(24-30-16-8-3-9-17-30)39-36(42)43-26-31-18-10-22-37-25-31/h1-10,12-18,22,25,32-33H,11,19-21,23-24,26-27H2,(H,38,41)(H,39,42)/t32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(Homo sapiens (Human)) | BDBM85070

(Galanin, Porcine)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(C)C(N)=O |(22.15,12.84,;20.71,12.32,;20.44,10.8,;21.61,9.81,;18.99,10.28,;18.72,8.76,;19.9,7.77,;21.34,8.3,;19.63,6.25,;18.18,5.73,;20.8,5.26,;20.53,3.75,;19.09,3.22,;21.71,2.75,;21.44,1.24,;19.99,.71,;18.72,1.58,;17.5,.63,;18.03,-.82,;19.57,-.77,;23.16,3.28,;24.34,2.29,;24.07,.77,;25.79,2.81,;26.21,4.29,;27.75,4.34,;28.28,2.89,;27.06,1.95,;27.11,.41,;25.81,-.41,;28.47,-.32,;28.52,-1.86,;29.88,-2.58,;31.19,-1.77,;29.93,-4.12,;31.29,-4.85,;32.6,-4.03,;33.96,-4.76,;32.55,-2.49,;28.62,-4.93,;28.68,-6.47,;30.2,-6.69,;27.73,-7.69,;26.22,-8,;25.19,-6.86,;23.68,-7.17,;25.67,-5.39,;28.3,-9.11,;27.35,-10.33,;25.82,-10.11,;27.92,-11.76,;26.97,-12.97,;25.45,-12.75,;24.87,-11.32,;23.35,-11.1,;22.4,-12.32,;20.88,-12.1,;22.97,-13.75,;24.5,-13.96,;29.45,-11.97,;30.02,-13.4,;29.07,-14.61,;31.55,-13.62,;32.12,-15.05,;33.65,-15.26,;34.6,-14.05,;34.22,-16.69,;33.27,-17.9,;35.75,-16.91,;36.32,-18.34,;35.37,-19.55,;37.85,-18.56,;38.8,-17.34,;38.22,-15.91,;38.42,-19.98,;39.94,-20.2,;40.89,-18.99,;40.52,-21.63,;42.04,-21.85,;42.99,-20.64,;44.52,-20.85,;42.42,-19.21,;39.57,-22.84,;40.14,-24.27,;41.65,-23.95,;39.66,-25.74,;38.36,-26.55,;37,-25.82,;35.69,-26.64,;36.95,-24.28,;40.69,-26.88,;40.21,-28.35,;38.71,-28.66,;41.24,-29.49,;40.76,-30.96,;41.79,-32.1,;43.3,-31.78,;41.31,-33.57,;39.81,-33.88,;39.33,-35.35,;40.23,-36.59,;39.33,-37.83,;37.87,-37.36,;36.54,-38.13,;35.2,-37.36,;35.2,-35.82,;36.54,-35.05,;37.87,-35.82,;42.34,-34.71,;43.85,-34.39,;44.33,-32.93,;44.88,-35.54,;46.39,-35.22,;42.75,-29.17,;43.78,-30.32,;43.23,-27.71,;17.81,11.27,;16.36,10.75,;18.08,12.79,;16.9,13.78,;15.45,13.25,;14.28,14.25,;12.83,13.72,;14.55,15.76,;17.17,15.3,;15.99,16.29,;18.62,15.82,;18.89,17.34,;17.37,17.61,;16.85,19.05,;15.33,19.32,;17.84,20.23,;20.34,17.86,;21.52,16.87,;21.33,19.04,;22.85,18.77,;23.37,17.32,;24.89,17.05,;26,18.12,;27.36,17.39,;27.09,15.88,;25.56,15.66,;23.84,19.95,;25.35,19.68,;23.31,21.39,;24.31,22.57,;25.82,22.3,;26.81,23.48,;28.33,23.21,;29.32,24.39,;30.84,24.12,;31.83,25.3,;31.36,22.67,;23.78,24.02,;22.27,24.29,;24.77,25.2,;24.25,26.65,;22.73,26.92,;22.21,28.36,;25.24,27.82,;26.76,27.55,;24.72,29.27,;25.71,30.45,;27.23,30.18,;28.22,31.36,;29.73,31.09,;30.73,32.27,;30.2,33.71,;28.69,33.98,;27.69,32.81,;25.19,31.9,;26.18,33.08,;23.67,32.17,;23.14,33.62,;24.14,34.79,;23.61,36.24,;22.13,36.67,;22.08,38.21,;23.53,38.73,;24.48,37.52,;21.63,33.89,;21.1,35.33,;20.64,32.71,;19.12,32.98,;18.6,34.43,;17.42,35.42,;17.69,36.93,;15.97,34.89,;18.13,31.8,;18.65,30.35,;16.61,32.07,;15.62,30.89,;16.14,29.44,;15.15,28.27,;15.68,26.82,;14.69,25.64,;15.21,24.19,;14.1,31.16,;13.58,32.61,;13.11,29.98,;11.6,30.25,;11.07,31.7,;9.56,31.97,;9.03,33.42,;7.52,33.69,;6.52,32.51,;5.01,32.78,;7.05,31.06,;8.56,30.79,;10.6,29.08,;9.09,29.35,;11.13,27.63,;10.14,26.45,;8.62,26.72,;8.1,28.17,;7.63,25.54,;6.11,25.81,;5.59,27.26,;4.07,27.53,;3.55,28.98,;3.08,26.35,;5.12,24.63,;3.6,24.9,;5.64,23.19,;4.65,22.01,;3.14,22.28,;5.18,20.56,;4.19,19.38,;6.69,20.29,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526945
(CHEMBL4473806)Show SMILES ON(C(=O)SC[C@H](NC(=O)CC[C@H](NC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCCCCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C78H112Cl2N20O30S2/c79-47-7-11-49(12-8-47)99(129)77(127)131-45-53(73(121)93-43-71(117)118)97-67(113)17-15-51(75(123)124)95-69(115)29-41-91-65(111)27-39-89-63(109)25-37-87-61(107)23-35-85-59(105)21-33-83-57(103)19-31-81-55(101)5-3-1-2-4-6-56(102)82-32-20-58(104)84-34-22-60(106)86-36-24-62(108)88-38-26-64(110)90-40-28-66(112)92-42-30-70(116)96-52(76(125)126)16-18-68(114)98-54(74(122)94-44-72(119)120)46-132-78(128)100(130)50-13-9-48(80)10-14-50/h7-14,51-54,129-130H,1-6,15-46H2,(H,81,101)(H,82,102)(H,83,103)(H,84,104)(H,85,105)(H,86,106)(H,87,107)(H,88,108)(H,89,109)(H,90,110)(H,91,111)(H,92,112)(H,93,121)(H,94,122)(H,95,115)(H,96,116)(H,97,113)(H,98,114)(H,117,118)(H,119,120)(H,123,124)(H,125,126)/t51-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24459
(N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(C)n(-c5ccccc5)c4=O)cc3F)ccnc2c1 Show InChI InChI=1S/C28H23FN4O4/c1-17-26(28(35)33(32(17)2)19-7-5-4-6-8-19)27(34)31-18-9-12-25(22(29)15-18)37-24-13-14-30-23-16-20(36-3)10-11-21(23)24/h4-16H,1-3H3,(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Menin
(Homo sapiens (Human)) | BDBM50516569
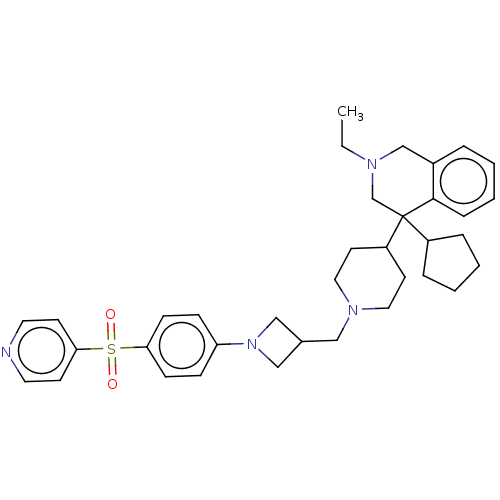
(CHEMBL4585198 | US10899738, Cpd. No 148)Show SMILES CCN1Cc2ccccc2C(C1)(C1CCCC1)C1CCN(CC2CN(C2)c2ccc(cc2)S(=O)(=O)c2ccncc2)CC1 Show InChI InChI=1S/C36H46N4O2S/c1-2-38-26-29-7-3-6-10-35(29)36(27-38,30-8-4-5-9-30)31-17-21-39(22-18-31)23-28-24-40(25-28)32-11-13-33(14-12-32)43(41,42)34-15-19-37-20-16-34/h3,6-7,10-16,19-20,28,30-31H,2,4-5,8-9,17-18,21-27H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAM-probe binding to Menin (unknown origin) after 60 mins by fluorescence polarization competitive binding assay |
J Med Chem 62: 6015-6034 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00021
BindingDB Entry DOI: 10.7270/Q2P84G8G |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24462
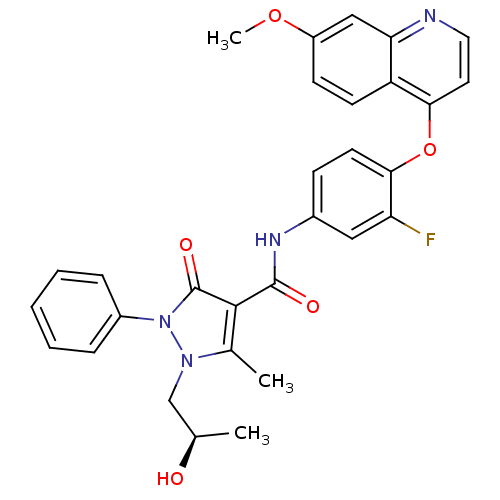
(N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(C[C@@H](C)O)n(-c5ccccc5)c4=O)cc3F)ccnc2c1 |r| Show InChI InChI=1S/C30H27FN4O5/c1-18(36)17-34-19(2)28(30(38)35(34)21-7-5-4-6-8-21)29(37)33-20-9-12-27(24(31)15-20)40-26-13-14-32-25-16-22(39-3)10-11-23(25)26/h4-16,18,36H,17H2,1-3H3,(H,33,37)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM304526
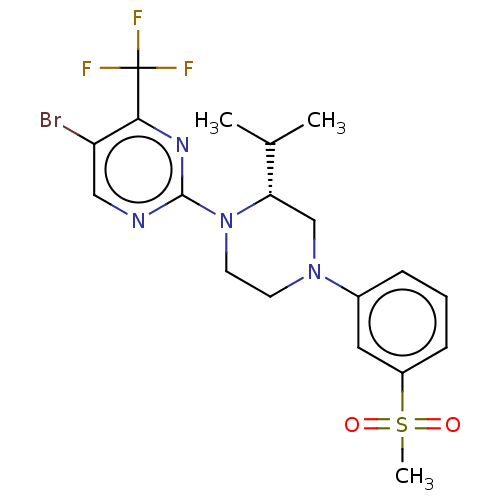
((R)-5-cyclopropyl-2-(4-(4-fluoro-3-(methylsulfonyl...)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(Br)c(n1)C(F)(F)F)c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H22BrF3N4O2S/c1-12(2)16-11-26(13-5-4-6-14(9-13)30(3,28)29)7-8-27(16)18-24-10-15(20)17(25-18)19(21,22)23/h4-6,9-10,12,16H,7-8,11H2,1-3H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... |
US Patent US10144715 (2018)
BindingDB Entry DOI: 10.7270/Q2PV6NFD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM304444
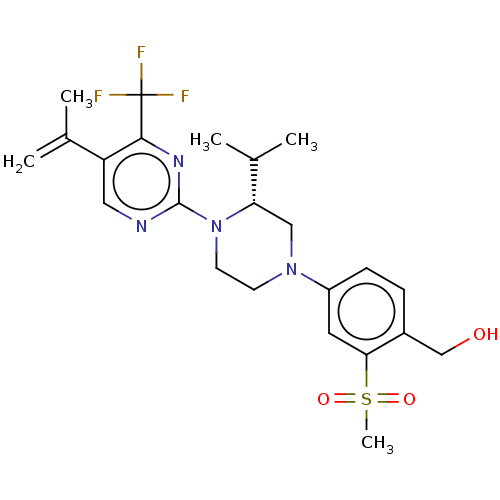
((R)-(4-(3-isopropyl-4-(5-(prop-1-en-2-yl)-4-(trifl...)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(C(C)=C)c(n1)C(F)(F)F)c1ccc(CO)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C23H29F3N4O3S/c1-14(2)18-11-27-22(28-21(18)23(24,25)26)30-9-8-29(12-19(30)15(3)4)17-7-6-16(13-31)20(10-17)34(5,32)33/h6-7,10-11,15,19,31H,1,8-9,12-13H2,2-5H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... |
US Patent US10144715 (2018)
BindingDB Entry DOI: 10.7270/Q2PV6NFD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM304452

((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...)Show SMILES CSCc1cnc(nc1C(F)(F)F)N1CCN(C[C@H]1C(C)C)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H26F4N4O2S2/c1-13(2)17-11-28(15-5-6-16(22)18(9-15)33(4,30)31)7-8-29(17)20-26-10-14(12-32-3)19(27-20)21(23,24)25/h5-6,9-10,13,17H,7-8,11-12H2,1-4H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... |
US Patent US10144715 (2018)
BindingDB Entry DOI: 10.7270/Q2PV6NFD |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177016
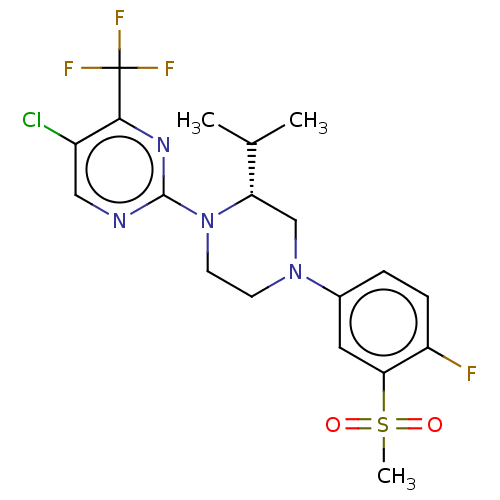
(CHEMBL3814501)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(Cl)c(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21ClF4N4O2S/c1-11(2)15-10-27(12-4-5-14(21)16(8-12)31(3,29)30)6-7-28(15)18-25-9-13(20)17(26-18)19(22,23)24/h4-5,8-9,11,15H,6-7,10H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526943
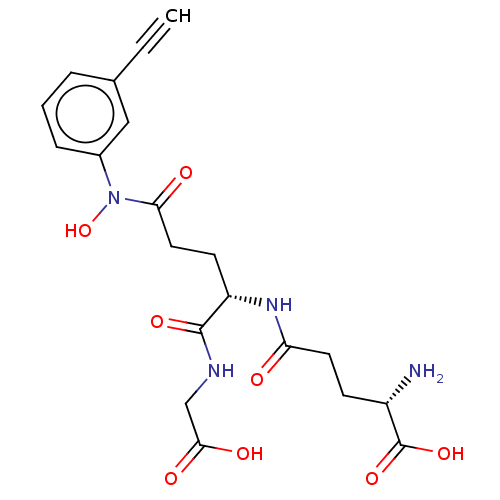
(CHEMBL4436073)Show SMILES N[C@@H](CCC(=O)N[C@@H](CCC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H24N4O8/c1-2-12-4-3-5-13(10-12)24(32)17(26)9-7-15(19(29)22-11-18(27)28)23-16(25)8-6-14(21)20(30)31/h1,3-5,10,14-15,32H,6-9,11,21H2,(H,22,29)(H,23,25)(H,27,28)(H,30,31)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177012
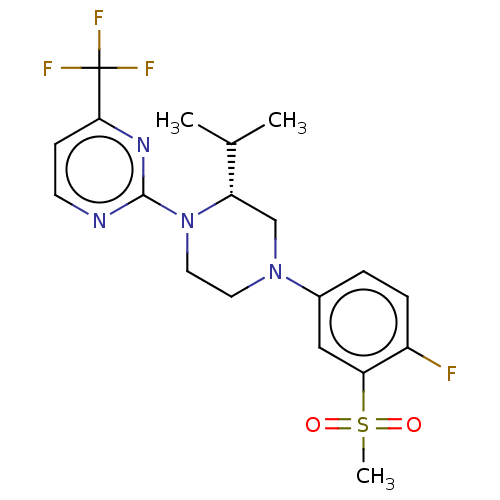
(CHEMBL3814153 | US10144715, Compound 7-32)Show SMILES CC(C)[C@@H]1CN(CCN1c1nccc(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H22F4N4O2S/c1-12(2)15-11-26(13-4-5-14(20)16(10-13)30(3,28)29)8-9-27(15)18-24-7-6-17(25-18)19(21,22)23/h4-7,10,12,15H,8-9,11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192752
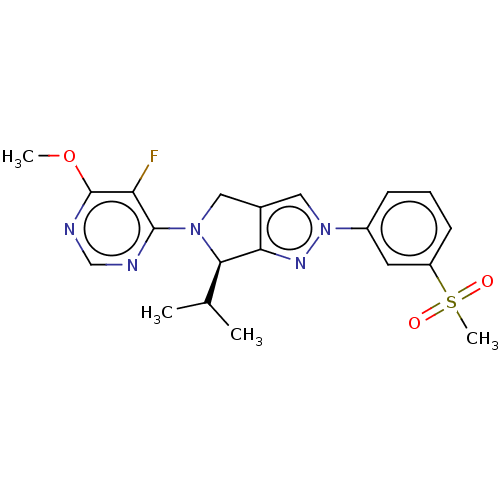
(CHEMBL3905741)Show SMILES COc1ncnc(N2Cc3cn(nc3[C@H]2C(C)C)-c2cccc(c2)S(C)(=O)=O)c1F |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(9-25(18)19-16(21)20(29-3)23-11-22-19)10-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-8,10-12,18H,9H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272598
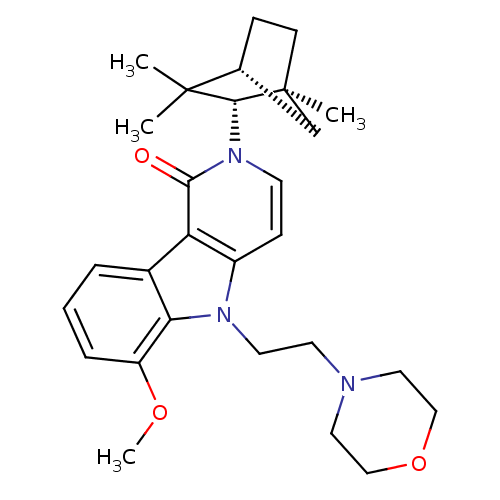
(6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...)Show SMILES COc1cccc2c1n(CCN1CCOCC1)c1ccn([C@H]3[C@@]4(C)CC[C@H](C4)C3(C)C)c(=O)c21 |r| Show InChI InChI=1S/C28H37N3O3/c1-27(2)19-8-10-28(3,18-19)26(27)31-11-9-21-23(25(31)32)20-6-5-7-22(33-4)24(20)30(21)13-12-29-14-16-34-17-15-29/h5-7,9,11,19,26H,8,10,12-18H2,1-4H3/t19-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177012

(CHEMBL3814153 | US10144715, Compound 7-32)Show SMILES CC(C)[C@@H]1CN(CCN1c1nccc(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H22F4N4O2S/c1-12(2)15-11-26(13-4-5-14(20)16(10-13)30(3,28)29)8-9-27(15)18-24-7-6-17(25-18)19(21,22)23/h4-7,10,12,15H,8-9,11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... |
US Patent US10144715 (2018)
BindingDB Entry DOI: 10.7270/Q2PV6NFD |
More data for this
Ligand-Target Pair | |
Menin
(Homo sapiens (Human)) | BDBM50516582
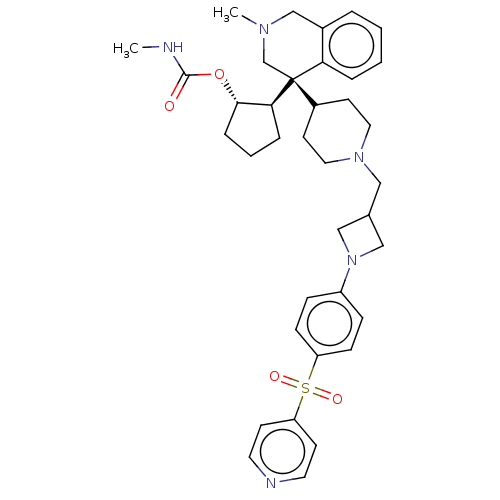
(CHEMBL4449381)Show SMILES [H][C@@]1(CCC[C@@H]1OC(=O)NC)[C@@]1(CN(C)Cc2ccccc12)C1CCN(CC2CN(C2)c2ccc(cc2)S(=O)(=O)c2ccncc2)CC1 |r| Show InChI InChI=1S/C37H47N5O4S/c1-38-36(43)46-35-9-5-8-34(35)37(26-40(2)25-28-6-3-4-7-33(28)37)29-16-20-41(21-17-29)22-27-23-42(24-27)30-10-12-31(13-11-30)47(44,45)32-14-18-39-19-15-32/h3-4,6-7,10-15,18-19,27,29,34-35H,5,8-9,16-17,20-26H2,1-2H3,(H,38,43)/t34-,35-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAM-probe binding to Menin (unknown origin) after 60 mins by fluorescence polarization competitive binding assay |
J Med Chem 62: 6015-6034 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00021
BindingDB Entry DOI: 10.7270/Q2P84G8G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cruzipain
(Trypanosoma cruzi) | BDBM50108847
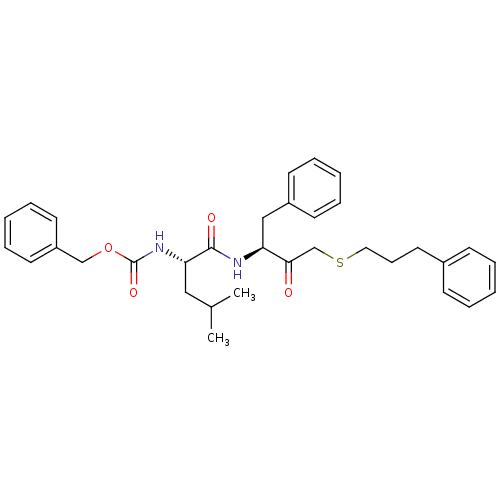
(CHEMBL162474 | benzyl (S)-4-methyl-1-oxo-1-((S)-3-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)CSCCCc1ccccc1 Show InChI InChI=1S/C33H40N2O4S/c1-25(2)21-30(35-33(38)39-23-28-17-10-5-11-18-28)32(37)34-29(22-27-15-8-4-9-16-27)31(36)24-40-20-12-19-26-13-6-3-7-14-26/h3-11,13-18,25,29-30H,12,19-24H2,1-2H3,(H,34,37)(H,35,38)/t29-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM304505
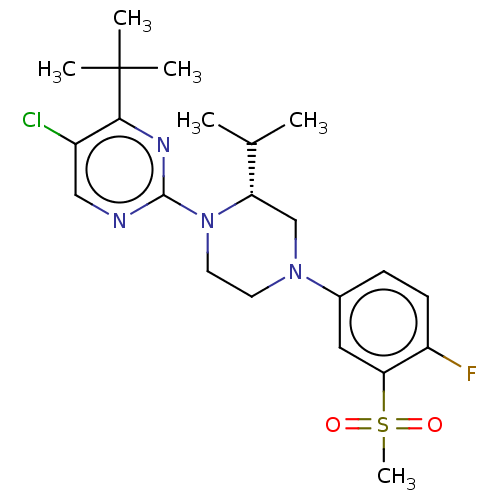
((R)-5-chloro-2-(4-(4-fluoro-3-(methylsulfonyl)phen...)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(Cl)c(n1)C(C)(C)C)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H30ClFN4O2S/c1-14(2)18-13-27(15-7-8-17(24)19(11-15)31(6,29)30)9-10-28(18)21-25-12-16(23)20(26-21)22(3,4)5/h7-8,11-12,14,18H,9-10,13H2,1-6H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... |
US Patent US10144715 (2018)
BindingDB Entry DOI: 10.7270/Q2PV6NFD |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24460
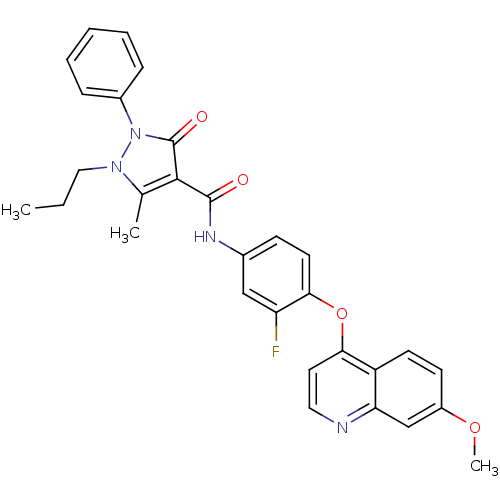
(N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...)Show SMILES CCCn1c(C)c(C(=O)Nc2ccc(Oc3ccnc4cc(OC)ccc34)c(F)c2)c(=O)n1-c1ccccc1 Show InChI InChI=1S/C30H27FN4O4/c1-4-16-34-19(2)28(30(37)35(34)21-8-6-5-7-9-21)29(36)33-20-10-13-27(24(31)17-20)39-26-14-15-32-25-18-22(38-3)11-12-23(25)26/h5-15,17-18H,4,16H2,1-3H3,(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.02 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(Homo sapiens (Human)) | BDBM85073

(CAS_3043476 | Galantide (M15) | NSC_3043476)Show InChI InChI=1S/C23H30N2O3/c1-19-10-12-20(13-11-19)28-17-16-25(21-8-4-5-9-22(21)27-2)23(26)18-24-14-6-3-7-15-24/h4-5,8-13H,3,6-7,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50108849
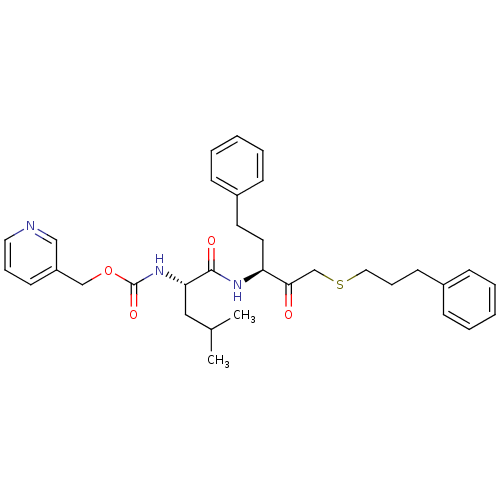
(CHEMBL321974 | {(S)-3-Methyl-1-[(S)-2-oxo-1-phenet...)Show SMILES CC(C)C[C@H](NC(=O)OCc1cccnc1)C(=O)N[C@@H](CCc1ccccc1)C(=O)CSCCCc1ccccc1 Show InChI InChI=1S/C33H41N3O4S/c1-25(2)21-30(36-33(39)40-23-28-15-9-19-34-22-28)32(38)35-29(18-17-27-13-7-4-8-14-27)31(37)24-41-20-10-16-26-11-5-3-6-12-26/h3-9,11-15,19,22,25,29-30H,10,16-18,20-21,23-24H2,1-2H3,(H,35,38)(H,36,39)/t29-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Affinity for cysteine protease (Cruzipain) of Chagas' disease |
Bioorg Med Chem Lett 12: 2993-6 (2002)
BindingDB Entry DOI: 10.7270/Q2W37VPP |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24463
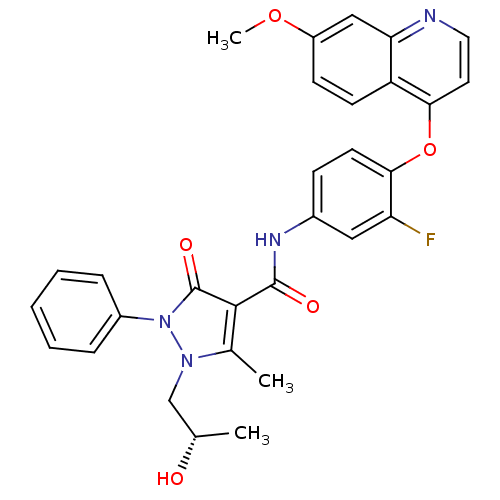
(N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(C[C@H](C)O)n(-c5ccccc5)c4=O)cc3F)ccnc2c1 |r| Show InChI InChI=1S/C30H27FN4O5/c1-18(36)17-34-19(2)28(30(38)35(34)21-7-5-4-6-8-21)29(37)33-20-9-12-27(24(31)15-20)40-26-13-14-32-25-16-22(39-3)10-11-23(25)26/h4-16,18,36H,17H2,1-3H3,(H,33,37)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor [1078-1345,V1092I,L1272V]
(Homo sapiens (Human)) | BDBM24466

(1-(2-hydroxy-2-methylpropyl)-N-{5-[(7-methoxyquino...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CC(C)(C)O)n(-c5ccccc5)c4=O)nc3)ccnc2c1 Show InChI InChI=1S/C30H29N5O5/c1-19-27(29(37)35(20-8-6-5-7-9-20)34(19)18-30(2,3)38)28(36)33-26-13-11-22(17-32-26)40-25-14-15-31-24-16-21(39-4)10-12-23(24)25/h5-17,38H,18H2,1-4H3,(H,32,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50108849

(CHEMBL321974 | {(S)-3-Methyl-1-[(S)-2-oxo-1-phenet...)Show SMILES CC(C)C[C@H](NC(=O)OCc1cccnc1)C(=O)N[C@@H](CCc1ccccc1)C(=O)CSCCCc1ccccc1 Show InChI InChI=1S/C33H41N3O4S/c1-25(2)21-30(36-33(39)40-23-28-15-9-19-34-22-28)32(38)35-29(18-17-27-13-7-4-8-14-27)31(37)24-41-20-10-16-26-11-5-3-6-12-26/h3-9,11-15,19,22,25,29-30H,10,16-18,20-21,23-24H2,1-2H3,(H,35,38)(H,36,39)/t29-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24466

(1-(2-hydroxy-2-methylpropyl)-N-{5-[(7-methoxyquino...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CC(C)(C)O)n(-c5ccccc5)c4=O)nc3)ccnc2c1 Show InChI InChI=1S/C30H29N5O5/c1-19-27(29(37)35(20-8-6-5-7-9-20)34(19)18-30(2,3)38)28(36)33-26-13-11-22(17-32-26)40-25-14-15-31-24-16-21(39-4)10-12-23(24)25/h5-17,38H,18H2,1-4H3,(H,32,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177010
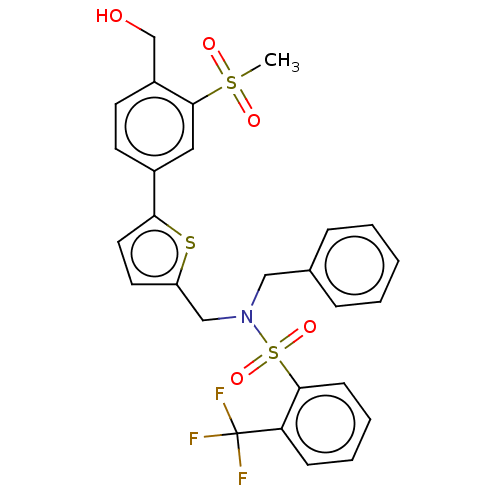
(CHEMBL3814006)Show SMILES CS(=O)(=O)c1cc(ccc1CO)-c1ccc(CN(Cc2ccccc2)S(=O)(=O)c2ccccc2C(F)(F)F)s1 Show InChI InChI=1S/C27H24F3NO5S3/c1-38(33,34)26-15-20(11-12-21(26)18-32)24-14-13-22(37-24)17-31(16-19-7-3-2-4-8-19)39(35,36)25-10-6-5-9-23(25)27(28,29)30/h2-15,32H,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50108857
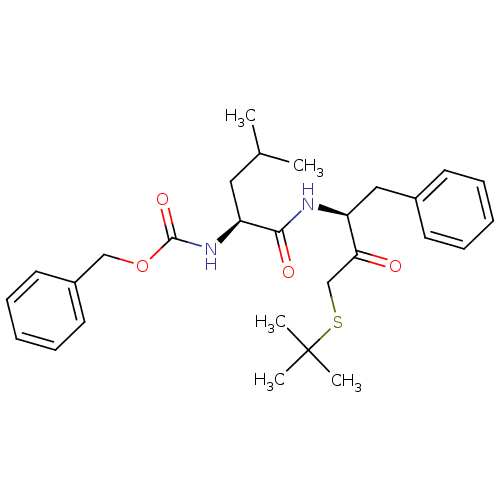
(CHEMBL160000 | [1-(1-Benzyl-3-tert-butylsulfanyl-2...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)CSC(C)(C)C Show InChI InChI=1S/C28H38N2O4S/c1-20(2)16-24(30-27(33)34-18-22-14-10-7-11-15-22)26(32)29-23(17-21-12-8-6-9-13-21)25(31)19-35-28(3,4)5/h6-15,20,23-24H,16-19H2,1-5H3,(H,29,32)(H,30,33)/t23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24464
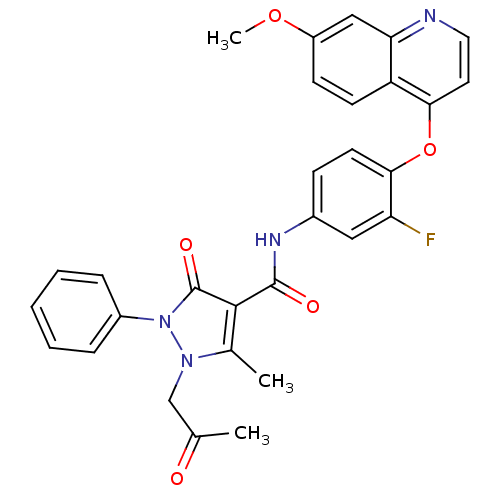
(N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CC(C)=O)n(-c5ccccc5)c4=O)cc3F)ccnc2c1 Show InChI InChI=1S/C30H25FN4O5/c1-18(36)17-34-19(2)28(30(38)35(34)21-7-5-4-6-8-21)29(37)33-20-9-12-27(24(31)15-20)40-26-13-14-32-25-16-22(39-3)10-11-23(25)26/h4-16H,17H2,1-3H3,(H,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen
| Assay Description
In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... |
J Med Chem 51: 3688-91 (2008)
Article DOI: 10.1021/jm800401t
BindingDB Entry DOI: 10.7270/Q2FF3QNG |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146493
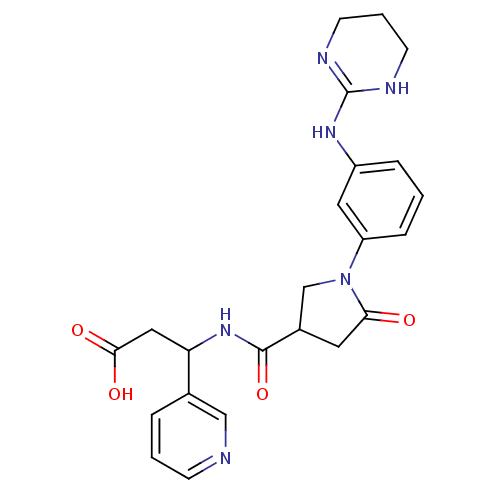
(3-({5-Oxo-1-[3-(1,4,5,6-tetrahydro-pyrimidin-2-yla...)Show SMILES OC(=O)CC(NC(=O)C1CN(C(=O)C1)c1cccc(NC2=NCCCN2)c1)c1cccnc1 |t:21| Show InChI InChI=1S/C23H26N6O4/c30-20-10-16(22(33)28-19(12-21(31)32)15-4-2-7-24-13-15)14-29(20)18-6-1-5-17(11-18)27-23-25-8-3-9-26-23/h1-2,4-7,11,13,16,19H,3,8-10,12,14H2,(H,28,33)(H,31,32)(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards vitronectin receptor (AlphaV-beta3 integrin). |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
Menin
(Homo sapiens (Human)) | BDBM50516571

(CHEMBL4592636 | US10899738, Cpd. No 50)Show SMILES O=S(=O)(c1ccncc1)c1ccc(cc1)N1CC(CN2CCC(CC2)C2(CN(CC3CC3)Cc3ccccc23)C2CCCC2)C1 Show InChI InChI=1S/C38H48N4O2S/c43-45(44,36-15-19-39-20-16-36)35-13-11-34(12-14-35)42-25-30(26-42)24-40-21-17-33(18-22-40)38(32-6-2-3-7-32)28-41(23-29-9-10-29)27-31-5-1-4-8-37(31)38/h1,4-5,8,11-16,19-20,29-30,32-33H,2-3,6-7,9-10,17-18,21-28H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAM-probe binding to Menin (unknown origin) after 60 mins by fluorescence polarization competitive binding assay |
J Med Chem 62: 6015-6034 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00021
BindingDB Entry DOI: 10.7270/Q2P84G8G |
More data for this
Ligand-Target Pair | |
Menin
(Homo sapiens (Human)) | BDBM50516570
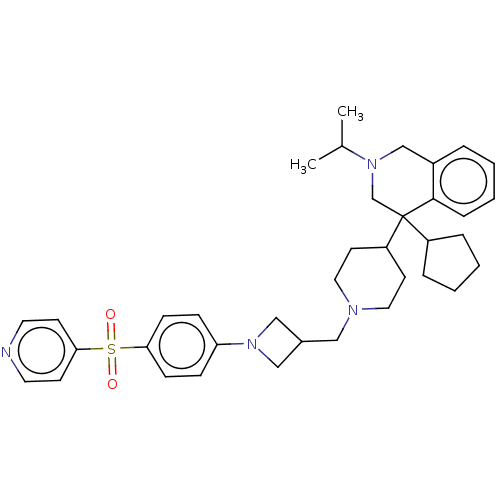
(CHEMBL4551865 | US10899738, Cpd. No 48)Show SMILES CC(C)N1Cc2ccccc2C(C1)(C1CCCC1)C1CCN(CC2CN(C2)c2ccc(cc2)S(=O)(=O)c2ccncc2)CC1 Show InChI InChI=1S/C37H48N4O2S/c1-28(2)41-26-30-7-3-6-10-36(30)37(27-41,31-8-4-5-9-31)32-17-21-39(22-18-32)23-29-24-40(25-29)33-11-13-34(14-12-33)44(42,43)35-15-19-38-20-16-35/h3,6-7,10-16,19-20,28-29,31-32H,4-5,8-9,17-18,21-27H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAM-probe binding to Menin (unknown origin) after 60 mins by fluorescence polarization competitive binding assay |
J Med Chem 62: 6015-6034 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00021
BindingDB Entry DOI: 10.7270/Q2P84G8G |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50108846

(CHEMBL161038 | [1-(3-tert-Butylsulfanyl-2-oxo-1-ph...)Show SMILES CC(C)(C)SCC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1cccnc1 Show InChI InChI=1S/C31H37N3O4S/c1-31(2,3)39-22-28(35)26(17-16-23-11-6-4-7-12-23)33-29(36)27(19-24-13-8-5-9-14-24)34-30(37)38-21-25-15-10-18-32-20-25/h4-15,18,20,26-27H,16-17,19,21-22H2,1-3H3,(H,33,36)(H,34,37)/t26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(Homo sapiens (Human)) | BDBM85168

(M32)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:111.115,131.138,95.103,84.88,68.80,52.59,20.28,4.4,2.2,145.150,161.166,162.169,179.184,188.193,199.204,wD:115.131,103.111,89.95,60.67,41.42,30.39,8.17,137.142,153.158,154.160,168.173,(13.69,11.51,;13.72,9.97,;12.46,9.14,;11.11,9.89,;12.54,7.63,;11.23,6.8,;9.89,7.48,;9.85,9.02,;8.62,6.65,;8.66,5.14,;10.01,4.43,;10.12,2.93,;11.51,2.18,;12.74,3.05,;14.16,2.37,;12.66,4.59,;11.27,5.26,;7.28,7.36,;5.97,6.57,;5.97,4.98,;4.67,7.32,;4.63,8.82,;5.93,9.65,;7.36,9.02,;8.39,10.12,;7.71,11.47,;6.13,11.19,;3.36,6.53,;2.02,7.16,;2.02,8.62,;.63,6.37,;.59,4.82,;1.94,3.99,;1.94,2.45,;3.24,1.66,;3.2,.08,;4.55,-.67,;1.86,-.67,;-.71,7.2,;-2.06,6.49,;-3.56,7.12,;-2.06,5.02,;-.75,4.11,;-1.19,2.65,;-2.69,2.61,;-3.16,4.03,;-4.63,4.43,;-4.98,5.93,;-5.77,3.36,;-7.24,3.84,;-8.27,2.73,;-7.91,1.19,;-9.73,3.08,;-10.12,4.59,;-9.02,5.7,;-9.41,7.2,;-7.51,5.26,;-10.84,2.06,;-12.34,2.37,;-12.66,3.8,;-13.41,1.27,;-13.05,-.2,;-11.59,-.59,;-10.48,.51,;-11.15,-2.06,;-14.87,1.7,;-15.98,.67,;-15.62,-.87,;-17.56,1.07,;-17.92,2.61,;-16.85,3.64,;-15.35,3.28,;-14.24,4.39,;-14.6,5.85,;-13.49,6.96,;-16.1,6.29,;-17.24,5.22,;-18.59,0,;-20.05,.44,;-20.45,1.9,;-21.12,-.67,;-22.54,-.28,;-23.65,-1.34,;-23.26,-2.81,;-25.19,-.99,;-25.55,.55,;-26.26,-2.02,;-27.73,-1.62,;-28.08,-.16,;-28.83,-2.69,;-28.44,-4.15,;-26.97,-4.63,;-30.3,-2.25,;-31.44,-3.28,;-31.09,-4.71,;-32.95,-2.81,;-33.3,-1.34,;-32.19,-.28,;-30.73,-.71,;-32.55,1.23,;-34.05,-3.92,;-35.56,-3.56,;-35.87,-2.14,;-36.63,-4.71,;-36.27,-6.13,;-34.8,-6.57,;-33.7,-5.5,;-34.41,-8.03,;-38.09,-4.23,;-39.23,-5.3,;-38.84,-6.76,;-40.7,-4.87,;-41.85,-5.93,;-43.27,-5.54,;-43.66,-4.03,;-44.46,-6.6,;-44.18,-8.11,;-42.64,-8.54,;-41.45,-7.59,;-40.11,-8.39,;-40.54,-9.93,;-39.71,-11.27,;-40.46,-12.66,;-41.96,-12.66,;-42.87,-11.35,;-42.12,-10.05,;-45.92,-6.13,;-47.07,-7.2,;-46.71,-8.66,;-48.57,-6.76,;-49.68,-7.83,;-41.05,-3.36,;-42.6,-2.89,;-39.91,-2.29,;13.88,6.88,;13.88,5.42,;15.19,7.67,;16.53,6.88,;16.53,5.38,;17.92,4.67,;19.18,5.46,;17.96,3.12,;17.84,7.71,;17.84,9.18,;19.18,7.04,;20.45,7.75,;20.49,9.25,;21.79,10.09,;21.83,11.59,;23.14,9.25,;21.79,7.04,;21.79,5.46,;23.14,7.75,;24.44,7,;24.48,5.5,;23.18,4.71,;25.79,4.75,;25.83,3.24,;25.71,7.75,;25.71,9.29,;27.01,6.92,;28.32,7.71,;28.32,9.25,;27.01,10.05,;29.66,10.01,;29.66,6.96,;29.66,5.46,;30.97,7.67,;32.31,6.88,;32.27,5.38,;33.62,4.59,;33.62,3.08,;34.88,2.37,;34.92,.83,;36.27,.04,;33.62,0,;33.66,7.63,;33.66,9.1,;35,6.88,;36.27,7.67,;36.27,9.18,;37.53,9.97,;37.53,11.47,;38.8,12.26,;36.19,12.26,;37.61,6.92,;37.61,5.38,;38.88,7.75,;40.18,7.04,;40.26,5.5,;41.61,4.71,;41.69,3.16,;42.99,2.49,;43.07,.95,;44.46,.16,;41.77,.04,;41.53,7.87,;41.49,9.25,;42.91,7.24,;44.22,7.99,;44.22,9.57,;45.56,10.32,;45.6,11.87,;46.99,12.66,;48.33,11.83,;49.68,12.58,;48.29,10.32,;46.91,9.53,;45.52,7.24,;46.79,8.07,;45.52,5.66,)| Show InChI InChI=1S/C136H209N41O34/c1-16-70(11)108(130(208)171-99(58-104(140)186)123(201)166-93(51-69(9)10)125(203)174-109(71(12)17-2)131(209)176-111(74(15)180)132(210)162-87(28-22-46-151-136(146)147)115(193)160-88(42-43-102(138)184)118(196)159-85(26-20-44-149-134(142)143)116(194)163-89(112(141)190)52-75-30-36-80(181)37-31-75)173-126(204)95(54-77-34-40-82(183)41-35-77)167-122(200)97(56-79-61-148-65-155-79)168-117(195)86(27-21-45-150-135(144)145)161-129(207)101-29-23-47-177(101)107(189)63-154-114(192)90(48-66(3)4)164-119(197)91(49-67(5)6)165-121(199)94(53-76-32-38-81(182)39-33-76)158-106(188)62-153-113(191)72(13)156-128(206)100(64-178)172-124(202)98(57-103(139)185)169-120(198)92(50-68(7)8)170-133(211)110(73(14)179)175-127(205)96(157-105(187)59-137)55-78-60-152-84-25-19-18-24-83(78)84/h18-19,24-25,30-41,60-61,65-74,85-101,108-111,152,178-183H,16-17,20-23,26-29,42-59,62-64,137H2,1-15H3,(H2,138,184)(H2,139,185)(H2,140,186)(H2,141,190)(H,148,155)(H,153,191)(H,154,192)(H,156,206)(H,157,187)(H,158,188)(H,159,196)(H,160,193)(H,161,207)(H,162,210)(H,163,194)(H,164,197)(H,165,199)(H,166,201)(H,167,200)(H,168,195)(H,169,198)(H,170,211)(H,171,208)(H,172,202)(H,173,204)(H,174,203)(H,175,205)(H,176,209)(H4,142,143,149)(H4,144,145,150)(H4,146,147,151)/t70-,71-,72-,73+,74+,85+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,108-,109-,110-,111-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data






















































