 Found 3602 hits with Last Name = 'ju' and Initial = 'c'
Found 3602 hits with Last Name = 'ju' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin
(GUINEA PIG) | BDBM85050
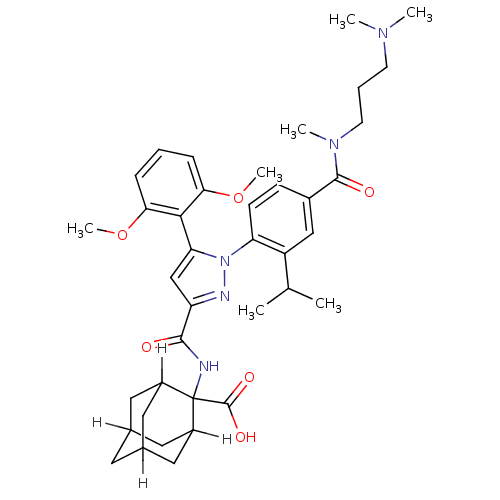
(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 802-12 (1997)
BindingDB Entry DOI: 10.7270/Q2RR1WR1 |
More data for this
Ligand-Target Pair | |
Neurotensin/neuromedin N
(Homo sapiens (Human)) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 802-12 (1997)
BindingDB Entry DOI: 10.7270/Q2RR1WR1 |
More data for this
Ligand-Target Pair | |
Neurotensin
(GUINEA PIG) | BDBM50248034
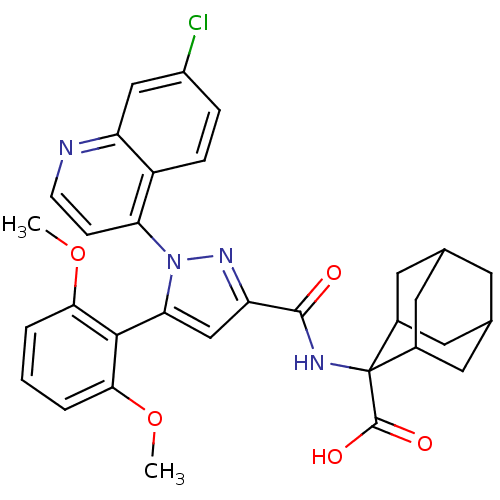
(2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:38:37:35:31.32.33,39:29:35:31.32.33,28:29:31.38.32:36.34.35,THB:38:32:29.37.36:35,39:29:31.38.32:36.34.35,28:29:35:31.32.33,33:32:29:36.34.35,33:34:29:31.38.32,(-7.63,.4,;-6.3,-.36,;-6.29,-1.9,;-7.62,-2.68,;-7.62,-4.22,;-6.29,-4.99,;-4.95,-4.22,;-3.61,-4.99,;-3.61,-6.53,;-4.95,-2.67,;-3.63,-1.9,;-2.22,-2.52,;-1.19,-1.37,;-1.97,-.04,;-3.47,-.36,;-4.62,.66,;-6.08,.17,;-7.22,1.19,;-6.91,2.71,;-5.45,3.19,;-5.14,4.69,;-3.68,5.16,;-3.37,6.67,;-2.53,4.14,;-2.85,2.64,;-4.31,2.16,;.35,-1.53,;.98,-2.93,;1.24,-.27,;2.78,-.43,;3.43,1.2,;4.84,1.23,;6.02,2.07,;5.46,3.5,;3.95,3.52,;2.87,2.58,;3.44,1.99,;4.02,.52,;5.54,.51,;3.42,-1.84,;2.51,-3.09,;4.95,-2,)| Show InChI InChI=1S/C32H31ClN4O5/c1-41-27-4-3-5-28(42-2)29(27)26-16-24(36-37(26)25-8-9-34-23-15-21(33)6-7-22(23)25)30(38)35-32(31(39)40)19-11-17-10-18(13-19)14-20(32)12-17/h3-9,15-20H,10-14H2,1-2H3,(H,35,38)(H,39,40) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 802-12 (1997)
BindingDB Entry DOI: 10.7270/Q2RR1WR1 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinant thymidine phosphorylase TP |
Bioorg Med Chem Lett 13: 107-10 (2002)
BindingDB Entry DOI: 10.7270/Q2GM87V9 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50067004
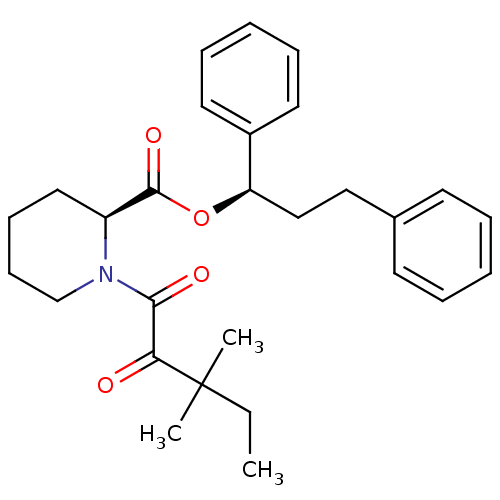
((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-20-12-11-17-23(29)27(32)33-24(22-15-9-6-10-16-22)19-18-21-13-7-5-8-14-21/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wu£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of FKBP12 |
J Med Chem 54: 277-83 (2011)
Article DOI: 10.1021/jm101156y
BindingDB Entry DOI: 10.7270/Q2GF0VG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM520995
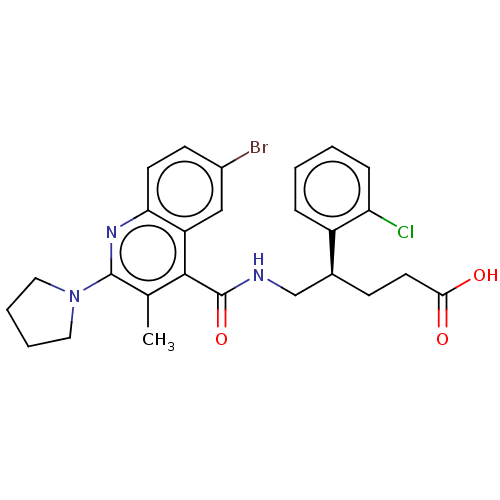
((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50335225

((S)-1-Phenylmethanesulfonyl-piperidine-2-carboxyli...)Show SMILES COc1cc(CCCOC(=O)[C@@H]2CCCCN2S(=O)(=O)Cc2ccccc2)cc(OC)c1OC Show InChI InChI=1S/C25H33NO7S/c1-30-22-16-20(17-23(31-2)24(22)32-3)12-9-15-33-25(27)21-13-7-8-14-26(21)34(28,29)18-19-10-5-4-6-11-19/h4-6,10-11,16-17,21H,7-9,12-15,18H2,1-3H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wu£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of FKBP12 |
J Med Chem 54: 277-83 (2011)
Article DOI: 10.1021/jm101156y
BindingDB Entry DOI: 10.7270/Q2GF0VG2 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50201010
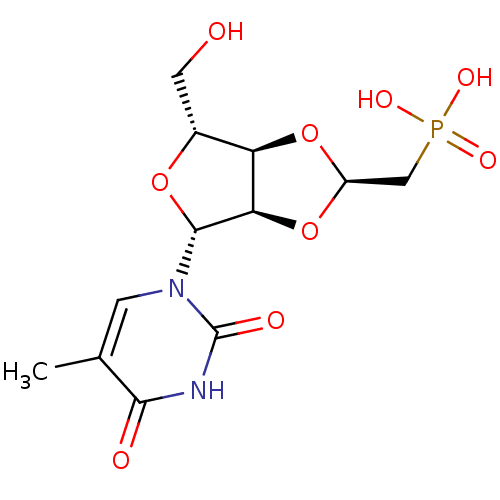
(((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)[C@H]3O[C@@H](CP(O)(O)=O)O[C@@H]23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H17N2O9P/c1-5-2-14(12(17)13-10(5)16)11-9-8(6(3-15)21-11)22-7(23-9)4-24(18,19)20/h2,6-9,11,15H,3-4H2,1H3,(H,13,16,17)(H2,18,19,20)/t6-,7-,8-,9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay |
J Med Chem 49: 7807-15 (2006)
Article DOI: 10.1021/jm060428u
BindingDB Entry DOI: 10.7270/Q2FB52KF |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50201013
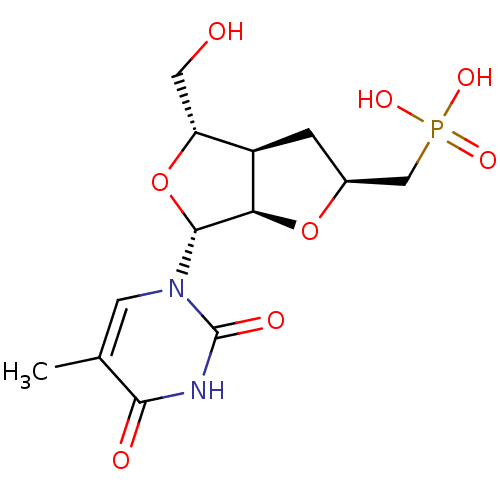
(((2S,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)[C@H]3C[C@@H](CP(O)(O)=O)O[C@@H]23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C13H19N2O8P/c1-6-3-15(13(18)14-11(6)17)12-10-8(9(4-16)23-12)2-7(22-10)5-24(19,20)21/h3,7-10,12,16H,2,4-5H2,1H3,(H,14,17,18)(H2,19,20,21)/t7-,8+,9+,10+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay |
J Med Chem 49: 7807-15 (2006)
Article DOI: 10.1021/jm060428u
BindingDB Entry DOI: 10.7270/Q2FB52KF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032737

(CHEMBL3354718)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)C1=CCOCC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C25H19F2N3O3/c26-20-12-16(14-5-8-31-9-6-14)11-19-22(20)33-21-4-3-15(17-2-1-7-29-23(17)27)10-18(21)25(19)13-32-24(28)30-25/h1-5,7,10-12H,6,8-9,13H2,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061780

(CHEMBL3394226)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)N1CCC(F)(F)C1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C23H17F4N5O2/c24-19-13(2-1-6-29-19)12-3-4-16-14(8-12)23(11-33-21(28)31-23)15-9-17(30-20(25)18(15)34-16)32-7-5-22(26,27)10-32/h1-4,6,8-9H,5,7,10-11H2,(H2,28,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061775
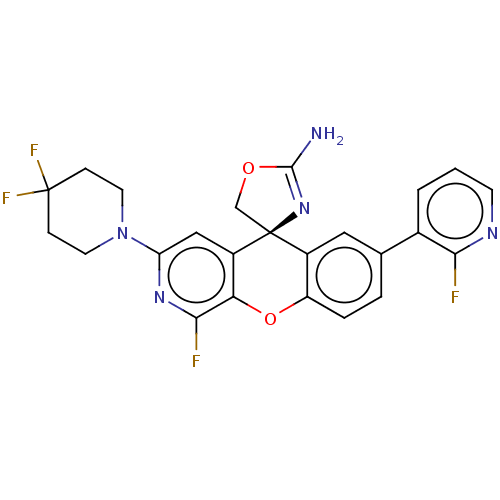
(CHEMBL3394225)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)N1CCC(F)(F)CC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H19F4N5O2/c25-20-14(2-1-7-30-20)13-3-4-17-15(10-13)24(12-34-22(29)32-24)16-11-18(31-21(26)19(16)35-17)33-8-5-23(27,28)6-9-33/h1-4,7,10-11H,5-6,8-9,12H2,(H2,29,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061587

(CHEMBL3394051)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)N1CC[C@@H](F)C1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C23H19F2N5O2/c24-14-5-7-30(11-14)20-9-17-19(10-28-20)32-18-4-3-13(15-2-1-6-27-21(15)25)8-16(18)23(17)12-31-22(26)29-23/h1-4,6,8-10,14H,5,7,11-12H2,(H2,26,29)/t14-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50201015
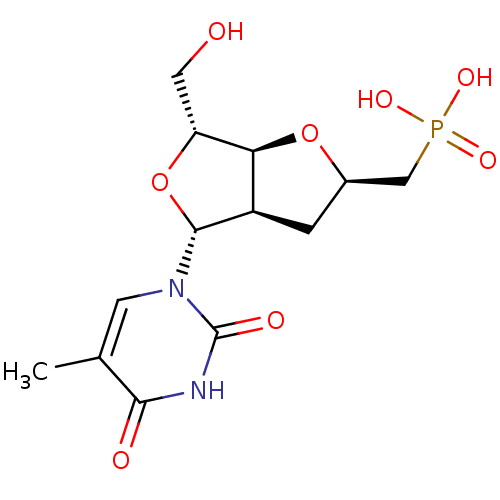
(((2R,3aR,4R,6R,6aS)-6-(hydroxymethyl)-4-(5-methyl-...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)[C@H]3O[C@@H](CP(O)(O)=O)C[C@@H]23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C13H19N2O8P/c1-6-3-15(13(18)14-11(6)17)12-8-2-7(5-24(19,20)21)22-10(8)9(4-16)23-12/h3,7-10,12,16H,2,4-5H2,1H3,(H,14,17,18)(H2,19,20,21)/t7-,8-,9-,10+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay |
J Med Chem 49: 7807-15 (2006)
Article DOI: 10.1021/jm060428u
BindingDB Entry DOI: 10.7270/Q2FB52KF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061595

(CHEMBL3394042)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)C1CCOCC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H21FN4O3/c25-22-16(2-1-7-27-22)15-3-4-20-17(10-15)24(13-31-23(26)29-24)18-11-19(28-12-21(18)32-20)14-5-8-30-9-6-14/h1-4,7,10-12,14H,5-6,8-9,13H2,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061598

(CHEMBL3394045)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)[C@H]1CCCCO1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H21FN4O3/c25-22-15(4-3-8-27-22)14-6-7-19-16(10-14)24(13-31-23(26)29-24)17-11-18(28-12-21(17)32-19)20-5-1-2-9-30-20/h3-4,6-8,10-12,20H,1-2,5,9,13H2,(H2,26,29)/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061582
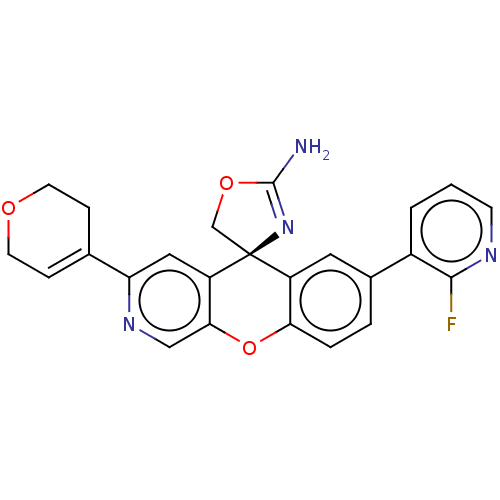
(CHEMBL3394039)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)C1=CCOCC1)-c1cccnc1F |r,t:1,23| Show InChI InChI=1S/C24H19FN4O3/c25-22-16(2-1-7-27-22)15-3-4-20-17(10-15)24(13-31-23(26)29-24)18-11-19(28-12-21(18)32-20)14-5-8-30-9-6-14/h1-5,7,10-12H,6,8-9,13H2,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061583

(CHEMBL3394047)Show SMILES CC1(C)CN(CCO1)c1cc2c(Oc3ccc(cc3[C@@]22COC(N)=N2)-c2cccnc2F)cn1 |r,c:26| Show InChI InChI=1S/C25H24FN5O3/c1-24(2)13-31(8-9-33-24)21-11-18-20(12-29-21)34-19-6-5-15(16-4-3-7-28-22(16)26)10-17(19)25(18)14-32-23(27)30-25/h3-7,10-12H,8-9,13-14H2,1-2H3,(H2,27,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061593
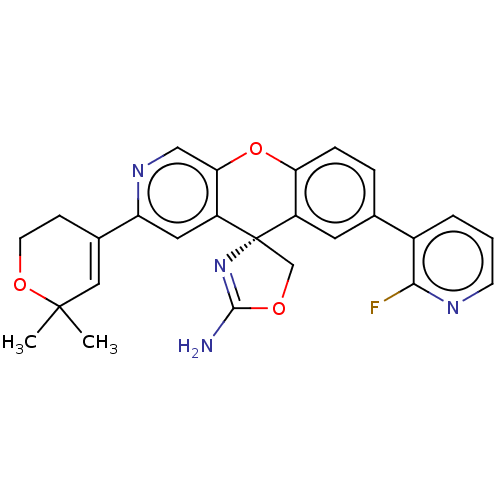
(CHEMBL3394040)Show SMILES CC1(C)OCCC(=C1)c1cc2c(Oc3ccc(cc3[C@@]22COC(N)=N2)-c2cccnc2F)cn1 |r,c:6,26| Show InChI InChI=1S/C26H23FN4O3/c1-25(2)12-16(7-9-33-25)20-11-19-22(13-30-20)34-21-6-5-15(17-4-3-8-29-23(17)27)10-18(21)26(19)14-32-24(28)31-26/h3-6,8,10-13H,7,9,14H2,1-2H3,(H2,28,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061586
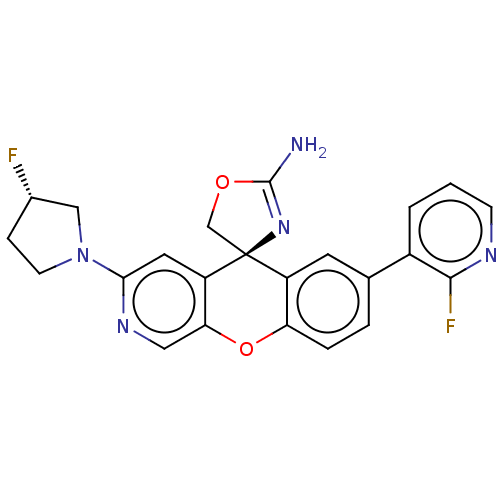
(CHEMBL3394050)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)N1CC[C@H](F)C1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C23H19F2N5O2/c24-14-5-7-30(11-14)20-9-17-19(10-28-20)32-18-4-3-13(15-2-1-6-27-21(15)25)8-16(18)23(17)12-31-22(26)29-23/h1-4,6,8-10,14H,5,7,11-12H2,(H2,26,29)/t14-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061584
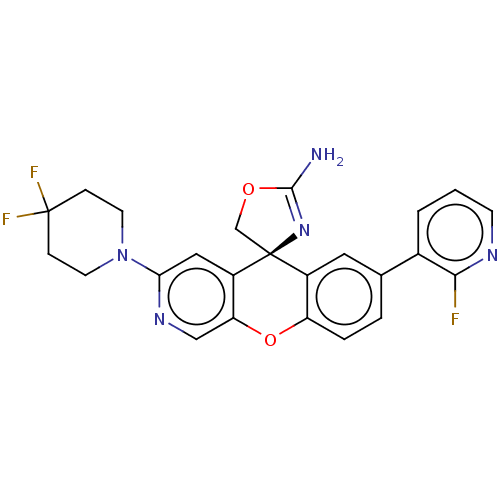
(CHEMBL3394048)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)N1CCC(F)(F)CC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H20F3N5O2/c25-21-15(2-1-7-29-21)14-3-4-18-16(10-14)24(13-33-22(28)31-24)17-11-20(30-12-19(17)34-18)32-8-5-23(26,27)6-9-32/h1-4,7,10-12H,5-6,8-9,13H2,(H2,28,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061588
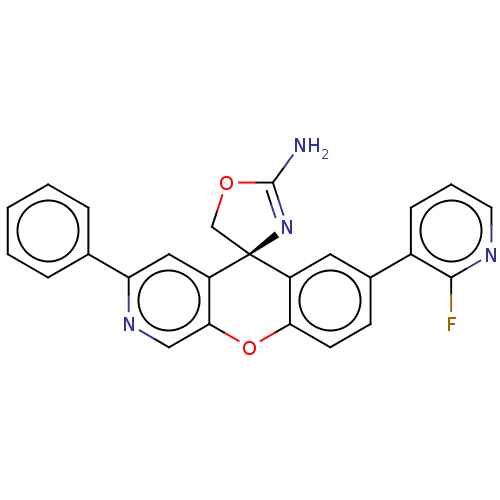
(CHEMBL3394052)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)-c1ccccc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C25H17FN4O2/c26-23-17(7-4-10-28-23)16-8-9-21-18(11-16)25(14-31-24(27)30-25)19-12-20(29-13-22(19)32-21)15-5-2-1-3-6-15/h1-13H,14H2,(H2,27,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061589
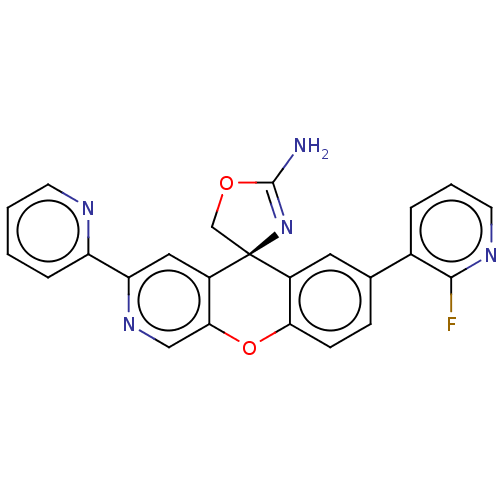
(CHEMBL3394053)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)-c1ccccn1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H16FN5O2/c25-22-15(4-3-9-28-22)14-6-7-20-16(10-14)24(13-31-23(26)30-24)17-11-19(29-12-21(17)32-20)18-5-1-2-8-27-18/h1-12H,13H2,(H2,26,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061590

(CHEMBL3394054)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)-c1cccnc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H16FN5O2/c25-22-16(4-2-8-28-22)14-5-6-20-17(9-14)24(13-31-23(26)30-24)18-10-19(29-12-21(18)32-20)15-3-1-7-27-11-15/h1-12H,13H2,(H2,26,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061591
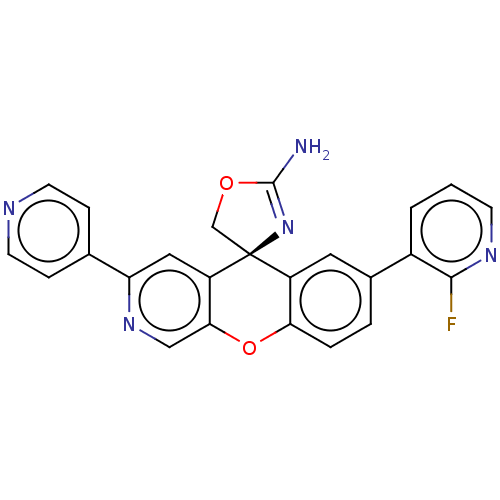
(CHEMBL3394055)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)-c1ccncc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H16FN5O2/c25-22-16(2-1-7-28-22)15-3-4-20-17(10-15)24(13-31-23(26)30-24)18-11-19(29-12-21(18)32-20)14-5-8-27-9-6-14/h1-12H,13H2,(H2,26,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061592

(CHEMBL3394056)Show SMILES Cc1cc(ccn1)-c1cc2c(Oc3ccc(cc3[C@@]22COC(N)=N2)-c2cccnc2F)cn1 |r,c:25| Show InChI InChI=1S/C25H18FN5O2/c1-14-9-16(6-8-28-14)20-11-19-22(12-30-20)33-21-5-4-15(17-3-2-7-29-23(17)26)10-18(21)25(19)13-32-24(27)31-25/h2-12H,13H2,1H3,(H2,27,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061580
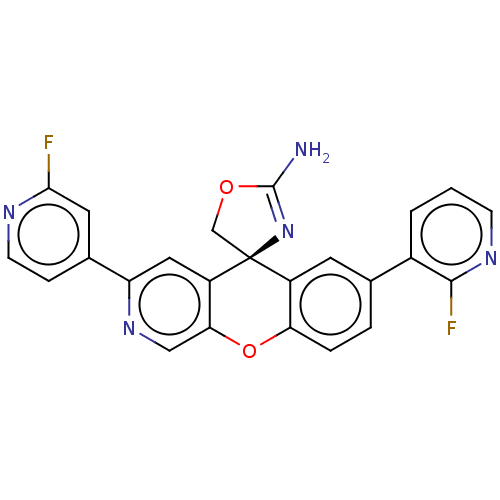
(CHEMBL3394057)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)-c1ccnc(F)c1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H15F2N5O2/c25-21-9-14(5-7-28-21)18-10-17-20(11-30-18)33-19-4-3-13(15-2-1-6-29-22(15)26)8-16(19)24(17)12-32-23(27)31-24/h1-11H,12H2,(H2,27,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061581
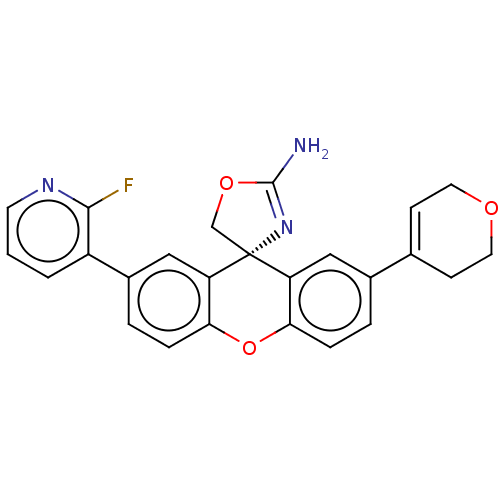
(CHEMBL3394058)Show SMILES NC1=N[C@]2(CO1)c1cc(ccc1Oc1ccc(cc21)-c1cccnc1F)C1=CCOCC1 |r,t:1,31| Show InChI InChI=1S/C25H20FN3O3/c26-23-18(2-1-9-28-23)17-4-6-22-20(13-17)25(14-31-24(27)29-25)19-12-16(3-5-21(19)32-22)15-7-10-30-11-8-15/h1-7,9,12-13H,8,10-11,14H2,(H2,27,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061585
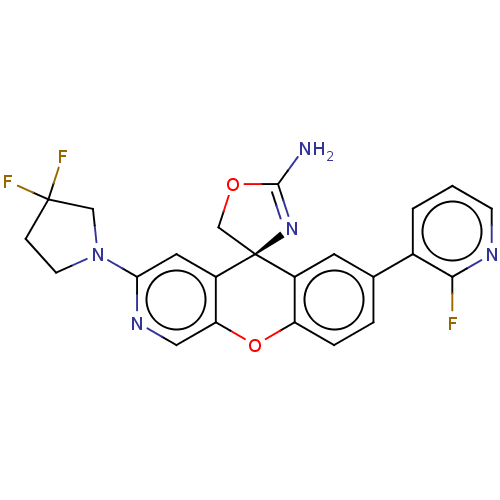
(CHEMBL3394049)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)N1CCC(F)(F)C1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C23H18F3N5O2/c24-20-14(2-1-6-28-20)13-3-4-17-15(8-13)23(12-32-21(27)30-23)16-9-19(29-10-18(16)33-17)31-7-5-22(25,26)11-31/h1-4,6,8-10H,5,7,11-12H2,(H2,27,30)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061594
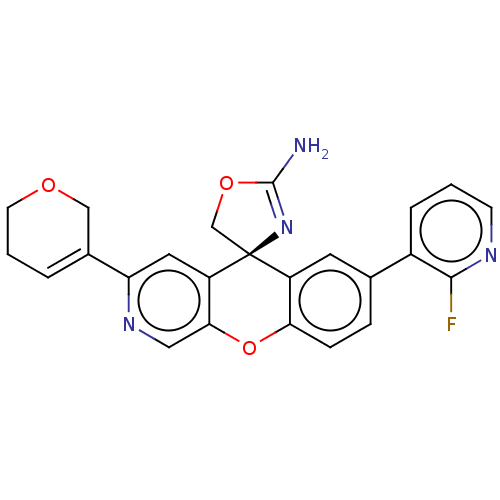
(CHEMBL3394041)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1cnc(cc21)C1=CCCOC1)-c1cccnc1F |r,t:1,23| Show InChI InChI=1S/C24H19FN4O3/c25-22-16(4-1-7-27-22)14-5-6-20-17(9-14)24(13-31-23(26)29-24)18-10-19(28-11-21(18)32-20)15-3-2-8-30-12-15/h1,3-7,9-11H,2,8,12-13H2,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061965
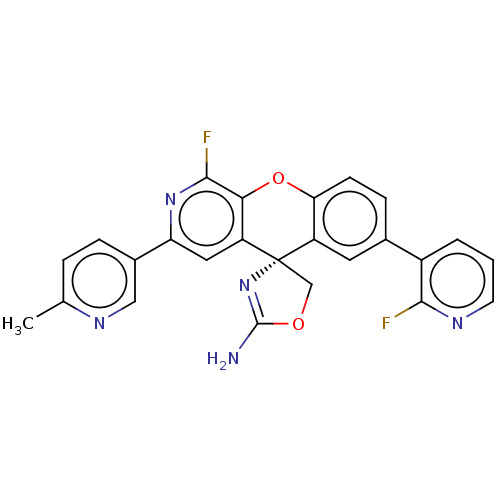
(CHEMBL3394213)Show SMILES Cc1ccc(cn1)-c1cc2c(Oc3ccc(cc3[C@@]22COC(N)=N2)-c2cccnc2F)c(F)n1 |r,c:25| Show InChI InChI=1S/C25H17F2N5O2/c1-13-4-5-15(11-30-13)19-10-18-21(23(27)31-19)34-20-7-6-14(16-3-2-8-29-22(16)26)9-17(20)25(18)12-33-24(28)32-25/h2-11H,12H2,1H3,(H2,28,32)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061893

(CHEMBL3394212)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)-c1ccncc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H15F2N5O2/c25-21-15(2-1-7-29-21)14-3-4-19-16(10-14)24(12-32-23(27)31-24)17-11-18(13-5-8-28-9-6-13)30-22(26)20(17)33-19/h1-11H,12H2,(H2,27,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061890
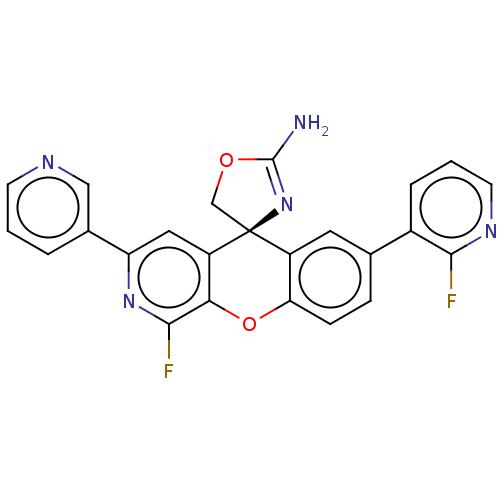
(CHEMBL3394211)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)-c1cccnc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H15F2N5O2/c25-21-15(4-2-8-29-21)13-5-6-19-16(9-13)24(12-32-23(27)31-24)17-10-18(14-3-1-7-28-11-14)30-22(26)20(17)33-19/h1-11H,12H2,(H2,27,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061874
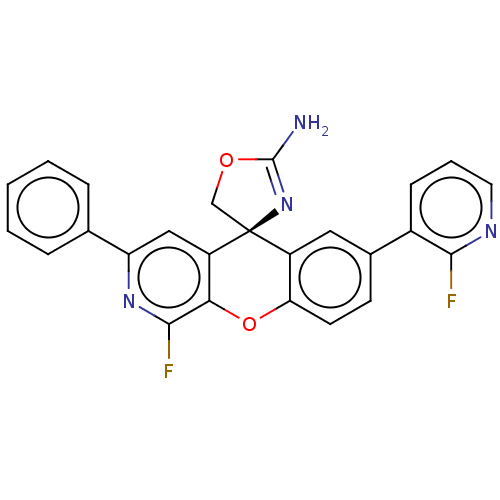
(CHEMBL3394210)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)-c1ccccc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C25H16F2N4O2/c26-22-16(7-4-10-29-22)15-8-9-20-17(11-15)25(13-32-24(28)31-25)18-12-19(14-5-2-1-3-6-14)30-23(27)21(18)33-20/h1-12H,13H2,(H2,28,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061835

(CHEMBL3394228)Show SMILES Cc1cc(on1)-c1cc2c(Oc3ccc(cc3[C@@]22COC(N)=N2)-c2cccnc2F)c(F)n1 |r,c:24| Show InChI InChI=1S/C23H15F2N5O3/c1-11-7-18(33-30-11)16-9-15-19(21(25)28-16)32-17-5-4-12(13-3-2-6-27-20(13)24)8-14(17)23(15)10-31-22(26)29-23/h2-9H,10H2,1H3,(H2,26,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061831

(CHEMBL3394227)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)N1CC[C@@H](F)C1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C23H18F3N5O2/c24-13-5-7-31(10-13)18-9-16-19(21(26)29-18)33-17-4-3-12(14-2-1-6-28-20(14)25)8-15(17)23(16)11-32-22(27)30-23/h1-4,6,8-9,13H,5,7,10-11H2,(H2,27,30)/t13-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061738
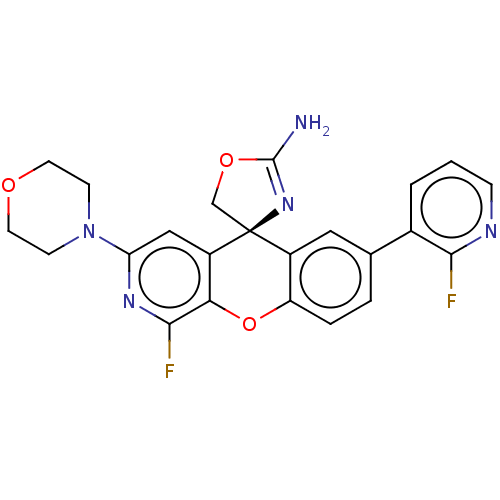
(CHEMBL3394224)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)N1CCOCC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C23H19F2N5O3/c24-20-14(2-1-5-27-20)13-3-4-17-15(10-13)23(12-32-22(26)29-23)16-11-18(28-21(25)19(16)33-17)30-6-8-31-9-7-30/h1-5,10-11H,6-9,12H2,(H2,26,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061737

(CHEMBL3394223)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)C1CCOCC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H20F2N4O3/c25-21-15(2-1-7-28-21)14-3-4-19-16(10-14)24(12-32-23(27)30-24)17-11-18(13-5-8-31-9-6-13)29-22(26)20(17)33-19/h1-4,7,10-11,13H,5-6,8-9,12H2,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061736
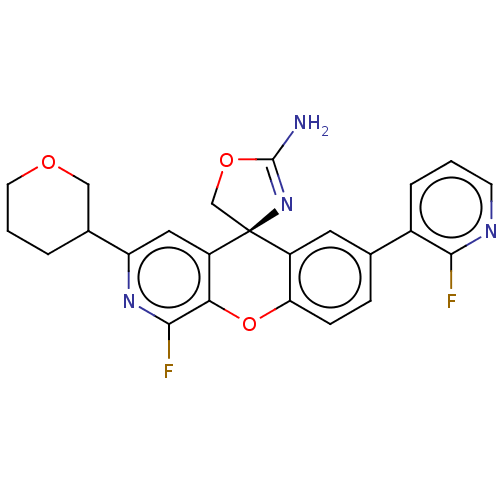
(CHEMBL3394222)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)C1CCCOC1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H20F2N4O3/c25-21-15(4-1-7-28-21)13-5-6-19-16(9-13)24(12-32-23(27)30-24)17-10-18(14-3-2-8-31-11-14)29-22(26)20(17)33-19/h1,4-7,9-10,14H,2-3,8,11-12H2,(H2,27,30)/t14?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061735
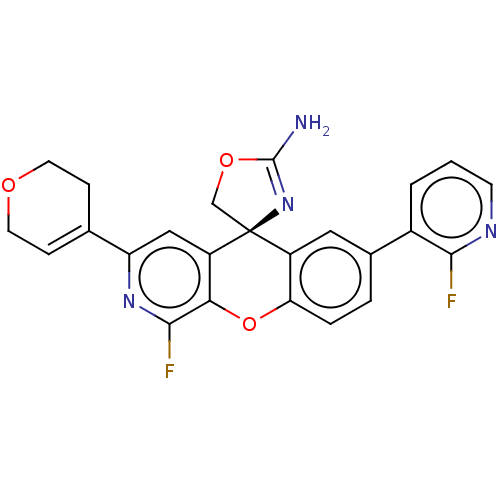
(CHEMBL3394221)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)C1=CCOCC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C24H18F2N4O3/c25-21-15(2-1-7-28-21)14-3-4-19-16(10-14)24(12-32-23(27)30-24)17-11-18(13-5-8-31-9-6-13)29-22(26)20(17)33-19/h1-5,7,10-11H,6,8-9,12H2,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061734
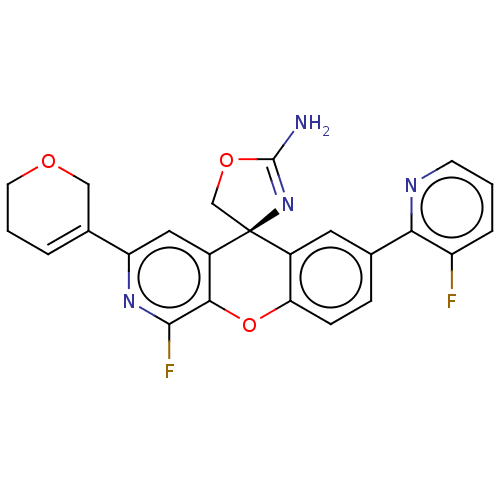
(CHEMBL3394220)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)C1=CCCOC1)-c1ncccc1F |r,t:1,24| Show InChI InChI=1S/C24H18F2N4O3/c25-17-4-1-7-28-20(17)13-5-6-19-15(9-13)24(12-32-23(27)30-24)16-10-18(14-3-2-8-31-11-14)29-22(26)21(16)33-19/h1,3-7,9-10H,2,8,11-12H2,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061733

(CHEMBL3394219)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)C1=CCCCO1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C24H18F2N4O3/c25-21-14(4-3-8-28-21)13-6-7-18-15(10-13)24(12-32-23(27)30-24)16-11-17(19-5-1-2-9-31-19)29-22(26)20(16)33-18/h3-8,10-11H,1-2,9,12H2,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061732

(CHEMBL3394218)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)C1=COCCC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C24H18F2N4O3/c25-21-15(4-1-7-28-21)13-5-6-19-16(9-13)24(12-32-23(27)30-24)17-10-18(14-3-2-8-31-11-14)29-22(26)20(17)33-19/h1,4-7,9-11H,2-3,8,12H2,(H2,27,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061731
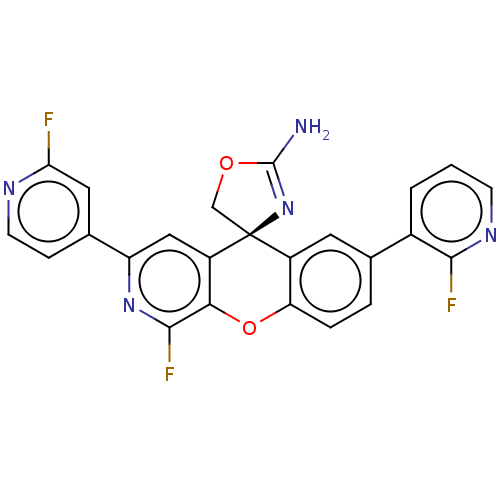
(CHEMBL3394217)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)-c1ccnc(F)c1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H14F3N5O2/c25-19-9-13(5-7-29-19)17-10-16-20(22(27)31-17)34-18-4-3-12(14-2-1-6-30-21(14)26)8-15(18)24(16)11-33-23(28)32-24/h1-10H,11H2,(H2,28,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061728
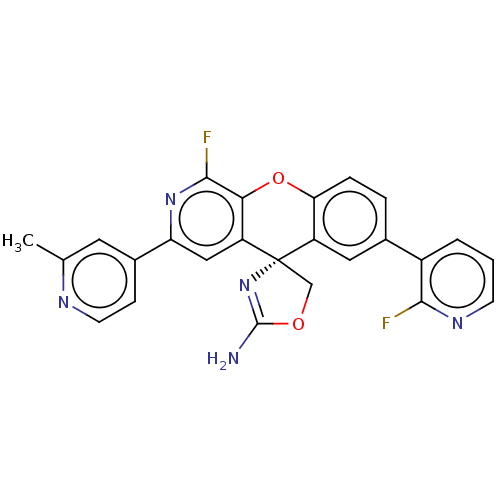
(CHEMBL3394214)Show SMILES Cc1cc(ccn1)-c1cc2c(Oc3ccc(cc3[C@@]22COC(N)=N2)-c2cccnc2F)c(F)n1 |r,c:25| Show InChI InChI=1S/C25H17F2N5O2/c1-13-9-15(6-8-29-13)19-11-18-21(23(27)31-19)34-20-5-4-14(16-3-2-7-30-22(16)26)10-17(20)25(18)12-33-24(28)32-25/h2-11H,12H2,1H3,(H2,28,32)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061729
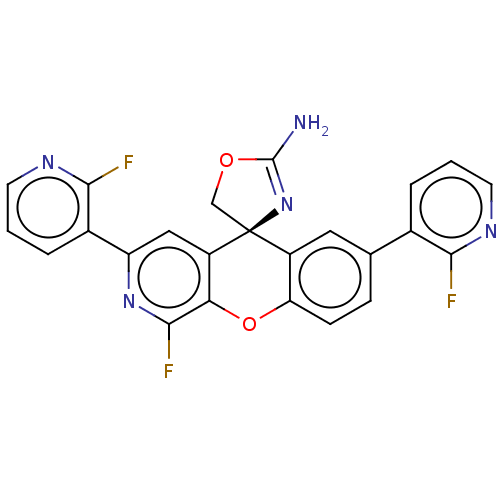
(CHEMBL3394215)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)-c1cccnc1F)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H14F3N5O2/c25-20-13(3-1-7-29-20)12-5-6-18-15(9-12)24(11-33-23(28)32-24)16-10-17(31-22(27)19(16)34-18)14-4-2-8-30-21(14)26/h1-10H,11H2,(H2,28,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061730

(CHEMBL3394216)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)nc(cc21)-c1ccc(F)nc1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C24H14F3N5O2/c25-19-6-4-13(10-30-19)17-9-16-20(22(27)31-17)34-18-5-3-12(14-2-1-7-29-21(14)26)8-15(18)24(16)11-33-23(28)32-24/h1-10H,11H2,(H2,28,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method |
ACS Med Chem Lett 6: 210-5 (2015)
Article DOI: 10.1021/ml500458t
BindingDB Entry DOI: 10.7270/Q2CN75JG |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50201012

(((2R,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)[C@H]3C[C@H](CP(O)(O)=O)O[C@@H]23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C13H19N2O8P/c1-6-3-15(13(18)14-11(6)17)12-10-8(9(4-16)23-12)2-7(22-10)5-24(19,20)21/h3,7-10,12,16H,2,4-5H2,1H3,(H,14,17,18)(H2,19,20,21)/t7-,8-,9-,10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay |
J Med Chem 49: 7807-15 (2006)
Article DOI: 10.1021/jm060428u
BindingDB Entry DOI: 10.7270/Q2FB52KF |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50201011
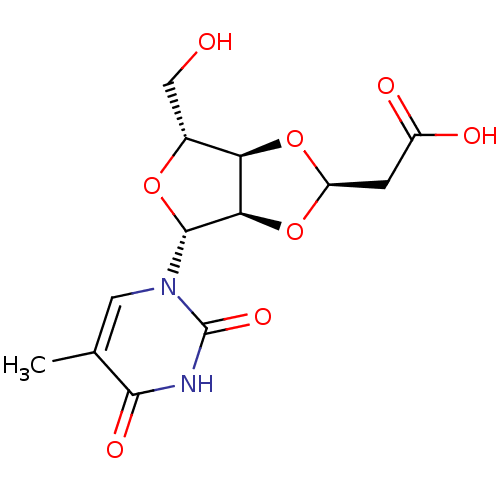
(2-((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methy...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)[C@H]3O[C@@H](CC(O)=O)O[C@@H]23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C13H16N2O8/c1-5-3-15(13(20)14-11(5)19)12-10-9(6(4-16)21-12)22-8(23-10)2-7(17)18/h3,6,8-10,12,16H,2,4H2,1H3,(H,17,18)(H,14,19,20)/t6-,8-,9-,10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay |
J Med Chem 49: 7807-15 (2006)
Article DOI: 10.1021/jm060428u
BindingDB Entry DOI: 10.7270/Q2FB52KF |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121756

((benzylamino)[(2,4-dioxo-1,2,3,4-tetrahydrothieno[...)Show SMILES NC(NCc1ccccc1)=[NH+]c1csc2c1[nH]c(=O)[nH]c2=O |w:10.11| Show InChI InChI=1S/C14H13N5O2S/c15-13(16-6-8-4-2-1-3-5-8)17-9-7-22-11-10(9)18-14(21)19-12(11)20/h1-5,7H,6H2,(H3,15,16,17)(H2,18,19,20,21)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinant thymidine phosphorylase TP |
Bioorg Med Chem Lett 13: 107-10 (2002)
BindingDB Entry DOI: 10.7270/Q2GM87V9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data