 Found 676 hits with Last Name = 'juteau' and Initial = 'h'
Found 676 hits with Last Name = 'juteau' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85177
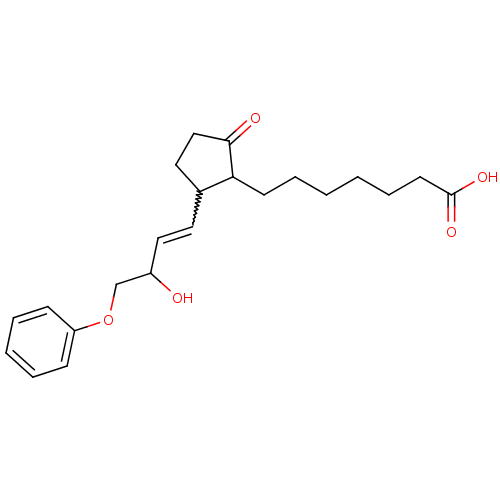
(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85603
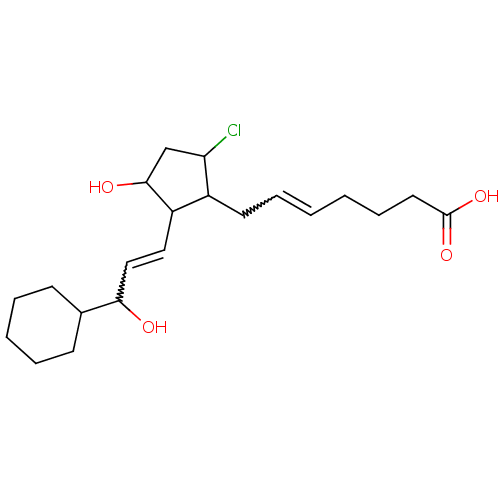
(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85183
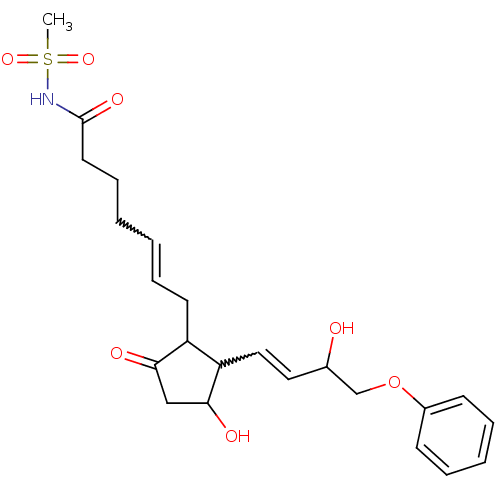
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85175
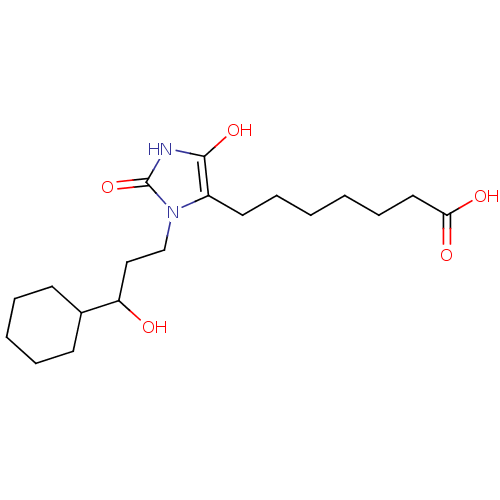
(BW245C | CAS_72814-32-5 | NSC_3080928)Show SMILES OC(CCn1c(CCCCCCC(O)=O)c(O)[nH]c1=O)C1CCCCC1 Show InChI InChI=1S/C19H32N2O5/c22-16(14-8-4-3-5-9-14)12-13-21-15(18(25)20-19(21)26)10-6-1-2-7-11-17(23)24/h14,16,22,25H,1-13H2,(H,20,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50085910
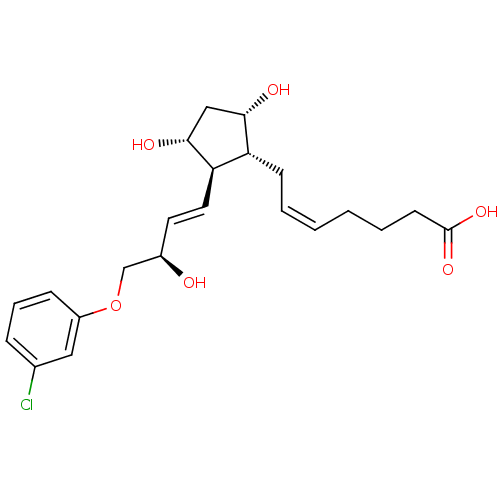
((Z)-7-{(1R,2R,3R,5S)-2-[(E)-(R)-4-(3-Chloro-phenox...)Show SMILES O[C@@H](COc1cccc(Cl)c1)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H29ClO6/c23-15-6-5-7-17(12-15)29-14-16(24)10-11-19-18(20(25)13-21(19)26)8-3-1-2-4-9-22(27)28/h1,3,5-7,10-12,16,18-21,24-26H,2,4,8-9,13-14H2,(H,27,28)/b3-1-,11-10+/t16-,18-,19-,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193922
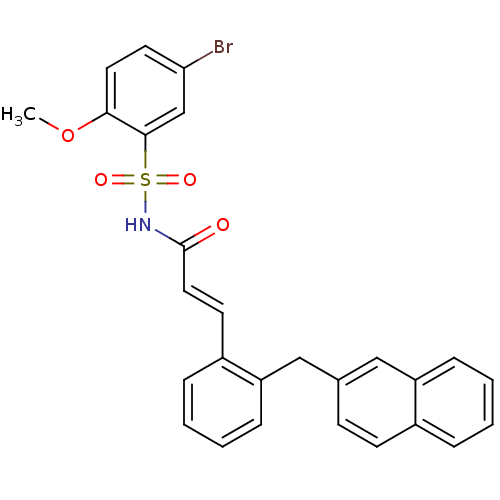
(CHEMBL218071 | N-(5-bromo-2-methoxyphenylsulfonyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C27H22BrNO4S/c1-33-25-14-13-24(28)18-26(25)34(31,32)29-27(30)15-12-21-7-3-5-9-23(21)17-19-10-11-20-6-2-4-8-22(20)16-19/h2-16,18H,17H2,1H3,(H,29,30)/b15-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193920
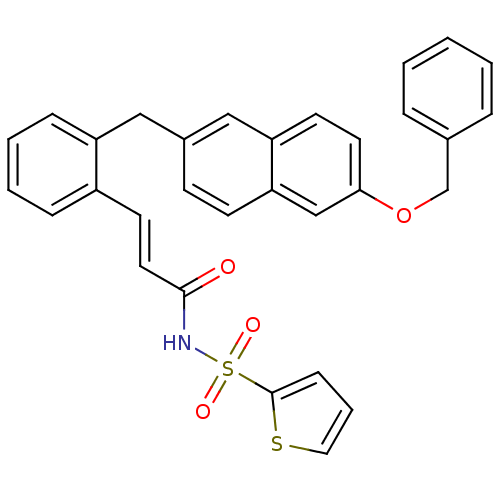
(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C31H25NO4S2/c33-30(32-38(34,35)31-11-6-18-37-31)17-15-25-9-4-5-10-26(25)19-24-12-13-28-21-29(16-14-27(28)20-24)36-22-23-7-2-1-3-8-23/h1-18,20-21H,19,22H2,(H,32,33)/b17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193935

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C34H28BrNO5S/c1-40-32-17-15-30(35)22-33(32)42(38,39)36-34(37)18-14-26-9-5-6-10-27(26)19-25-11-12-29-21-31(16-13-28(29)20-25)41-23-24-7-3-2-4-8-24/h2-18,20-22H,19,23H2,1H3,(H,36,37)/b18-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193935

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C34H28BrNO5S/c1-40-32-17-15-30(35)22-33(32)42(38,39)36-34(37)18-14-26-9-5-6-10-27(26)19-25-11-12-29-21-31(16-13-28(29)20-25)41-23-24-7-3-2-4-8-24/h2-18,20-22H,19,23H2,1H3,(H,36,37)/b18-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193921
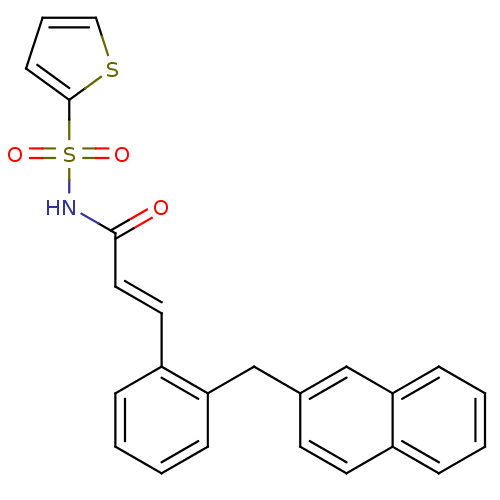
(3-(2-(naphthalen-2-ylmethyl)phenyl)-N-(thiophen-2-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C24H19NO3S2/c26-23(25-30(27,28)24-10-5-15-29-24)14-13-20-7-2-4-9-22(20)17-18-11-12-19-6-1-3-8-21(19)16-18/h1-16H,17H2,(H,25,26)/b14-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193938

(3-(2-(4-(benzyloxy)-3-methoxycinnamyl)phenyl)-N-(t...)Show SMILES COc1cc(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-13,15-21H,14,22H2,1H3,(H,31,32)/b11-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193918
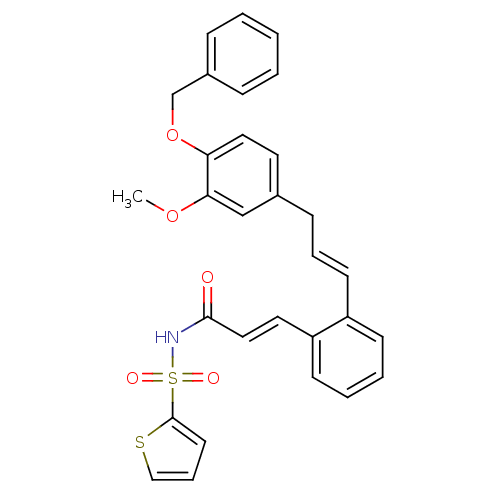
(3-(2-((E)-3-(4-(benzyloxy)-3-methoxyphenyl)prop-1-...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-10,12-21H,11,22H2,1H3,(H,31,32)/b14-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM82213
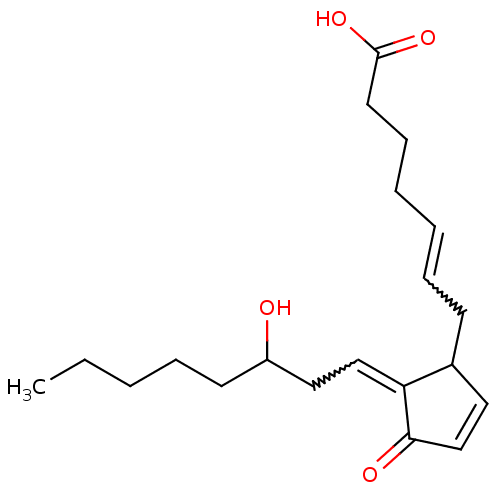
(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85603

(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM85173
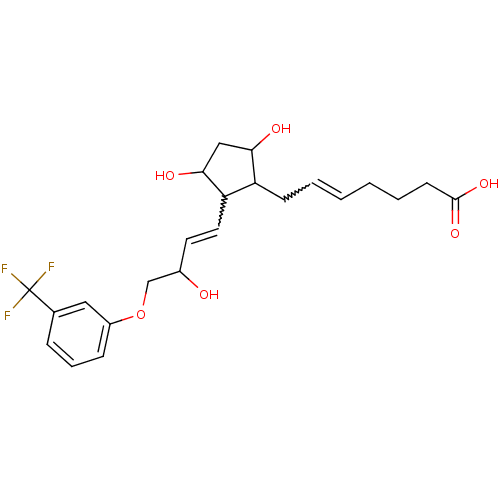
(CAS_40666-16-8 | FLUPROSTENOL | NSC_5311100)Show SMILES OC(COc1cccc(c1)C(F)(F)F)C=CC1C(O)CC(O)C1CC=CCCCC(O)=O |w:15.16,24.25| Show InChI InChI=1S/C23H29F3O6/c24-23(25,26)15-6-5-7-17(12-15)32-14-16(27)10-11-19-18(20(28)13-21(19)29)8-3-1-2-4-9-22(30)31/h1,3,5-7,10-12,16,18-21,27-29H,2,4,8-9,13-14H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193920

(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C31H25NO4S2/c33-30(32-38(34,35)31-11-6-18-37-31)17-15-25-9-4-5-10-26(25)19-24-12-13-28-21-29(16-14-27(28)20-24)36-22-23-7-2-1-3-8-23/h1-18,20-21H,19,22H2,(H,32,33)/b17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193923
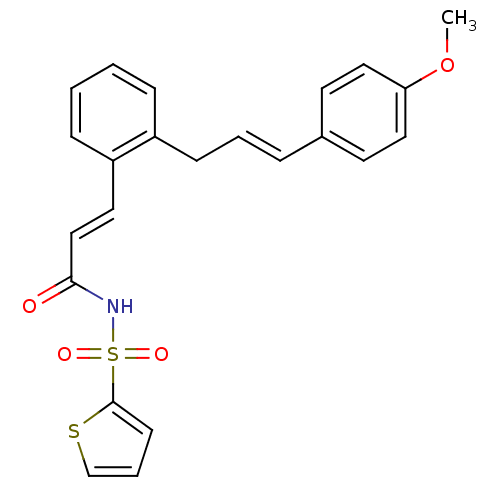
(3-(2-(4-methoxycinnamyl)phenyl)-N-(thiophen-2-ylsu...)Show SMILES COc1ccc(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C23H21NO4S2/c1-28-21-14-11-18(12-15-21)6-4-9-19-7-2-3-8-20(19)13-16-22(25)24-30(26,27)23-10-5-17-29-23/h2-8,10-17H,9H2,1H3,(H,24,25)/b6-4+,16-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193937

(3-(2-((E)-3-(4-methoxyphenyl)prop-1-enyl)phenyl)-N...)Show SMILES COc1ccc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C23H21NO4S2/c1-28-21-14-11-18(12-15-21)6-4-9-19-7-2-3-8-20(19)13-16-22(25)24-30(26,27)23-10-5-17-29-23/h2-5,7-17H,6H2,1H3,(H,24,25)/b9-4+,16-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50240648
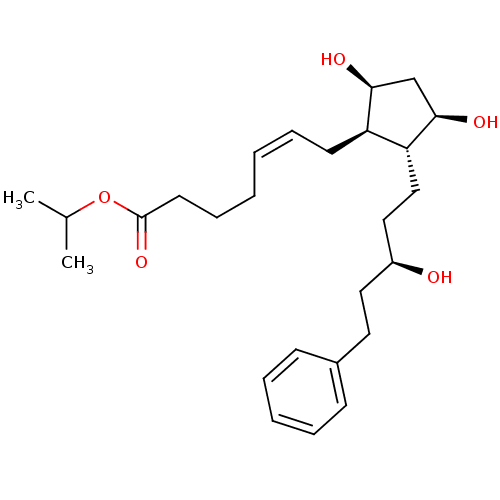
(LATANOPROST (FREE ACID) | PhXA 41 | Xalatan | isop...)Show SMILES CC(C)OC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1CC[C@@H](O)CCc1ccccc1 |r| Show InChI InChI=1S/C26H40O5/c1-19(2)31-26(30)13-9-4-3-8-12-22-23(25(29)18-24(22)28)17-16-21(27)15-14-20-10-6-5-7-11-20/h3,5-8,10-11,19,21-25,27-29H,4,9,12-18H2,1-2H3/b8-3-/t21-,22+,23+,24-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193926

(3-(2-(2-(2,6-dichlorobenzyloxy)-5-methylcinnamyl)p...)Show SMILES Cc1ccc(OCc2c(Cl)cccc2Cl)c(\C=C\Cc2ccccc2\C=C\C(O)=O)c1 Show InChI InChI=1S/C26H22Cl2O3/c1-18-12-14-25(31-17-22-23(27)10-5-11-24(22)28)21(16-18)9-4-8-19-6-2-3-7-20(19)13-15-26(29)30/h2-7,9-16H,8,17H2,1H3,(H,29,30)/b9-4+,15-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50020300
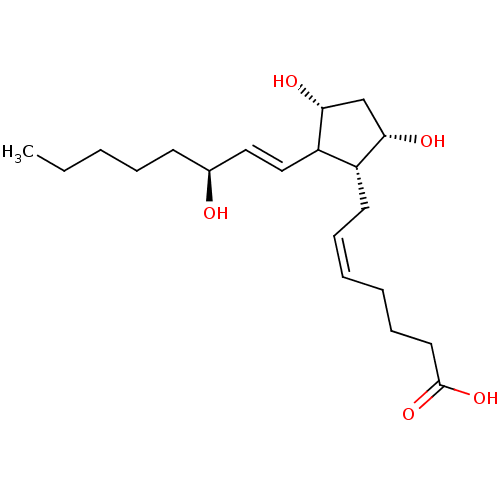
((S-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-oct-1-enyl...)Show SMILES CCCCC[C@H](O)\C=C\C1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17?,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193924
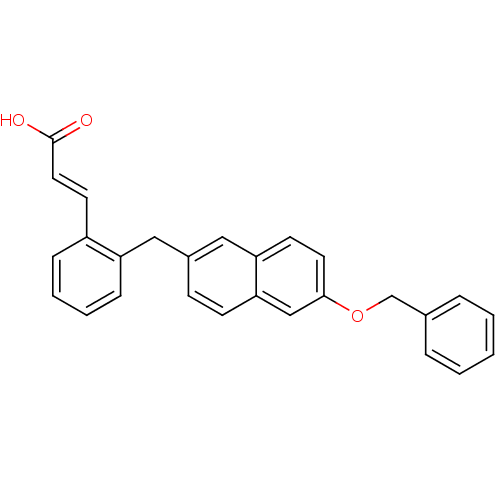
(3-(2-((6-(benzyloxy)naphthalen-2-yl)methyl)phenyl)...)Show SMILES OC(=O)\C=C\c1ccccc1Cc1ccc2cc(OCc3ccccc3)ccc2c1 Show InChI InChI=1S/C27H22O3/c28-27(29)15-13-22-8-4-5-9-23(22)16-21-10-11-25-18-26(14-12-24(25)17-21)30-19-20-6-2-1-3-7-20/h1-15,17-18H,16,19H2,(H,28,29)/b15-13+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 2A1
(Homo sapiens (Human)) | BDBM50008781
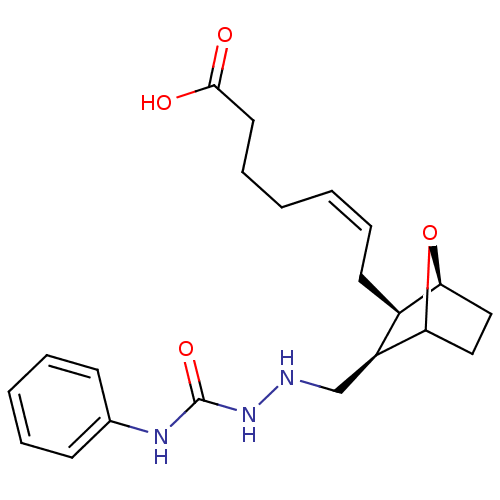
(7-(3-(2-ethyl-N-phenylhydrazinecarboxamide)-7-oxa-...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(O2)[C@H]1CNNC(=O)Nc1ccccc1 Show InChI InChI=1S/C21H29N3O4/c25-20(26)11-7-2-1-6-10-16-17(19-13-12-18(16)28-19)14-22-24-21(27)23-15-8-4-3-5-9-15/h1,3-6,8-9,16-19,22H,2,7,10-14H2,(H,25,26)(H2,23,24,27)/b6-1-/t16-,17+,18+,19?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50085910

((Z)-7-{(1R,2R,3R,5S)-2-[(E)-(R)-4-(3-Chloro-phenox...)Show SMILES O[C@@H](COc1cccc(Cl)c1)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H29ClO6/c23-15-6-5-7-17(12-15)29-14-16(24)10-11-19-18(20(25)13-21(19)26)8-3-1-2-4-9-22(27)28/h1,3,5-7,10-12,16,18-21,24-26H,2,4,8-9,13-14H2,(H,27,28)/b3-1-,11-10+/t16-,18-,19-,20+,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85605
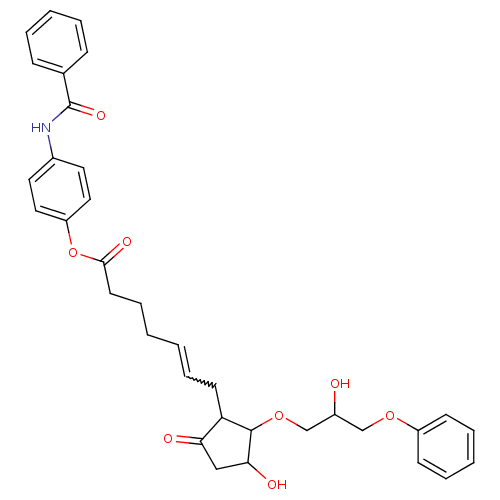
(GR 63799)Show SMILES OC(COC1C(O)CC(=O)C1CC=CCCCC(=O)Oc1ccc(NC(=O)c2ccccc2)cc1)COc1ccccc1 |w:12.12| Show InChI InChI=1S/C34H37NO8/c36-26(22-41-27-13-7-4-8-14-27)23-42-33-29(30(37)21-31(33)38)15-9-1-2-10-16-32(39)43-28-19-17-25(18-20-28)35-34(40)24-11-5-3-6-12-24/h1,3-9,11-14,17-20,26,29,31,33,36,38H,2,10,15-16,21-23H2,(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193925
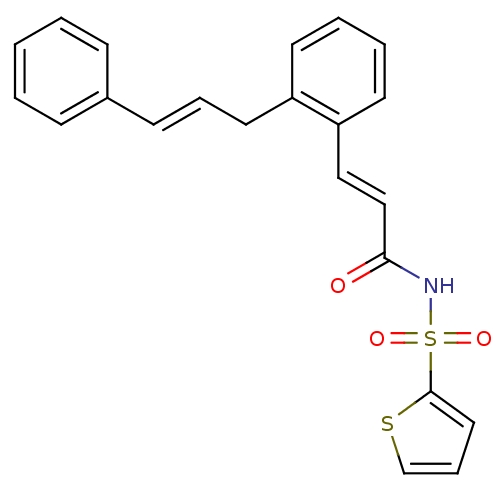
(3-(2-cinnamylphenyl)-N-(thiophen-2-ylsulfonyl)acry...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1C\C=C\c1ccccc1 Show InChI InChI=1S/C22H19NO3S2/c24-21(23-28(25,26)22-14-7-17-27-22)16-15-20-12-5-4-11-19(20)13-6-10-18-8-2-1-3-9-18/h1-12,14-17H,13H2,(H,23,24)/b10-6+,16-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193930
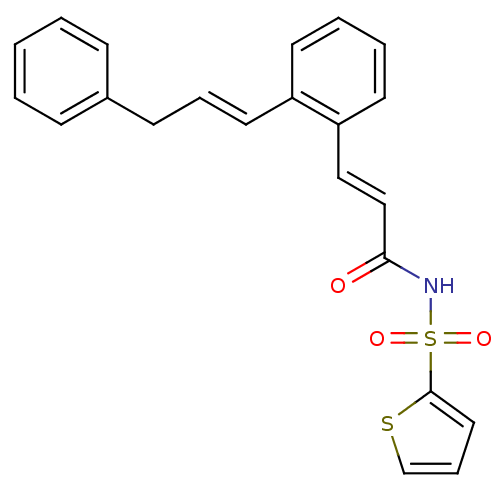
(3-(2-((E)-3-phenylprop-1-enyl)phenyl)-N-(thiophen-...)Show SMILES O=C(NS(=O)(=O)c1cccs1)\C=C\c1ccccc1\C=C\Cc1ccccc1 Show InChI InChI=1S/C22H19NO3S2/c24-21(23-28(25,26)22-14-7-17-27-22)16-15-20-12-5-4-11-19(20)13-6-10-18-8-2-1-3-9-18/h1-9,11-17H,10H2,(H,23,24)/b13-6+,16-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM85603

(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM82213

(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193922

(CHEMBL218071 | N-(5-bromo-2-methoxyphenylsulfonyl)...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)NC(=O)\C=C\c1ccccc1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C27H22BrNO4S/c1-33-25-14-13-24(28)18-26(25)34(31,32)29-27(30)15-12-21-7-3-5-9-23(21)17-19-10-11-20-6-2-4-8-22(20)16-19/h2-16,18H,17H2,1H3,(H,29,30)/b15-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor in presence of HSA |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85606
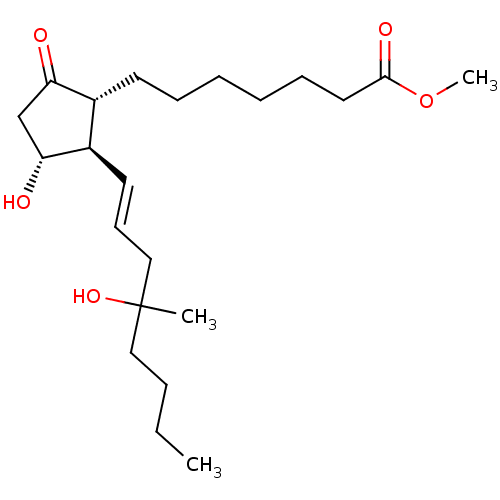
(CAS_59122-46-2 | MISOPROSTOL (Free Acid))Show SMILES CCCCC(C)(O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C22H38O5/c1-4-5-14-22(2,26)15-10-12-18-17(19(23)16-20(18)24)11-8-6-7-9-13-21(25)27-3/h10,12,17-18,20,24,26H,4-9,11,13-16H2,1-3H3/b12-10+/t17-,18-,20-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50159774
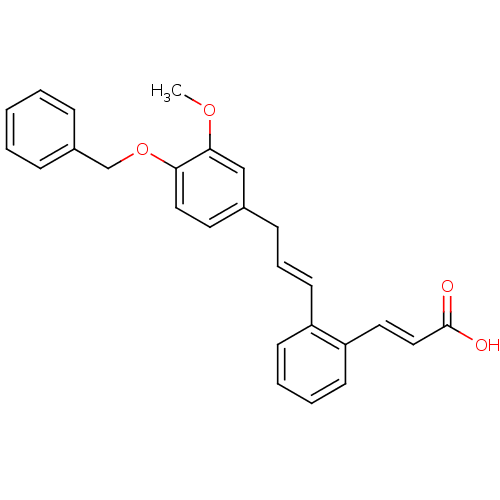
((E)-3-{2-[(E)-3-(4-Benzyloxy-3-methoxy-phenyl)-pro...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-9,11-18H,10,19H2,1H3,(H,27,28)/b13-7+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193931
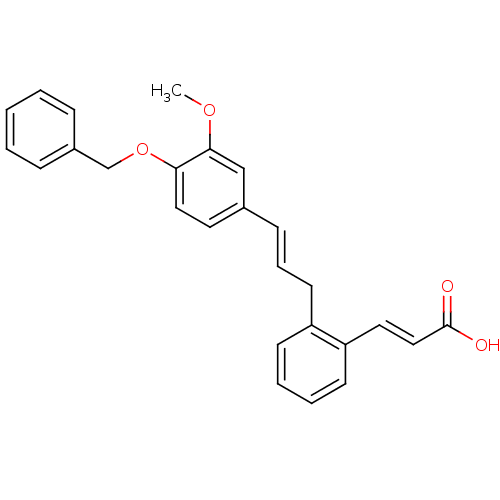
(3-(2-(4-(benzyloxy)-3-methoxycinnamyl)phenyl)acryl...)Show SMILES COc1cc(\C=C\Cc2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-12,14-18H,13,19H2,1H3,(H,27,28)/b10-7+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193929
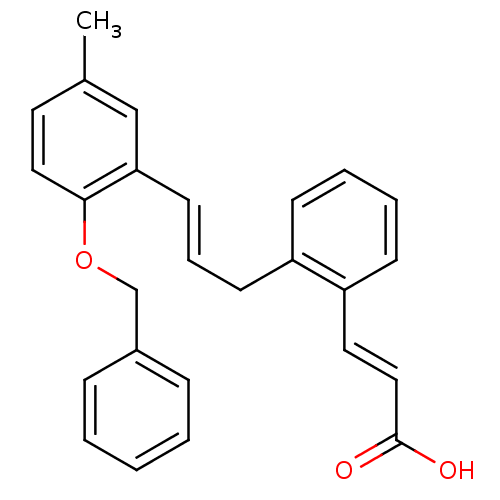
(3-(2-(2-(benzyloxy)-5-methylcinnamyl)phenyl)acryli...)Show SMILES Cc1ccc(OCc2ccccc2)c(\C=C\Cc2ccccc2\C=C\C(O)=O)c1 Show InChI InChI=1S/C26H24O3/c1-20-14-16-25(29-19-21-8-3-2-4-9-21)24(18-20)13-7-12-22-10-5-6-11-23(22)15-17-26(27)28/h2-11,13-18H,12,19H2,1H3,(H,27,28)/b13-7+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193928

(3-(2-((E)-3-(2-(benzyloxy)-5-methylphenyl)prop-1-e...)Show SMILES Cc1ccc(OCc2ccccc2)c(C\C=C\c2ccccc2\C=C\C(O)=O)c1 Show InChI InChI=1S/C26H24O3/c1-20-14-16-25(29-19-21-8-3-2-4-9-21)24(18-20)13-7-12-22-10-5-6-11-23(22)15-17-26(27)28/h2-12,14-18H,13,19H2,1H3,(H,27,28)/b12-7+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193933

(3-(2-(2-(benzyloxy)-5-methylcinnamyl)phenyl)-N-(th...)Show SMILES Cc1ccc(OCc2ccccc2)c(\C=C\Cc2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)c1 Show InChI InChI=1S/C30H27NO4S2/c1-23-16-18-28(35-22-24-9-3-2-4-10-24)27(21-23)14-7-13-25-11-5-6-12-26(25)17-19-29(32)31-37(33,34)30-15-8-20-36-30/h2-12,14-21H,13,22H2,1H3,(H,31,32)/b14-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50193927
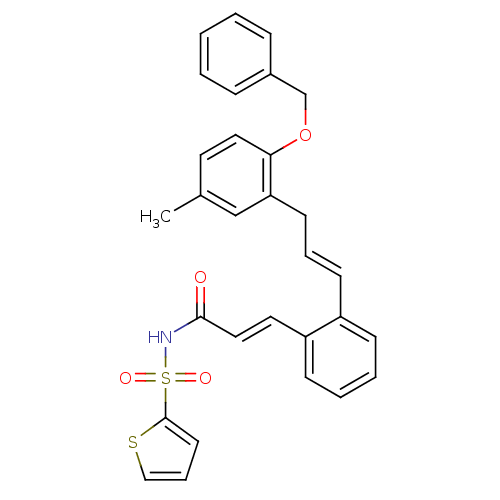
(3-(2-((E)-3-(2-(benzyloxy)-5-methylphenyl)prop-1-e...)Show SMILES Cc1ccc(OCc2ccccc2)c(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)c1 Show InChI InChI=1S/C30H27NO4S2/c1-23-16-18-28(35-22-24-9-3-2-4-10-24)27(21-23)14-7-13-25-11-5-6-12-26(25)17-19-29(32)31-37(33,34)30-15-8-20-36-30/h2-13,15-21H,14,22H2,1H3,(H,31,32)/b13-7+,19-17+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor |
Bioorg Med Chem Lett 16: 5639-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.025
BindingDB Entry DOI: 10.7270/Q2NS0VQ6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM85177

(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85338
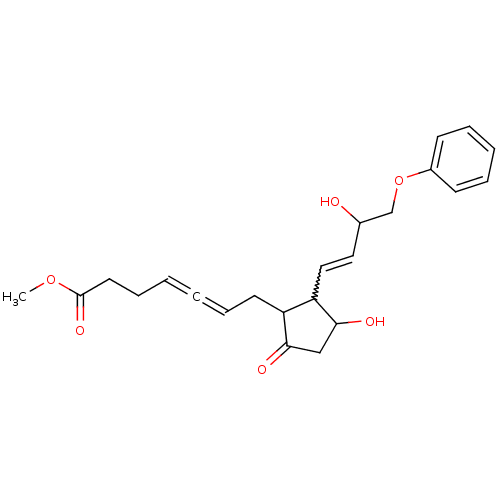
(CAS_73121-56-9 | ENPROSTIL | NSC_5282207)Show SMILES COC(=O)CCC=C=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:12.11,(-.79,-17.87,;.54,-17.1,;1.88,-17.87,;1.88,-19.41,;3.21,-17.1,;4.55,-17.87,;5.88,-17.1,;7.21,-17.87,;8.55,-18.64,;9.88,-17.87,;11.21,-18.64,;12.62,-18.02,;12.94,-16.51,;14.41,-16.03,;14.73,-14.53,;13.58,-13.5,;16.19,-14.05,;16.51,-12.55,;17.98,-12.07,;18.3,-10.56,;19.76,-10.09,;20.9,-11.12,;20.58,-12.62,;19.12,-13.1,;13.65,-19.16,;15.18,-19,;12.88,-20.49,;11.38,-20.17,;10.23,-21.2,)| Show InChI InChI=1S/C23H28O6/c1-28-23(27)12-8-3-2-7-11-19-20(22(26)15-21(19)25)14-13-17(24)16-29-18-9-5-4-6-10-18/h3-7,9-10,13-14,17,19-20,22,24,26H,8,11-12,15-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85599
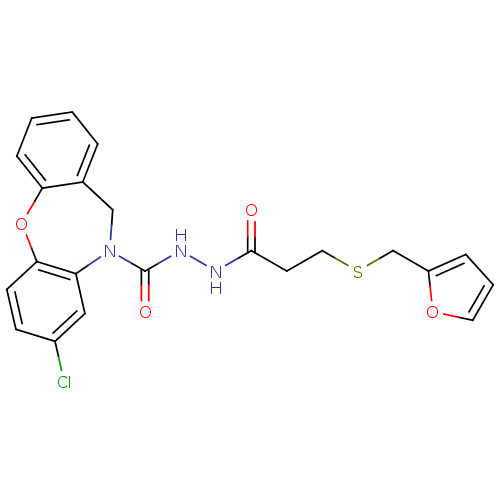
(CAS_146032-79-3 | SC-51322)Show SMILES Clc1ccc2Oc3ccccc3CN(C(=O)NNC(=O)CCSCc3ccco3)c2c1 Show InChI InChI=1S/C22H20ClN3O4S/c23-16-7-8-20-18(12-16)26(13-15-4-1-2-6-19(15)30-20)22(28)25-24-21(27)9-11-31-14-17-5-3-10-29-17/h1-8,10,12H,9,11,13-14H2,(H,24,27)(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50109546
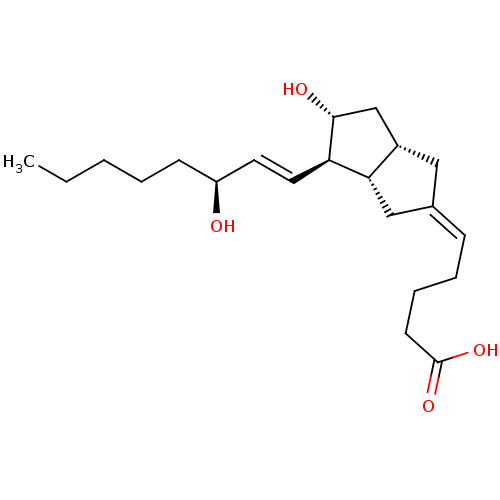
(5-[(3aS,4R,5R,6aS)-5-Hydroxy-4-((S)-3-hydroxy-oct-...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@@H]2C\C(C[C@H]12)=C\CCCC(O)=O Show InChI InChI=1S/C21H34O4/c1-2-3-4-8-17(22)10-11-18-19-13-15(7-5-6-9-21(24)25)12-16(19)14-20(18)23/h7,10-11,16-20,22-23H,2-6,8-9,12-14H2,1H3,(H,24,25)/b11-10+,15-7-/t16-,17-,18+,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50117700
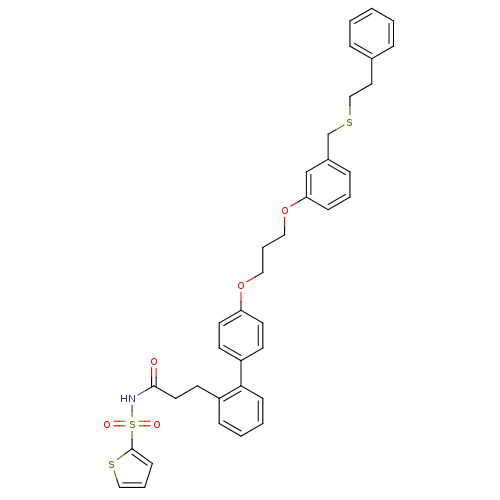
(CHEMBL87366 | Thiophene-2-sulfonic acid (3-{4'-[3-...)Show SMILES O=C(CCc1ccccc1-c1ccc(OCCCOc2cccc(CSCCc3ccccc3)c2)cc1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C37H37NO5S3/c39-36(38-46(40,41)37-15-7-25-45-37)21-18-31-12-4-5-14-35(31)32-16-19-33(20-17-32)42-23-8-24-43-34-13-6-11-30(27-34)28-44-26-22-29-9-2-1-3-10-29/h1-7,9-17,19-20,25,27H,8,18,21-24,26,28H2,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity at human Prostanoid EP3 receptor. |
Bioorg Med Chem Lett 12: 2583-6 (2002)
BindingDB Entry DOI: 10.7270/Q2QN663S |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM85179
(CAS_94079-80-8 | CICAPROST | NSC_72023)Show SMILES CCC#CCC(C)C(O)C#CC1C(O)CC2CC(CC12)=CCOCC(O)=O |w:20.22| Show InChI InChI=1S/C22H30O5/c1-3-4-5-6-15(2)20(23)8-7-18-19-12-16(9-10-27-14-22(25)26)11-17(19)13-21(18)24/h9,15,17-21,23-24H,3,6,10-14H2,1-2H3,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50097792
((E)-3-(2-Naphthalen-2-ylmethyl-phenyl)-acrylic aci...)Show InChI InChI=1S/C20H16O2/c21-20(22)12-11-17-6-2-4-8-19(17)14-15-9-10-16-5-1-3-7-18(16)13-15/h1-13H,14H2,(H,21,22)/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Antagonistic activity at Prostanoid EP3 receptor in human was determined |
Bioorg Med Chem Lett 11: 747-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GB239F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data