

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Mus musculus (mouse)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283355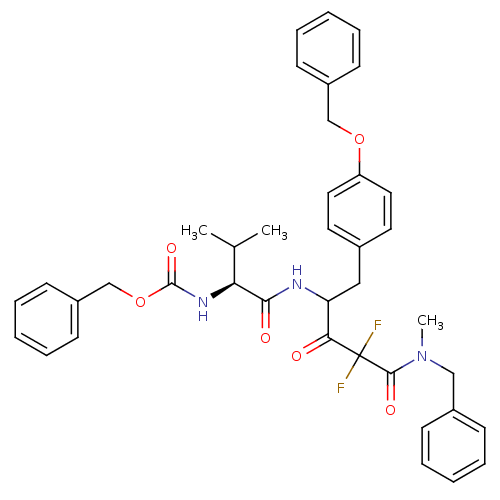 (CHEMBL311418 | {(S)-1-[3-(Benzyl-methyl-carbamoyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283358 (CHEMBL79799 | [(S)-1-(1-Benzyl-3-benzylcarbamoyl-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131550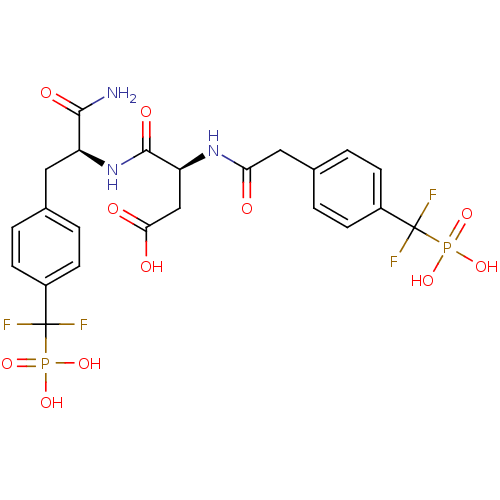 ((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283362 (CHEMBL311049 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037811 (CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was determined against HIV-1 protease | Bioorg Med Chem Lett 4: 1213-1218 (1994) Article DOI: 10.1016/S0960-894X(01)80332-2 BindingDB Entry DOI: 10.7270/Q24Q7TXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037811 (CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093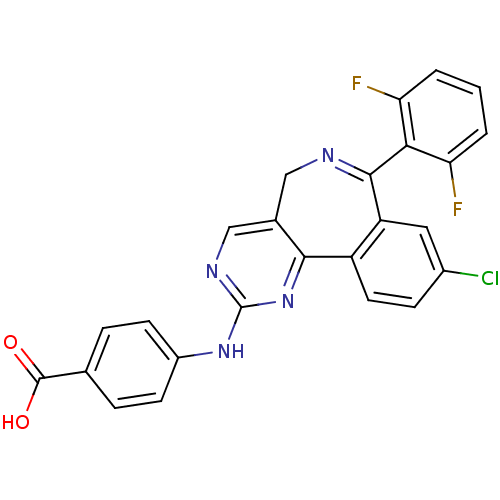 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282534 (CHEMBL21095 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3-((R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was determined against HIV-1 protease | Bioorg Med Chem Lett 4: 1213-1218 (1994) Article DOI: 10.1016/S0960-894X(01)80332-2 BindingDB Entry DOI: 10.7270/Q24Q7TXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13976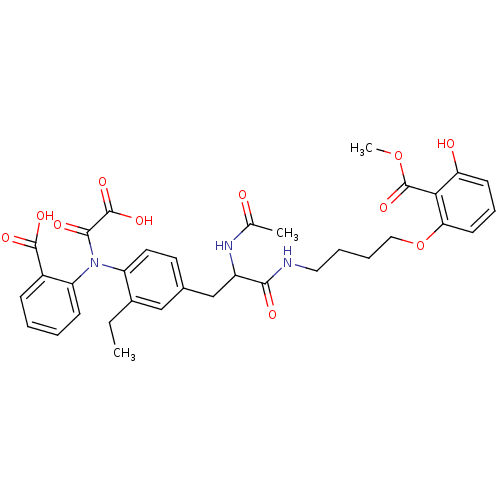 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131550 ((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131555 (2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283359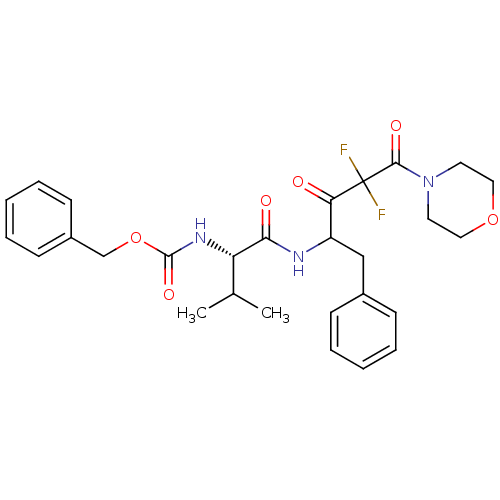 (CHEMBL83002 | [(S)-1-(1-Benzyl-3,3-difluoro-4-morp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131555 (2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM15819 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131553 (2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-nitro-pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131545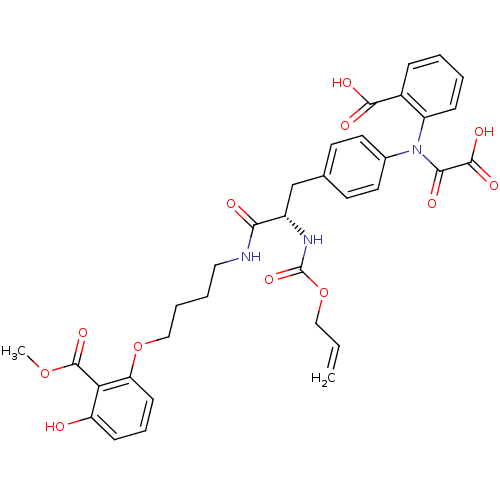 ((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131553 (2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-nitro-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13975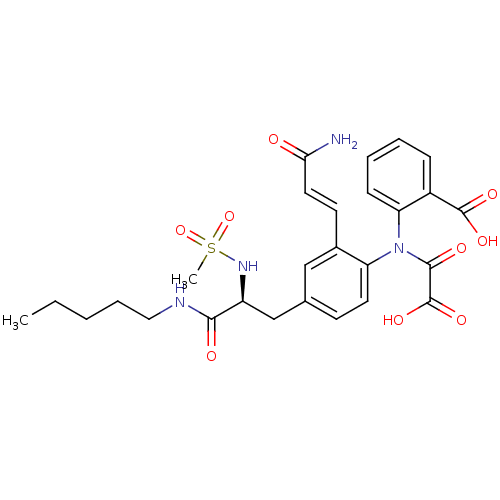 (2-({2-[(1E)-2-carbamoyleth-1-en-1-yl]-4-[(2S)-2-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13975 (2-({2-[(1E)-2-carbamoyleth-1-en-1-yl]-4-[(2S)-2-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131546 (2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-methylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM15819 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131544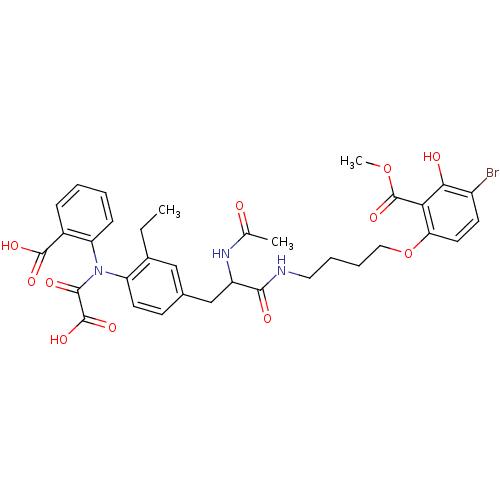 (6-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131545 ((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131548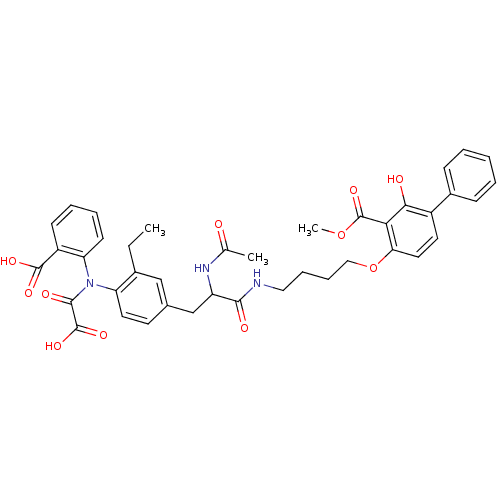 (4-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131546 (2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-methylcarba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131548 (4-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131551 (2-carboxy(2-ethyl-4-{2-methylcarboxamido-2-[4-(2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13971 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13953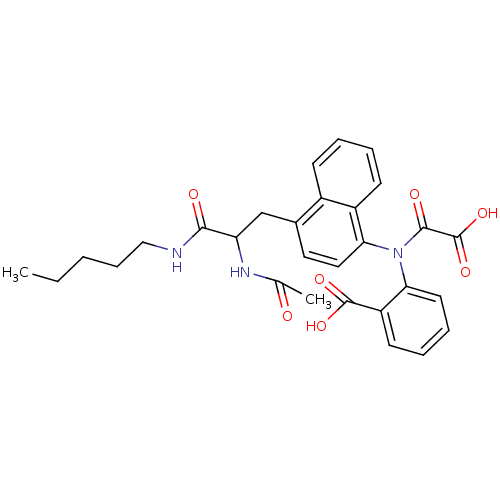 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13953 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13971 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13972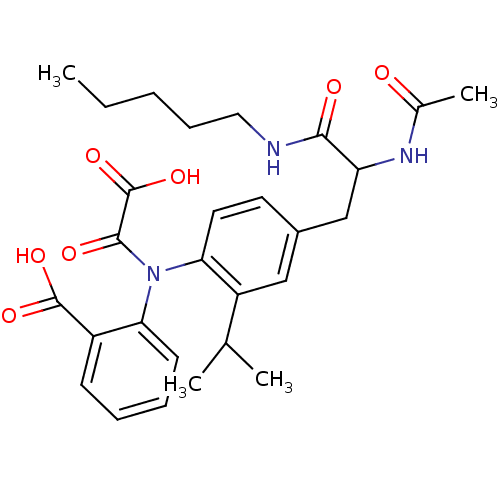 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13972 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13973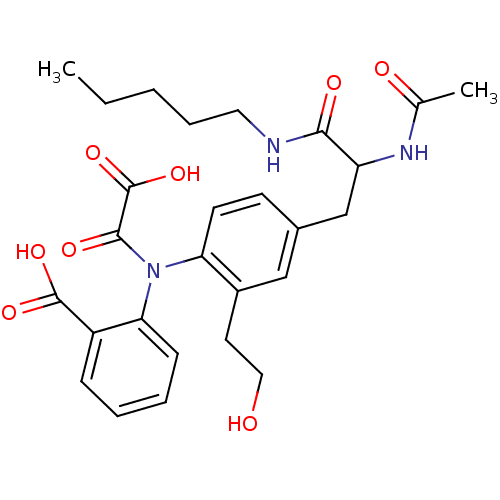 (2-({4-[2-acetamido-2-(pentylcarbamoyl)ethyl]-2-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13973 (2-({4-[2-acetamido-2-(pentylcarbamoyl)ethyl]-2-(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131551 (2-carboxy(2-ethyl-4-{2-methylcarboxamido-2-[4-(2-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13974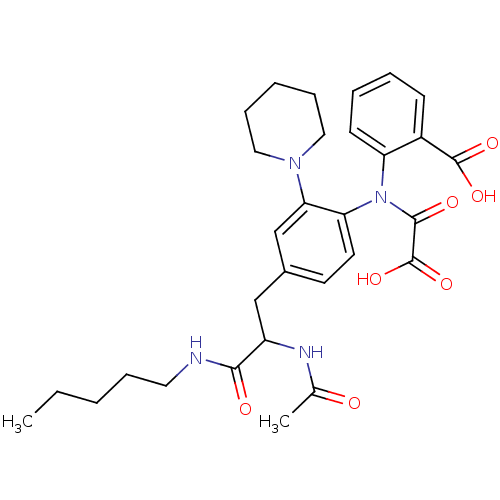 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| rRNA adenine N-6-methyltransferase (Streptococcus pneumoniae) | BDBM50081297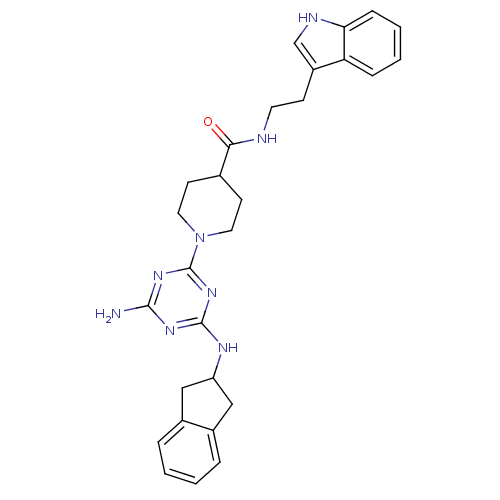 (1-[4-Amino-6-(indan-2-ylamino)-[1,3,5]triazin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against ErmAM methylase | J Med Chem 42: 3852-9 (1999) BindingDB Entry DOI: 10.7270/Q21Z43MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50131544 (6-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against T cell protein tyrosine phosphatase | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 3 catalytic subunit alpha (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against (calcineurin) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against SH-domain containing phosphotyrosine phosphatase-2 (Tyrosine phosphatase SHP2) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against CD45 tyrosine phosphatase was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| rRNA adenine N-6-methyltransferase (Streptococcus pneumoniae) | BDBM50081299 (CHEMBL125249 | N-(5-Fluoro-indan-2-yl)-6-piperidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against ErmAM methylase | J Med Chem 42: 3852-9 (1999) BindingDB Entry DOI: 10.7270/Q21Z43MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25 degree C (Cdc25 C) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| rRNA adenine N-6-methyltransferase (Streptococcus pneumoniae) | BDBM50081279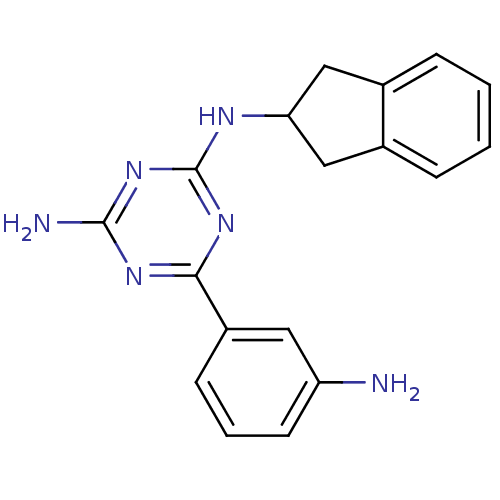 (6-(3-Amino-phenyl)-N-indan-2-yl-[1,3,5]triazine-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against ErmAM methylase | J Med Chem 42: 3852-9 (1999) BindingDB Entry DOI: 10.7270/Q21Z43MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| rRNA adenine N-6-methyltransferase (Streptococcus pneumoniae) | BDBM50081285 (CHEMBL124572 | N-(5-Methoxy-indan-2-yl)-6-piperidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against ErmAM methylase | J Med Chem 42: 3852-9 (1999) BindingDB Entry DOI: 10.7270/Q21Z43MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 604 total ) | Next | Last >> |