 Found 34 hits with Last Name = 'imming' and Initial = 'p'
Found 34 hits with Last Name = 'imming' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50054470
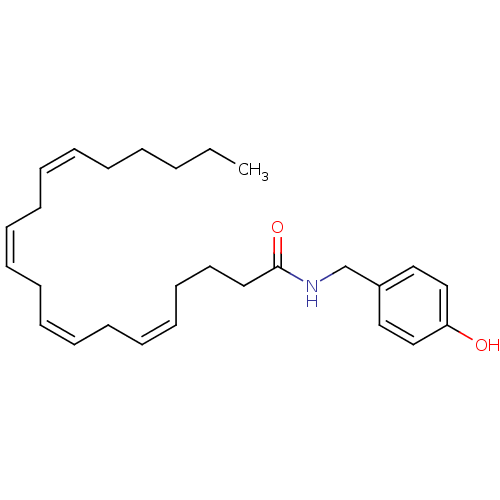
((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 4-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCc1ccc(O)cc1 Show InChI InChI=1S/C27H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(30)28-24-25-20-22-26(29)23-21-25/h6-7,9-10,12-13,15-16,20-23,29H,2-5,8,11,14,17-19,24H2,1H3,(H,28,30)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50054470

((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid 4-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCc1ccc(O)cc1 Show InChI InChI=1S/C27H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(30)28-24-25-20-22-26(29)23-21-25/h6-7,9-10,12-13,15-16,20-23,29H,2-5,8,11,14,17-19,24H2,1H3,(H,28,30)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50246638

(CHEMBL472897 | N-(1H-indazol-5-yl)icosa-5,8,11,14-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc2[nH]ncc2c1 Show InChI InChI=1S/C27H37N3O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(31)29-25-20-21-26-24(22-25)23-28-30-26/h6-7,9-10,12-13,15-16,20-23H,2-5,8,11,14,17-19H2,1H3,(H,28,30)(H,29,31)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50246639
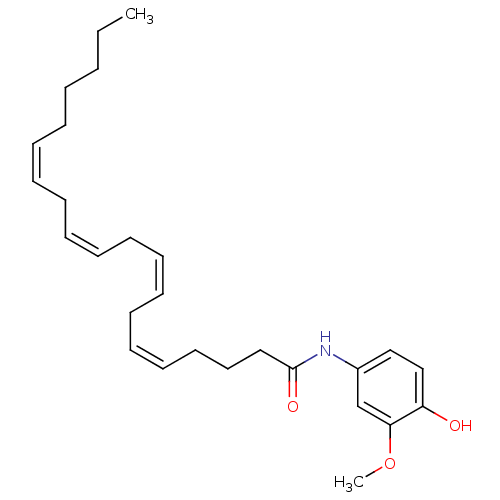
(CHEMBL472898 | N-(4-hydroxy-3-methoxyphenyl)icosa-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc(O)c(OC)c1 Show InChI InChI=1S/C27H39NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-27(30)28-24-21-22-25(29)26(23-24)31-2/h7-8,10-11,13-14,16-17,21-23,29H,3-6,9,12,15,18-20H2,1-2H3,(H,28,30)/b8-7-,11-10-,14-13-,17-16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50246638

(CHEMBL472897 | N-(1H-indazol-5-yl)icosa-5,8,11,14-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc2[nH]ncc2c1 Show InChI InChI=1S/C27H37N3O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-27(31)29-25-20-21-26-24(22-25)23-28-30-26/h6-7,9-10,12-13,15-16,20-23H,2-5,8,11,14,17-19H2,1H3,(H,28,30)(H,29,31)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50054471
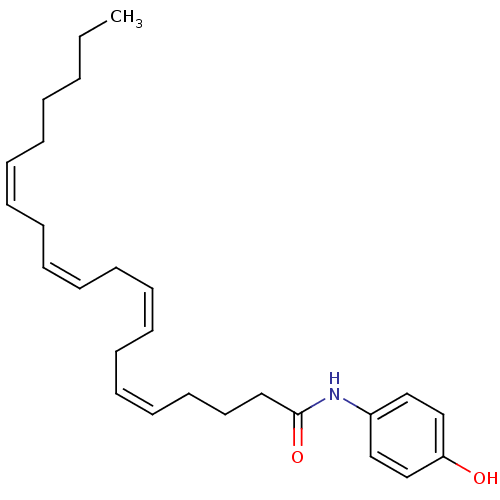
((5Z,8Z)-Icosa-5,8,11,14-tetraenoic acid (4-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc(O)cc1 Show InChI InChI=1S/C26H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-26(29)27-24-20-22-25(28)23-21-24/h6-7,9-10,12-13,15-16,20-23,28H,2-5,8,11,14,17-19H2,1H3,(H,27,29)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50246639

(CHEMBL472898 | N-(4-hydroxy-3-methoxyphenyl)icosa-...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc(O)c(OC)c1 Show InChI InChI=1S/C27H39NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-27(30)28-24-21-22-25(29)26(23-24)31-2/h7-8,10-11,13-14,16-17,21-23,29H,3-6,9,12,15,18-20H2,1-2H3,(H,28,30)/b8-7-,11-10-,14-13-,17-16- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50054471

((5Z,8Z)-Icosa-5,8,11,14-tetraenoic acid (4-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc(O)cc1 Show InChI InChI=1S/C26H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-26(29)27-24-20-22-25(28)23-21-24/h6-7,9-10,12-13,15-16,20-23,28H,2-5,8,11,14,17-19H2,1H3,(H,27,29)/b7-6-,10-9-,13-12-,16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50360023

(CHEMBL1928337)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C31H43N3O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23-26-29(35)32-30-27(2)33(3)34(31(30)36)28-24-21-20-22-25-28/h8-9,11-12,14-15,17-18,20-22,24-25H,4-7,10,13,16,19,23,26H2,1-3H3,(H,32,35)/b9-8-,12-11-,15-14-,18-17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK cells |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50360022
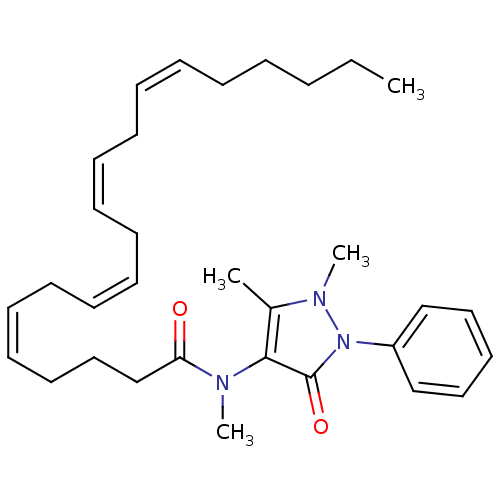
(CHEMBL1928336)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N(C)c1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C32H45N3O2/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-27-30(36)33(3)31-28(2)34(4)35(32(31)37)29-25-22-21-23-26-29/h9-10,12-13,15-16,18-19,21-23,25-26H,5-8,11,14,17,20,24,27H2,1-4H3/b10-9-,13-12-,16-15-,19-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50360023

(CHEMBL1928337)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C31H43N3O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23-26-29(35)32-30-27(2)33(3)34(31(30)36)28-24-21-20-22-25-28/h8-9,11-12,14-15,17-18,20-22,24-25H,4-7,10,13,16,19,23,26H2,1-3H3,(H,32,35)/b9-8-,12-11-,15-14-,18-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK cells |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50360022

(CHEMBL1928336)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N(C)c1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C32H45N3O2/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-27-30(36)33(3)31-28(2)34(4)35(32(31)37)29-25-22-21-23-26-29/h9-10,12-13,15-16,18-19,21-23,25-26H,5-8,11,14,17,20,24,27H2,1-4H3/b10-9-,13-12-,16-15-,19-18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK cells |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50396754
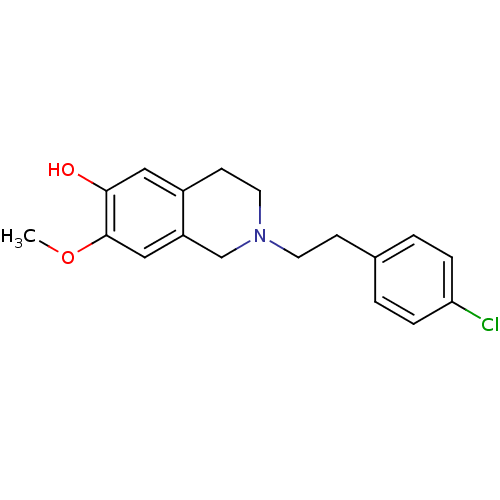
(CHEMBL2172347)Show InChI InChI=1S/C18H20ClNO2/c1-22-18-11-15-12-20(9-7-14(15)10-17(18)21)8-6-13-2-4-16(19)5-3-13/h2-5,10-11,21H,6-9,12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... |
J Med Chem 55: 7614-22 (2012)
Article DOI: 10.1021/jm3006096
BindingDB Entry DOI: 10.7270/Q2W95B9V |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50396753
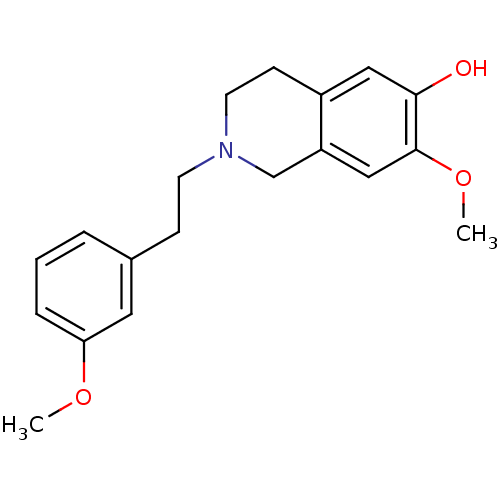
(CHEMBL2172349)Show InChI InChI=1S/C19H23NO3/c1-22-17-5-3-4-14(10-17)6-8-20-9-7-15-11-18(21)19(23-2)12-16(15)13-20/h3-5,10-12,21H,6-9,13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... |
J Med Chem 55: 7614-22 (2012)
Article DOI: 10.1021/jm3006096
BindingDB Entry DOI: 10.7270/Q2W95B9V |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50170646

(4-(2-{4-[(E)-3-(4-Chloro-phenyl)-allyl]-piperazin-...)Show SMILES OC(=O)c1ccc(CCN2CCN(C\C=C\c3ccc(Cl)cc3)CC2)cc1 Show InChI InChI=1S/C22H25ClN2O2/c23-21-9-5-18(6-10-21)2-1-12-24-14-16-25(17-15-24)13-11-19-3-7-20(8-4-19)22(26)27/h1-10H,11-17H2,(H,26,27)/b2-1+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... |
J Med Chem 55: 7614-22 (2012)
Article DOI: 10.1021/jm3006096
BindingDB Entry DOI: 10.7270/Q2W95B9V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50583542
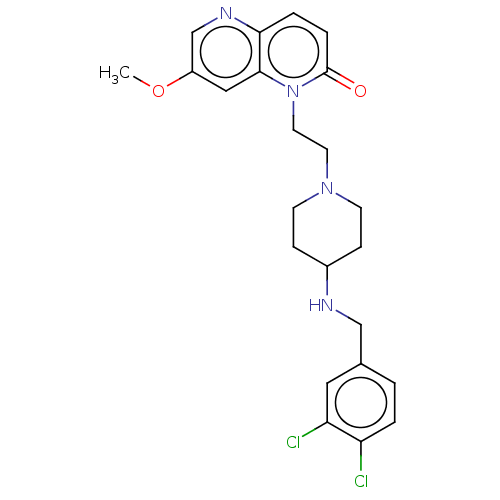
(CHEMBL5074804)Show SMILES COc1cnc2ccc(=O)n(CCN3CCC(CC3)NCc3ccc(Cl)c(Cl)c3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00549
BindingDB Entry DOI: 10.7270/Q2DV1PSX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50360022

(CHEMBL1928336)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N(C)c1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C32H45N3O2/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-27-30(36)33(3)31-28(2)34(4)35(32(31)37)29-25-22-21-23-26-29/h9-10,12-13,15-16,18-19,21-23,25-26H,5-8,11,14,17,20,24,27H2,1-4H3/b10-9-,13-12-,16-15-,19-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of TRPV1 |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50396752
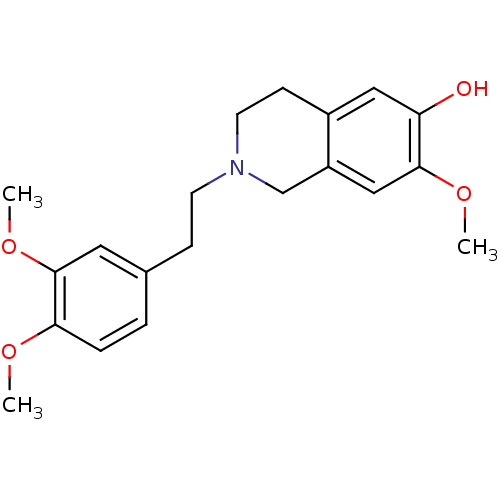
(CHEMBL2172350)Show InChI InChI=1S/C20H25NO4/c1-23-18-5-4-14(10-20(18)25-3)6-8-21-9-7-15-11-17(22)19(24-2)12-16(15)13-21/h4-5,10-12,22H,6-9,13H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... |
J Med Chem 55: 7614-22 (2012)
Article DOI: 10.1021/jm3006096
BindingDB Entry DOI: 10.7270/Q2W95B9V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PknB
(Mycobacterium tuberculosis) | BDBM50461791

(CHEMBL4226192)Show InChI InChI=1S/C12H12O4/c1-15-10-5-3-9(4-6-10)11(13)7-8-12(14)16-2/h3-8H,1-2H3/b8-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PknB (279 residues) expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in PknB autophospho... |
Bioorg Med Chem 26: 3166-3190 (2018)
Article DOI: 10.1016/j.bmc.2018.04.045
BindingDB Entry DOI: 10.7270/Q22R3V9B |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50360022

(CHEMBL1928336)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N(C)c1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C32H45N3O2/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-27-30(36)33(3)31-28(2)34(4)35(32(31)37)29-25-22-21-23-26-29/h9-10,12-13,15-16,18-19,21-23,25-26H,5-8,11,14,17,20,24,27H2,1-4H3/b10-9-,13-12-,16-15-,19-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 in C57BL/6 mouse brain homogenate |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50360022

(CHEMBL1928336)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N(C)c1c(C)n(C)n(-c2ccccc2)c1=O Show InChI InChI=1S/C32H45N3O2/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-27-30(36)33(3)31-28(2)34(4)35(32(31)37)29-25-22-21-23-26-29/h9-10,12-13,15-16,18-19,21-23,25-26H,5-8,11,14,17,20,24,27H2,1-4H3/b10-9-,13-12-,16-15-,19-18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in C57BL/6 mouse brain homogenate |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50246684
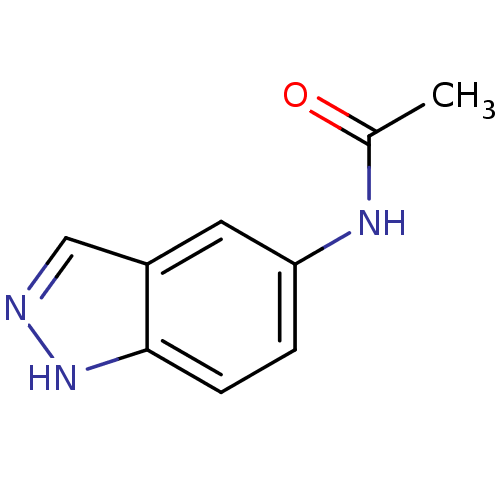
(CHEMBL503641 | N-(1H-indazol-5-yl)acetamide)Show InChI InChI=1S/C9H9N3O/c1-6(13)11-8-2-3-9-7(4-8)5-10-12-9/h2-5H,1H3,(H,10,12)(H,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50246684

(CHEMBL503641 | N-(1H-indazol-5-yl)acetamide)Show InChI InChI=1S/C9H9N3O/c1-6(13)11-8-2-3-9-7(4-8)5-10-12-9/h2-5H,1H3,(H,10,12)(H,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50360021

(CHEMBL1164701)Show InChI InChI=1S/C12H15N3O/c1-9-11(13-2)12(16)15(14(9)3)10-7-5-4-6-8-10/h4-8,13H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 in C57BL/6 mouse brain homogenate |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50246685
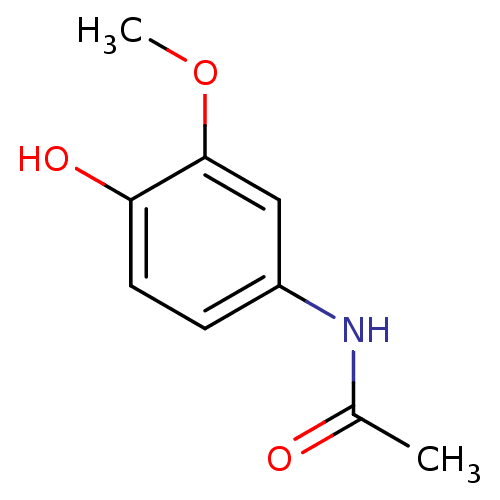
(CHEMBL498882 | N-(4-hydroxy-3-methoxyphenyl)acetam...)Show InChI InChI=1S/C9H11NO3/c1-6(11)10-7-3-4-8(12)9(5-7)13-2/h3-5,12H,1-2H3,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50246685

(CHEMBL498882 | N-(4-hydroxy-3-methoxyphenyl)acetam...)Show InChI InChI=1S/C9H11NO3/c1-6(11)10-7-3-4-8(12)9(5-7)13-2/h3-5,12H,1-2H3,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM26197
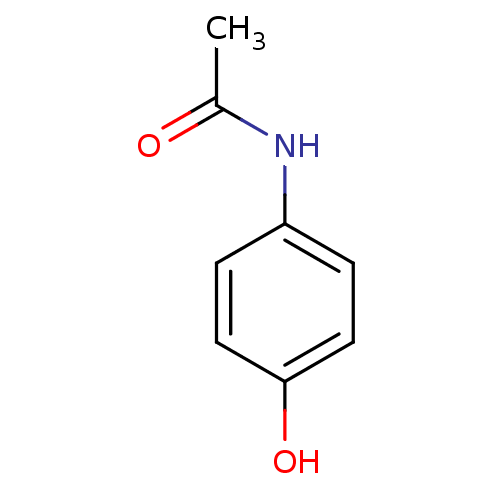
(CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...)Show InChI InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM26197

(CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...)Show InChI InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 2 in human whole blood assessed as prostaglandin H2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50583541
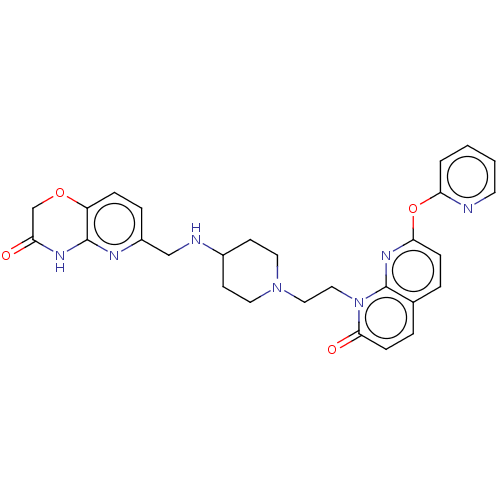
(CHEMBL5085925)Show SMILES O=C1COc2ccc(CNC3CCN(CCn4c5nc(Oc6ccccn6)ccc5ccc4=O)CC3)nc2N1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00549
BindingDB Entry DOI: 10.7270/Q2DV1PSX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50246685

(CHEMBL498882 | N-(4-hydroxy-3-methoxyphenyl)acetam...)Show InChI InChI=1S/C9H11NO3/c1-6(11)10-7-3-4-8(12)9(5-7)13-2/h3-5,12H,1-2H3,(H,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universitaet
Curated by ChEMBL
| Assay Description
Inhibition of cyclooxygenase 1 in human whole blood assessed as thromboxane B2 level |
J Med Chem 51: 7800-5 (2008)
Article DOI: 10.1021/jm800807k
BindingDB Entry DOI: 10.7270/Q2ST7PPX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50360021

(CHEMBL1164701)Show InChI InChI=1S/C12H15N3O/c1-9-11(13-2)12(16)15(14(9)3)10-7-5-4-6-8-10/h4-8,13H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in C57BL/6 mouse brain homogenate |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM85515
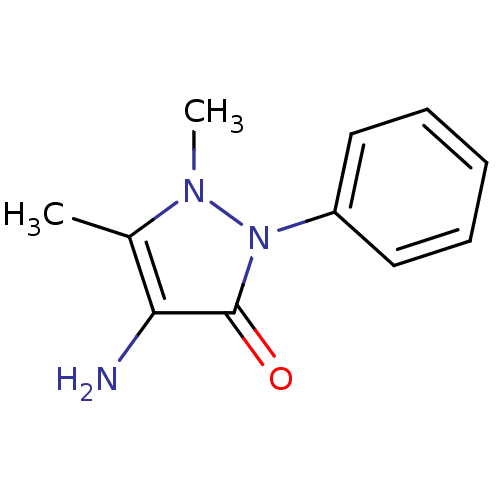
(Ampyrone | CAS_83-07-8 | EN300-17058 | NSC_2151 | ...)Show InChI InChI=1S/C11H13N3O/c1-8-10(12)11(15)14(13(8)2)9-6-4-3-5-7-9/h3-7H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 in C57BL/6 mouse brain homogenate |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM85515

(Ampyrone | CAS_83-07-8 | EN300-17058 | NSC_2151 | ...)Show InChI InChI=1S/C11H13N3O/c1-8-10(12)11(15)14(13(8)2)9-6-4-3-5-7-9/h3-7H,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in C57BL/6 mouse brain homogenate |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50054471

((5Z,8Z)-Icosa-5,8,11,14-tetraenoic acid (4-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)Nc1ccc(O)cc1 Show InChI InChI=1S/C26H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-26(29)27-24-20-22-25(28)23-21-24/h6-7,9-10,12-13,15-16,20-23,28H,2-5,8,11,14,17-19H2,1H3,(H,27,29)/b7-6-,10-9-,13-12-,16-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Philipps-Universit£t
Curated by ChEMBL
| Assay Description
Agonist activity at TRPV1 |
Bioorg Med Chem 20: 101-7 (2011)
Article DOI: 10.1016/j.bmc.2011.11.028
BindingDB Entry DOI: 10.7270/Q28S4QBQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data