 Found 629 hits with Last Name = 'kamau' and Initial = 'mg'
Found 629 hits with Last Name = 'kamau' and Initial = 'mg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337332
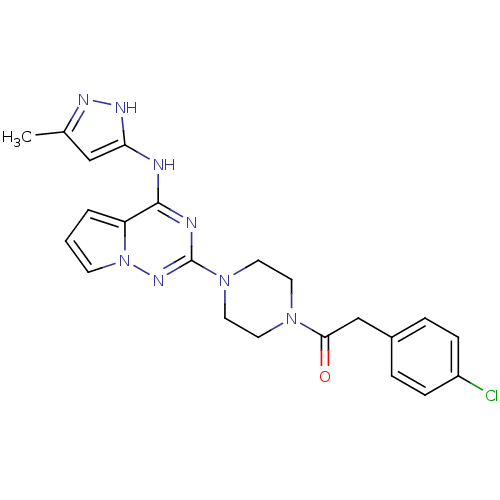
(2-(4-chlorophenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(Cl)cc2)[nH]n1 Show InChI InChI=1S/C22H23ClN8O/c1-15-13-19(27-26-15)24-21-18-3-2-8-31(18)28-22(25-21)30-11-9-29(10-12-30)20(32)14-16-4-6-17(23)7-5-16/h2-8,13H,9-12,14H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337333
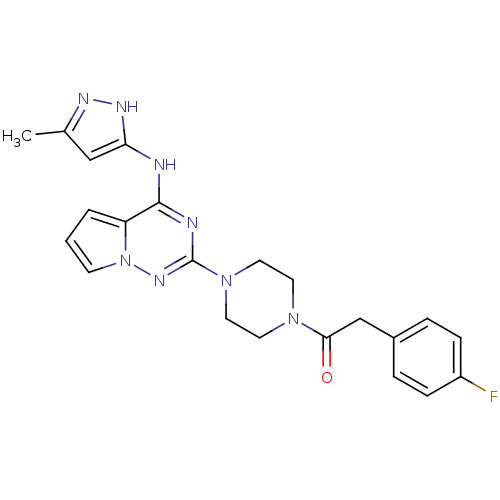
(2-(4-fluorophenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(F)cc2)[nH]n1 Show InChI InChI=1S/C22H23FN8O/c1-15-13-19(27-26-15)24-21-18-3-2-8-31(18)28-22(25-21)30-11-9-29(10-12-30)20(32)14-16-4-6-17(23)7-5-16/h2-8,13H,9-12,14H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516404
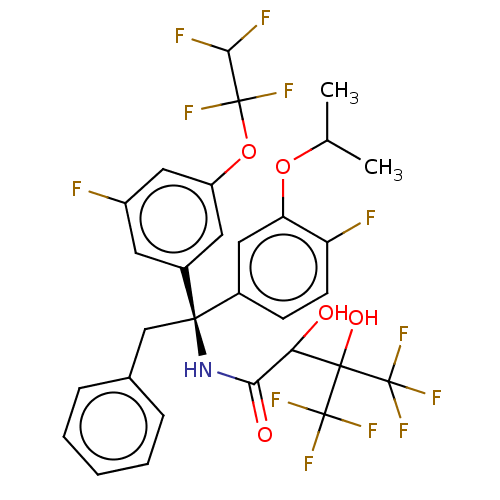
(CHEMBL4439823)Show SMILES CC(C)Oc1cc(ccc1F)[C@@](Cc1ccccc1)(NC(=O)C(O)C(O)(C(F)(F)F)C(F)(F)F)c1cc(F)cc(OC(F)(F)C(F)F)c1 |r| Show InChI InChI=1S/C30H25F12NO5/c1-15(2)47-22-12-17(8-9-21(22)32)26(14-16-6-4-3-5-7-16,18-10-19(31)13-20(11-18)48-28(35,36)25(33)34)43-24(45)23(44)27(46,29(37,38)39)30(40,41)42/h3-13,15,23,25,44,46H,14H2,1-2H3,(H,43,45)/t23?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337334
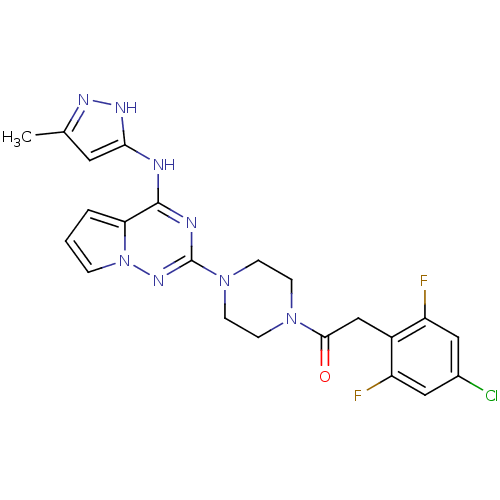
(2-(4-chloro-2,6-difluorophenyl)-1-(4-(4-(5-methyl-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2c(F)cc(Cl)cc2F)[nH]n1 Show InChI InChI=1S/C22H21ClF2N8O/c1-13-9-19(29-28-13)26-21-18-3-2-4-33(18)30-22(27-21)32-7-5-31(6-8-32)20(34)12-15-16(24)10-14(23)11-17(15)25/h2-4,9-11H,5-8,12H2,1H3,(H2,26,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337335

((4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2S(C)(=O)=O)[nH]n1 Show InChI InChI=1S/C22H24N8O3S/c1-15-14-19(26-25-15)23-20-17-7-5-9-30(17)27-22(24-20)29-12-10-28(11-13-29)21(31)16-6-3-4-8-18(16)34(2,32)33/h3-9,14H,10-13H2,1-2H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337336
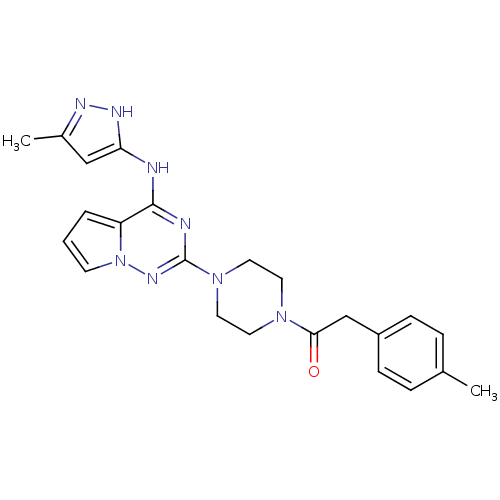
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(C)cc2)[nH]n1 Show InChI InChI=1S/C23H26N8O/c1-16-5-7-18(8-6-16)15-21(32)29-10-12-30(13-11-29)23-25-22(19-4-3-9-31(19)28-23)24-20-14-17(2)26-27-20/h3-9,14H,10-13,15H2,1-2H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337337
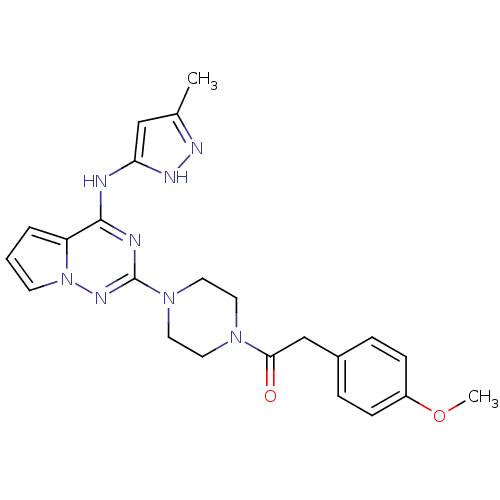
(2-(4-methoxyphenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3...)Show SMILES COc1ccc(CC(=O)N2CCN(CC2)c2nc(Nc3cc(C)n[nH]3)c3cccn3n2)cc1 Show InChI InChI=1S/C23H26N8O2/c1-16-14-20(27-26-16)24-22-19-4-3-9-31(19)28-23(25-22)30-12-10-29(11-13-30)21(32)15-17-5-7-18(33-2)8-6-17/h3-9,14H,10-13,15H2,1-2H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337338
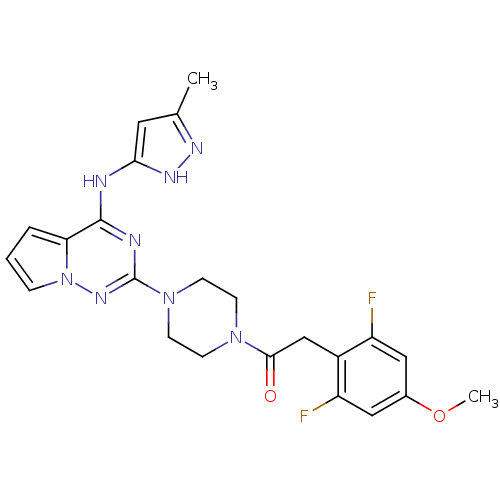
(2-(2,6-difluoro-4-methoxyphenyl)-1-(4-(4-(5-methyl...)Show SMILES COc1cc(F)c(CC(=O)N2CCN(CC2)c2nc(Nc3cc(C)n[nH]3)c3cccn3n2)c(F)c1 Show InChI InChI=1S/C23H24F2N8O2/c1-14-10-20(29-28-14)26-22-19-4-3-5-33(19)30-23(27-22)32-8-6-31(7-9-32)21(34)13-16-17(24)11-15(35-2)12-18(16)25/h3-5,10-12H,6-9,13H2,1-2H3,(H2,26,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516410
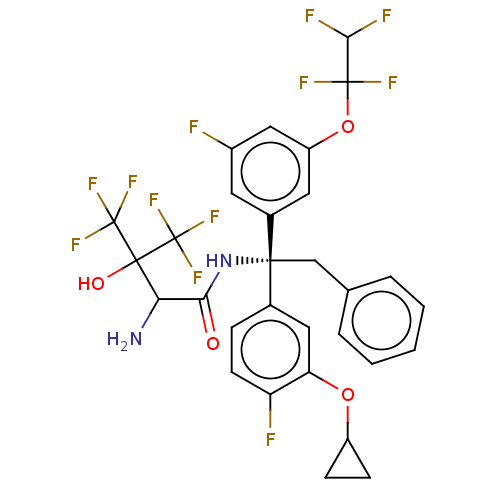
(CHEMBL4574163)Show SMILES NC(C(=O)N[C@](Cc1ccccc1)(c1ccc(F)c(OC2CC2)c1)c1cc(F)cc(OC(F)(F)C(F)F)c1)C(O)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C30H24F12N2O4/c31-18-10-17(11-20(13-18)48-28(35,36)25(33)34)26(14-15-4-2-1-3-5-15,16-6-9-21(32)22(12-16)47-19-7-8-19)44-24(45)23(43)27(46,29(37,38)39)30(40,41)42/h1-6,9-13,19,23,25,46H,7-8,14,43H2,(H,44,45)/t23?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337339
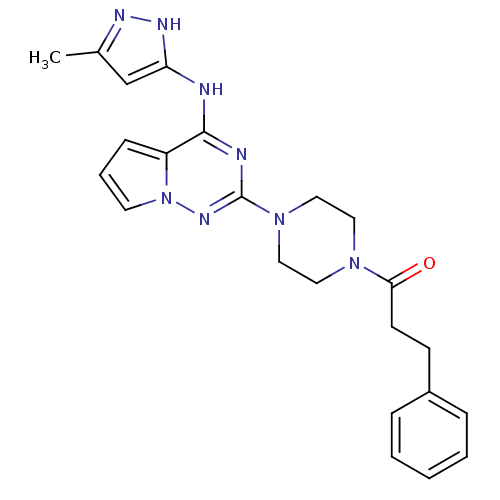
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)CCc2ccccc2)[nH]n1 Show InChI InChI=1S/C23H26N8O/c1-17-16-20(27-26-17)24-22-19-8-5-11-31(19)28-23(25-22)30-14-12-29(13-15-30)21(32)10-9-18-6-3-2-4-7-18/h2-8,11,16H,9-10,12-15H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337340

((2-bromophenyl)(4-(4-(5-methyl-1H-pyrazol-3-ylamin...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2Br)[nH]n1 Show InChI InChI=1S/C21H21BrN8O/c1-14-13-18(26-25-14)23-19-17-7-4-8-30(17)27-21(24-19)29-11-9-28(10-12-29)20(31)15-5-2-3-6-16(15)22/h2-8,13H,9-12H2,1H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337341
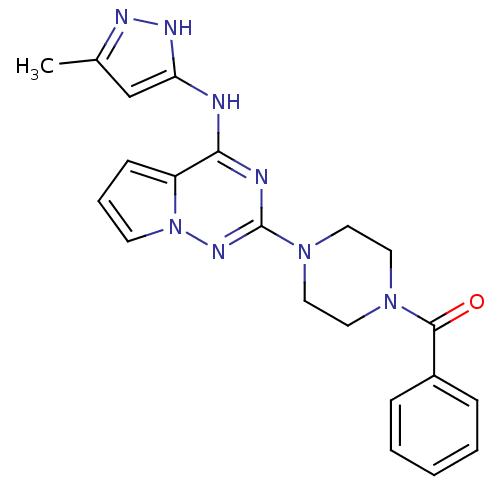
((4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2)[nH]n1 Show InChI InChI=1S/C21H22N8O/c1-15-14-18(25-24-15)22-19-17-8-5-9-29(17)26-21(23-19)28-12-10-27(11-13-28)20(30)16-6-3-2-4-7-16/h2-9,14H,10-13H2,1H3,(H2,22,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337342

(2-(2,6-difluorophenyl)-1-(4-(4-(5-methyl-1H-pyrazo...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(F)cc2F)[nH]n1 Show InChI InChI=1S/C22H22F2N8O/c1-14-11-19(28-27-14)25-21-18-3-2-6-32(18)29-22(26-21)31-9-7-30(8-10-31)20(33)12-15-4-5-16(23)13-17(15)24/h2-6,11,13H,7-10,12H2,1H3,(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337343
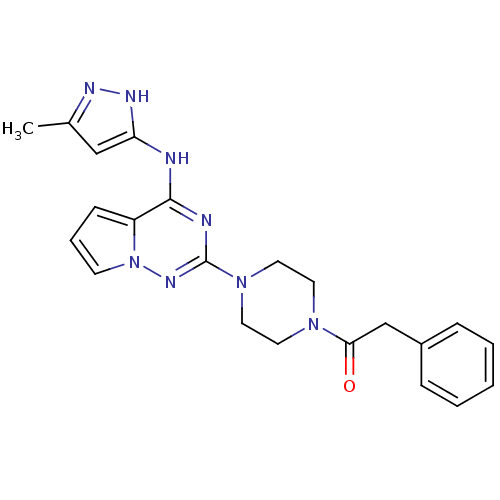
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccccc2)[nH]n1 Show InChI InChI=1S/C22H24N8O/c1-16-14-19(26-25-16)23-21-18-8-5-9-30(18)27-22(24-21)29-12-10-28(11-13-29)20(31)15-17-6-3-2-4-7-17/h2-9,14H,10-13,15H2,1H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516398

(CHEMBL4457286)Show SMILES CC(C)Oc1cc(ccc1F)[C@@](Cc1ccccc1)(NC(=O)C(N)C(O)(C(F)(F)F)C(F)(F)F)c1cc(F)cc(OC(F)(F)C(F)F)c1 |r| Show InChI InChI=1S/C30H26F12N2O4/c1-15(2)47-22-12-17(8-9-21(22)32)26(14-16-6-4-3-5-7-16,18-10-19(31)13-20(11-18)48-28(35,36)25(33)34)44-24(45)23(43)27(46,29(37,38)39)30(40,41)42/h3-13,15,23,25,46H,14,43H2,1-2H3,(H,44,45)/t23?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50094519
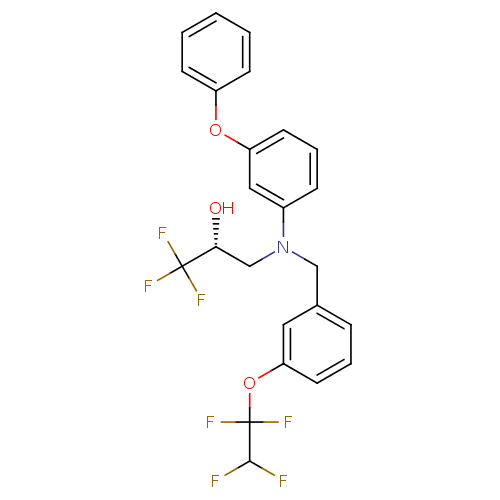
((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...)Show SMILES O[C@H](CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C24H20F7NO3/c25-22(26)24(30,31)35-20-11-4-6-16(12-20)14-32(15-21(33)23(27,28)29)17-7-5-10-19(13-17)34-18-8-2-1-3-9-18/h1-13,21-22,33H,14-15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337344
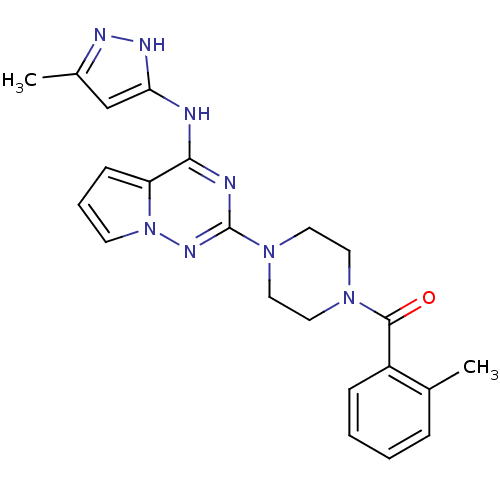
((4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2C)[nH]n1 Show InChI InChI=1S/C22H24N8O/c1-15-6-3-4-7-17(15)21(31)28-10-12-29(13-11-28)22-24-20(18-8-5-9-30(18)27-22)23-19-14-16(2)25-26-19/h3-9,14H,10-13H2,1-2H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337345
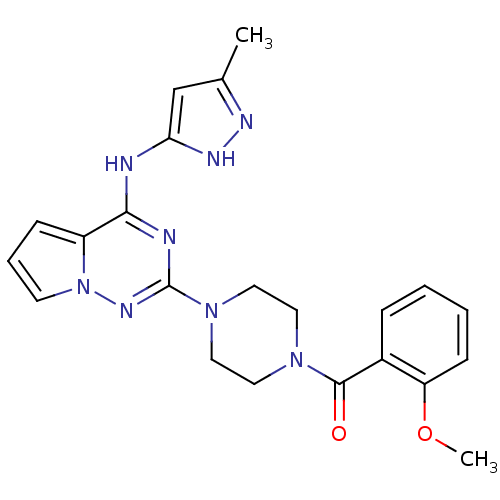
((2-methoxyphenyl)(4-(4-(5-methyl-1H-pyrazol-3-ylam...)Show SMILES COc1ccccc1C(=O)N1CCN(CC1)c1nc(Nc2cc(C)n[nH]2)c2cccn2n1 Show InChI InChI=1S/C22H24N8O2/c1-15-14-19(26-25-15)23-20-17-7-5-9-30(17)27-22(24-20)29-12-10-28(11-13-29)21(31)16-6-3-4-8-18(16)32-2/h3-9,14H,10-13H2,1-2H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337346
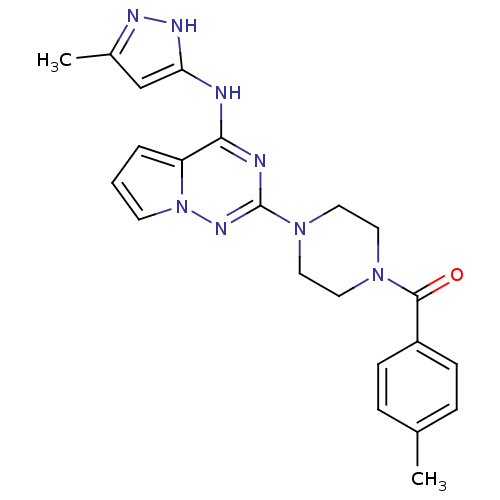
((4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccc(C)cc2)[nH]n1 Show InChI InChI=1S/C22H24N8O/c1-15-5-7-17(8-6-15)21(31)28-10-12-29(13-11-28)22-24-20(18-4-3-9-30(18)27-22)23-19-14-16(2)25-26-19/h3-9,14H,10-13H2,1-2H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337347
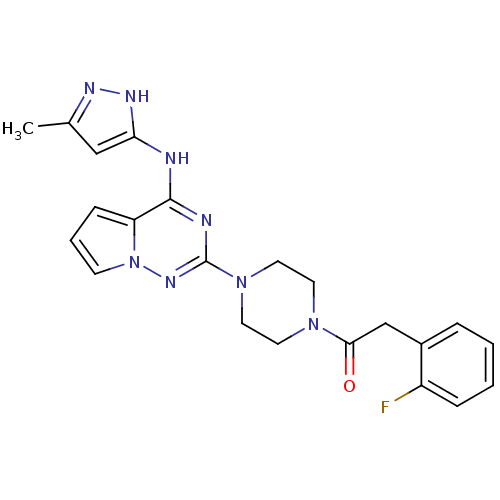
(2-(2-fluorophenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccccc2F)[nH]n1 Show InChI InChI=1S/C22H23FN8O/c1-15-13-19(27-26-15)24-21-18-7-4-8-31(18)28-22(25-21)30-11-9-29(10-12-30)20(32)14-16-5-2-3-6-17(16)23/h2-8,13H,9-12,14H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337348
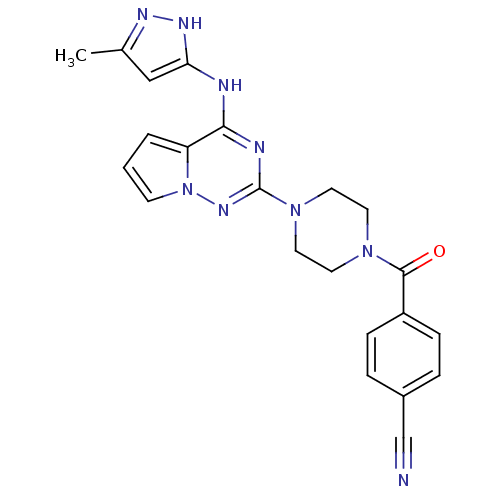
(4-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccc(cc2)C#N)[nH]n1 Show InChI InChI=1S/C22H21N9O/c1-15-13-19(27-26-15)24-20-18-3-2-8-31(18)28-22(25-20)30-11-9-29(10-12-30)21(32)17-6-4-16(14-23)5-7-17/h2-8,13H,9-12H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516405

(CHEMBL4552555)Show SMILES CC(C)Oc1cc(ccc1F)[C@@](Cc1ccccc1)(NCC(O)C(O)(C(F)(F)F)C(F)(F)F)c1cc(F)cc(OC(F)(F)C(F)F)c1 |r| Show InChI InChI=1S/C30H27F12NO4/c1-16(2)46-23-12-18(8-9-22(23)32)26(14-17-6-4-3-5-7-17,43-15-24(44)27(45,29(37,38)39)30(40,41)42)19-10-20(31)13-21(11-19)47-28(35,36)25(33)34/h3-13,16,24-25,43-45H,14-15H2,1-2H3/t24?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516406

(CHEMBL4469874)Show SMILES CC(C)(C)Oc1cc(ccc1F)[C@@](Cc1ccccc1)(NC(=O)C(N)C(O)(C(F)(F)F)C(F)(F)F)c1cc(F)cc(OC(F)(F)C(F)F)c1 |r| Show InChI InChI=1S/C31H28F12N2O4/c1-26(2,3)49-22-13-17(9-10-21(22)33)27(15-16-7-5-4-6-8-16,18-11-19(32)14-20(12-18)48-29(36,37)25(34)35)45-24(46)23(44)28(47,30(38,39)40)31(41,42)43/h4-14,23,25,47H,15,44H2,1-3H3,(H,45,46)/t23?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337349
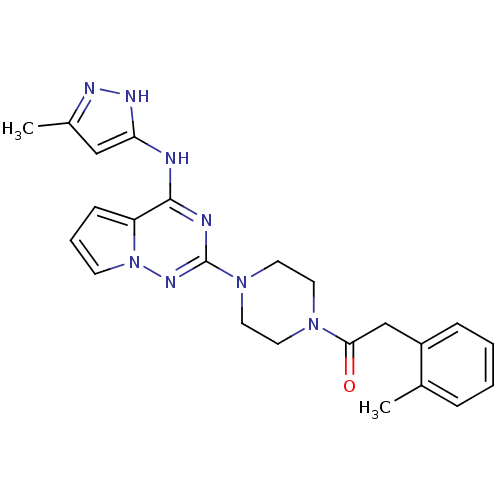
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccccc2C)[nH]n1 Show InChI InChI=1S/C23H26N8O/c1-16-6-3-4-7-18(16)15-21(32)29-10-12-30(13-11-29)23-25-22(19-8-5-9-31(19)28-23)24-20-14-17(2)26-27-20/h3-9,14H,10-13,15H2,1-2H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516400

(CHEMBL4439856)Show SMILES CC(C)Oc1cc(ccc1F)[C@@](Cc1ccccc1)(NC(=O)C(N)C(O)C(F)(F)F)c1cc(F)cc(OC(F)(F)C(F)F)c1 |r| Show InChI InChI=1S/C29H27F9N2O4/c1-15(2)43-22-12-17(8-9-21(22)31)27(14-16-6-4-3-5-7-16,40-25(42)23(39)24(41)28(34,35)36)18-10-19(30)13-20(11-18)44-29(37,38)26(32)33/h3-13,15,23-24,26,41H,14,39H2,1-2H3,(H,40,42)/t23?,24?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337350
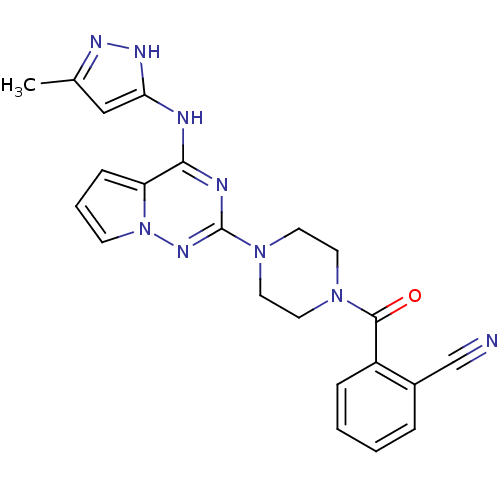
(2-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2C#N)[nH]n1 Show InChI InChI=1S/C22H21N9O/c1-15-13-19(27-26-15)24-20-18-7-4-8-31(18)28-22(25-20)30-11-9-29(10-12-30)21(32)17-6-3-2-5-16(17)14-23/h2-8,13H,9-12H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50392512
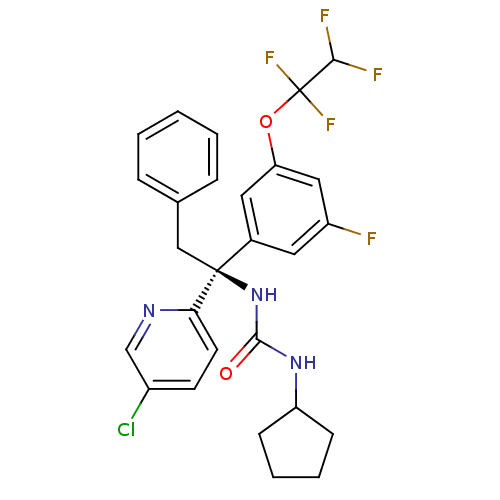
(CHEMBL2152165)Show SMILES FC(F)C(F)(F)Oc1cc(F)cc(c1)[C@](Cc1ccccc1)(NC(=O)NC1CCCC1)c1ccc(Cl)cn1 |r| Show InChI InChI=1S/C27H25ClF5N3O2/c28-19-10-11-23(34-16-19)26(15-17-6-2-1-3-7-17,36-25(37)35-21-8-4-5-9-21)18-12-20(29)14-22(13-18)38-27(32,33)24(30)31/h1-3,6-7,10-14,16,21,24H,4-5,8-9,15H2,(H2,35,36,37)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50392512

(CHEMBL2152165)Show SMILES FC(F)C(F)(F)Oc1cc(F)cc(c1)[C@](Cc1ccccc1)(NC(=O)NC1CCCC1)c1ccc(Cl)cn1 |r| Show InChI InChI=1S/C27H25ClF5N3O2/c28-19-10-11-23(34-16-19)26(15-17-6-2-1-3-7-17,36-25(37)35-21-8-4-5-9-21)18-12-20(29)14-22(13-18)38-27(32,33)24(30)31/h1-3,6-7,10-14,16,21,24H,4-5,8-9,15H2,(H2,35,36,37)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP using [3H]-CE/HDL by scintillation proximity assay |
Bioorg Med Chem Lett 24: 860-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.088
BindingDB Entry DOI: 10.7270/Q2WM1FW9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337351
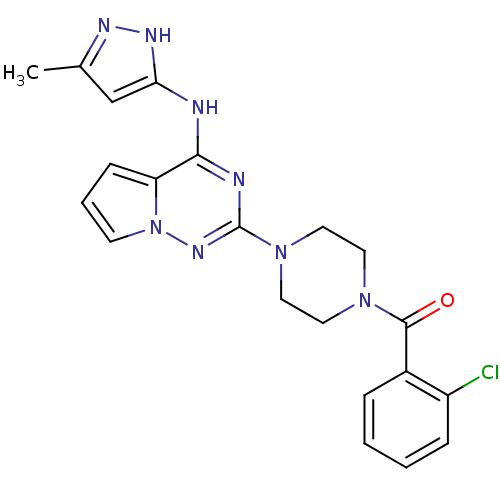
((2-chlorophenyl)(4-(4-(5-methyl-1H-pyrazol-3-ylami...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2Cl)[nH]n1 Show InChI InChI=1S/C21H21ClN8O/c1-14-13-18(26-25-14)23-19-17-7-4-8-30(17)27-21(24-19)29-11-9-28(10-12-29)20(31)15-5-2-3-6-16(15)22/h2-8,13H,9-12H2,1H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337352
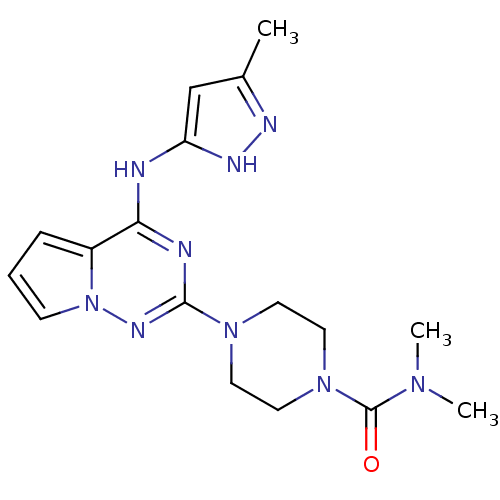
(CHEMBL1682370 | N,N-dimethyl-4-(4-(5-methyl-1H-pyr...)Show SMILES CN(C)C(=O)N1CCN(CC1)c1nc(Nc2cc(C)n[nH]2)c2cccn2n1 Show InChI InChI=1S/C17H23N9O/c1-12-11-14(21-20-12)18-15-13-5-4-6-26(13)22-16(19-15)24-7-9-25(10-8-24)17(27)23(2)3/h4-6,11H,7-10H2,1-3H3,(H2,18,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50376718
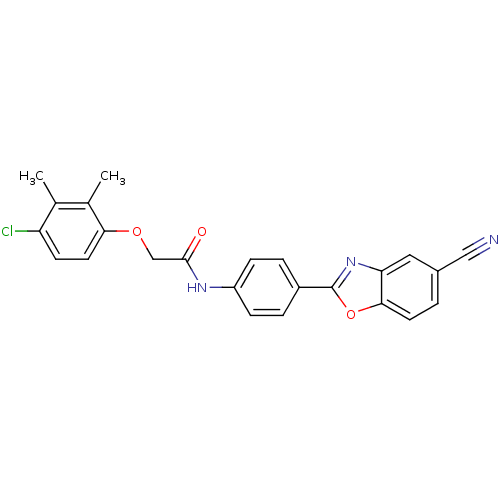
(CHEMBL261502)Show SMILES Cc1c(Cl)ccc(OCC(=O)Nc2ccc(cc2)-c2nc3cc(ccc3o2)C#N)c1C Show InChI InChI=1S/C24H18ClN3O3/c1-14-15(2)21(10-8-19(14)25)30-13-23(29)27-18-6-4-17(5-7-18)24-28-20-11-16(12-26)3-9-22(20)31-24/h3-11H,13H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50376712
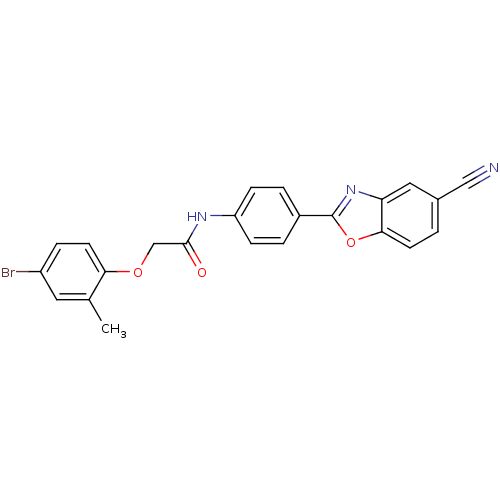
(CHEMBL259502)Show SMILES Cc1cc(Br)ccc1OCC(=O)Nc1ccc(cc1)-c1nc2cc(ccc2o1)C#N Show InChI InChI=1S/C23H16BrN3O3/c1-14-10-17(24)5-9-20(14)29-13-22(28)26-18-6-3-16(4-7-18)23-27-19-11-15(12-25)2-8-21(19)30-23/h2-11H,13H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516402
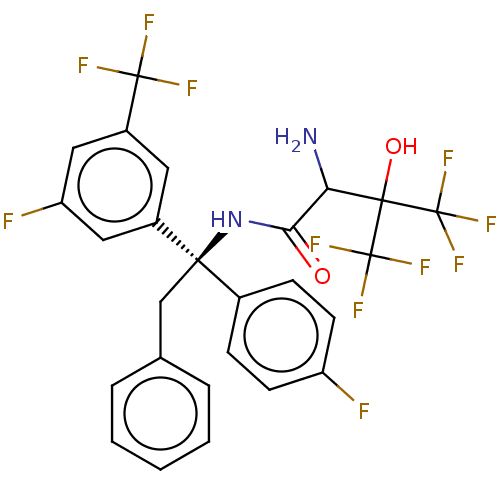
(CHEMBL4473304)Show SMILES NC(C(=O)N[C@](Cc1ccccc1)(c1ccc(F)cc1)c1cc(F)cc(c1)C(F)(F)F)C(O)(C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H19F11N2O2/c27-18-8-6-15(7-9-18)22(13-14-4-2-1-3-5-14,16-10-17(24(29,30)31)12-19(28)11-16)39-21(40)20(38)23(41,25(32,33)34)26(35,36)37/h1-12,20,41H,13,38H2,(H,39,40)/t20?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50312718
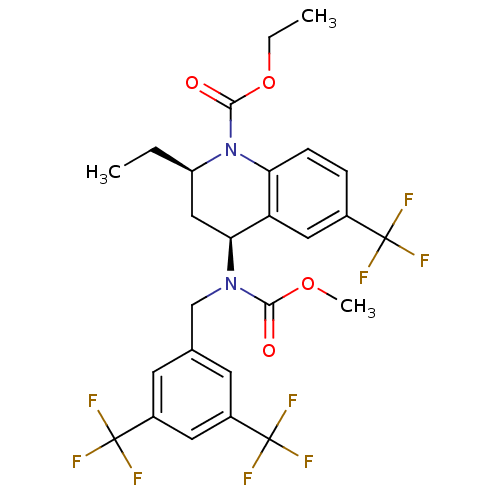
(CHEMBL479527 | torcetrapib)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)C(=O)OC)c2cc(ccc12)C(F)(F)F |r| Show InChI InChI=1S/C26H25F9N2O4/c1-4-18-12-21(19-11-15(24(27,28)29)6-7-20(19)37(18)23(39)41-5-2)36(22(38)40-3)13-14-8-16(25(30,31)32)10-17(9-14)26(33,34)35/h6-11,18,21H,4-5,12-13H2,1-3H3/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50376713
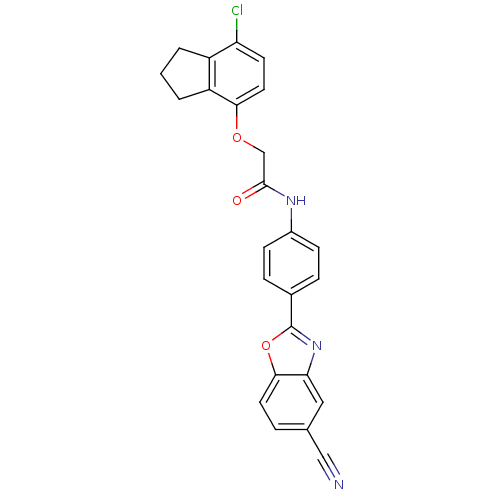
(CHEMBL259710)Show SMILES Clc1ccc(OCC(=O)Nc2ccc(cc2)-c2nc3cc(ccc3o2)C#N)c2CCCc12 Show InChI InChI=1S/C25H18ClN3O3/c26-20-9-11-22(19-3-1-2-18(19)20)31-14-24(30)28-17-7-5-16(6-8-17)25-29-21-12-15(13-27)4-10-23(21)32-25/h4-12H,1-3,14H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50394941
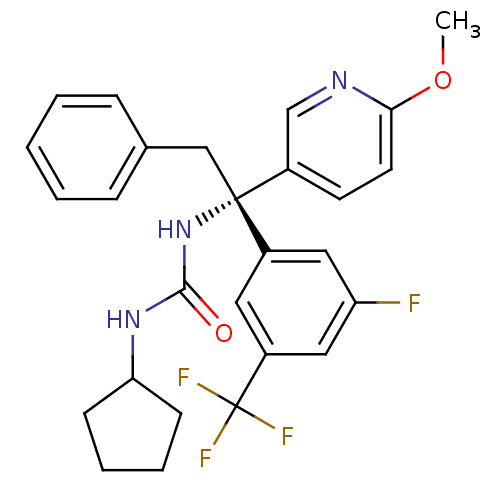
(CHEMBL2165719)Show SMILES COc1ccc(cn1)[C@@](Cc1ccccc1)(NC(=O)NC1CCCC1)c1cc(F)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H27F4N3O2/c1-36-24-12-11-19(17-32-24)26(16-18-7-3-2-4-8-18,34-25(35)33-23-9-5-6-10-23)20-13-21(27(29,30)31)15-22(28)14-20/h2-4,7-8,11-15,17,23H,5-6,9-10,16H2,1H3,(H2,33,34,35)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50394932
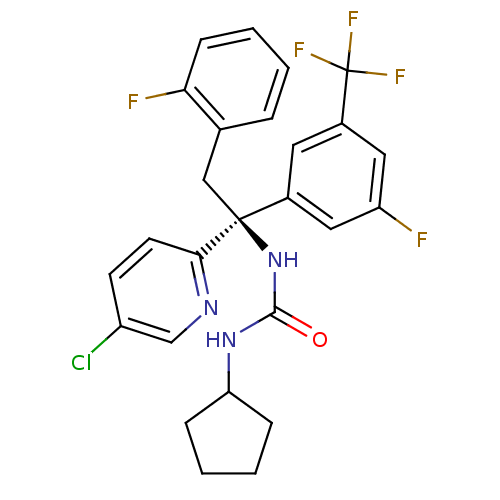
(CHEMBL2165691)Show SMILES Fc1cc(cc(c1)[C@](Cc1ccccc1F)(NC(=O)NC1CCCC1)c1ccc(Cl)cn1)C(F)(F)F |r| Show InChI InChI=1S/C26H23ClF5N3O/c27-19-9-10-23(33-15-19)25(14-16-5-1-4-8-22(16)29,35-24(36)34-21-6-2-3-7-21)17-11-18(26(30,31)32)13-20(28)12-17/h1,4-5,8-13,15,21H,2-3,6-7,14H2,(H2,34,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50376711
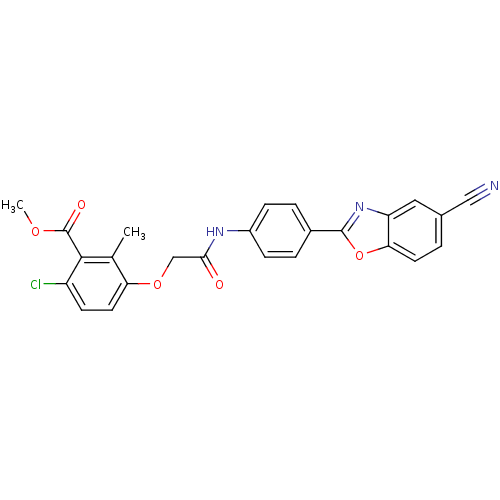
(CHEMBL259503)Show SMILES COC(=O)c1c(Cl)ccc(OCC(=O)Nc2ccc(cc2)-c2nc3cc(ccc3o2)C#N)c1C Show InChI InChI=1S/C25H18ClN3O5/c1-14-20(10-8-18(26)23(14)25(31)32-2)33-13-22(30)28-17-6-4-16(5-7-17)24-29-19-11-15(12-27)3-9-21(19)34-24/h3-11H,13H2,1-2H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50376719

(CHEMBL258795)Show SMILES FC(F)(F)c1cc(OCC(=O)Nc2ccc(cc2)-c2nc3cc(ccc3o2)C#N)ccc1Cl Show InChI InChI=1S/C23H13ClF3N3O3/c24-18-7-6-16(10-17(18)23(25,26)27)32-12-21(31)29-15-4-2-14(3-5-15)22-30-19-9-13(11-28)1-8-20(19)33-22/h1-10H,12H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50392499

(CHEMBL2152152)Show SMILES Fc1cc(cc(c1)[C@](Cc1ccccc1)(NC(=O)NC1CCCC1)c1ccc(Cl)cn1)C(F)(F)F |r| Show InChI InChI=1S/C26H24ClF4N3O/c27-20-10-11-23(32-16-20)25(15-17-6-2-1-3-7-17,34-24(35)33-22-8-4-5-9-22)18-12-19(26(29,30)31)14-21(28)13-18/h1-3,6-7,10-14,16,22H,4-5,8-9,15H2,(H2,33,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50447540
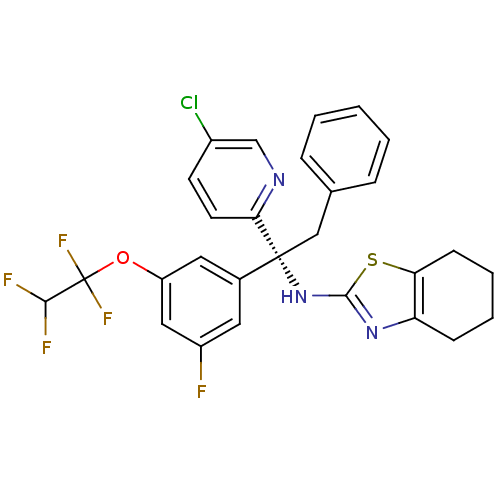
(CHEMBL3116159)Show SMILES FC(F)C(F)(F)Oc1cc(F)cc(c1)[C@@](Cc1ccccc1)(Nc1nc2CCCCc2s1)c1ccc(Cl)cn1 |r| Show InChI InChI=1S/C28H23ClF5N3OS/c29-19-10-11-24(35-16-19)27(15-17-6-2-1-3-7-17,37-26-36-22-8-4-5-9-23(22)39-26)18-12-20(30)14-21(13-18)38-28(33,34)25(31)32/h1-3,6-7,10-14,16,25H,4-5,8-9,15H2,(H,36,37)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP using [3H]-CE/HDL by scintillation proximity assay |
Bioorg Med Chem Lett 24: 860-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.088
BindingDB Entry DOI: 10.7270/Q2WM1FW9 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50447538

(CHEMBL3116157)Show SMILES Cc1sc(N[C@](Cc2ccccc2)(c2cc(F)cc(OC(F)(F)C(F)F)c2)c2ccc(Cl)cn2)nc1C(F)(F)F |r| Show InChI InChI=1S/C26H18ClF8N3OS/c1-14-21(25(31,32)33)37-23(40-14)38-24(12-15-5-3-2-4-6-15,20-8-7-17(27)13-36-20)16-9-18(28)11-19(10-16)39-26(34,35)22(29)30/h2-11,13,22H,12H2,1H3,(H,37,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP using [3H]-CE/HDL by scintillation proximity assay |
Bioorg Med Chem Lett 24: 860-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.088
BindingDB Entry DOI: 10.7270/Q2WM1FW9 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50392499

(CHEMBL2152152)Show SMILES Fc1cc(cc(c1)[C@](Cc1ccccc1)(NC(=O)NC1CCCC1)c1ccc(Cl)cn1)C(F)(F)F |r| Show InChI InChI=1S/C26H24ClF4N3O/c27-20-10-11-23(32-16-20)25(15-17-6-2-1-3-7-17,34-24(35)33-22-8-4-5-9-22)18-12-19(26(29,30)31)14-21(28)13-18/h1-3,6-7,10-14,16,22H,4-5,8-9,15H2,(H2,33,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP using [3H]-CE/HDL by scintillation proximity assay |
Bioorg Med Chem Lett 24: 860-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.088
BindingDB Entry DOI: 10.7270/Q2WM1FW9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337354
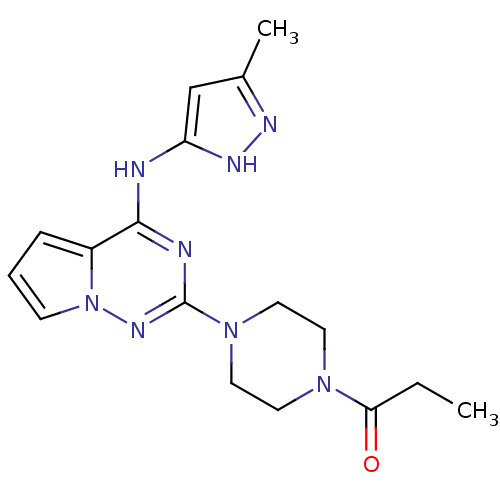
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES CCC(=O)N1CCN(CC1)c1nc(Nc2cc(C)n[nH]2)c2cccn2n1 Show InChI InChI=1S/C17H22N8O/c1-3-15(26)23-7-9-24(10-8-23)17-19-16(13-5-4-6-25(13)22-17)18-14-11-12(2)20-21-14/h4-6,11H,3,7-10H2,1-2H3,(H2,18,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337353
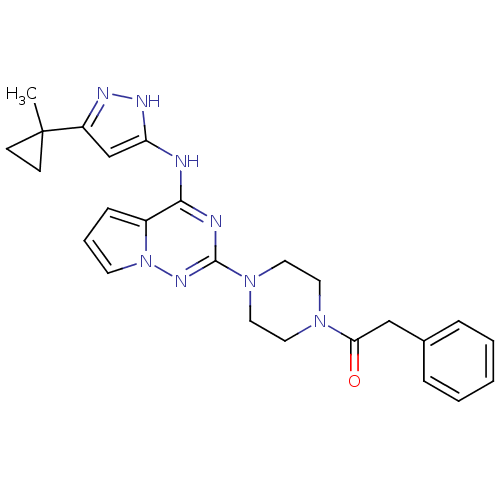
(1-(4-(4-(5-(1-methylcyclopropyl)-1H-pyrazol-3-ylam...)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccccc2)[nH]n1 Show InChI InChI=1S/C25H28N8O/c1-25(9-10-25)20-17-21(29-28-20)26-23-19-8-5-11-33(19)30-24(27-23)32-14-12-31(13-15-32)22(34)16-18-6-3-2-4-7-18/h2-8,11,17H,9-10,12-16H2,1H3,(H2,26,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50376693
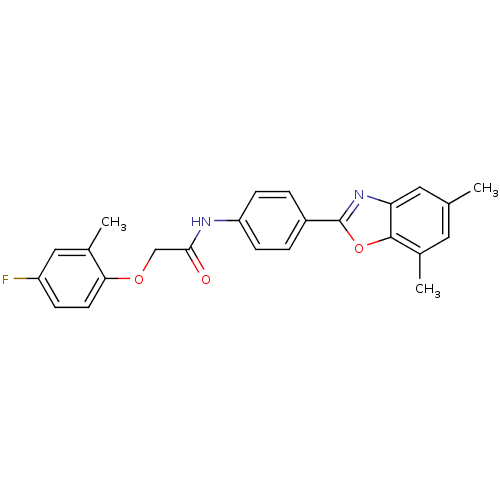
(CHEMBL409318)Show SMILES Cc1cc(C)c2oc(nc2c1)-c1ccc(NC(=O)COc2ccc(F)cc2C)cc1 Show InChI InChI=1S/C24H21FN2O3/c1-14-10-16(3)23-20(11-14)27-24(30-23)17-4-7-19(8-5-17)26-22(28)13-29-21-9-6-18(25)12-15(21)2/h4-12H,13H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CETP by scintillation proximity assay |
Bioorg Med Chem Lett 18: 2640-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.030
BindingDB Entry DOI: 10.7270/Q2V40W3V |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50394933
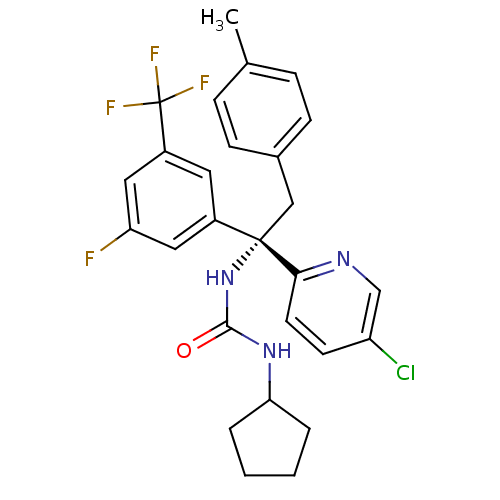
(CHEMBL2165690)Show SMILES Cc1ccc(C[C@](NC(=O)NC2CCCC2)(c2cc(F)cc(c2)C(F)(F)F)c2ccc(Cl)cn2)cc1 |r| Show InChI InChI=1S/C27H26ClF4N3O/c1-17-6-8-18(9-7-17)15-26(24-11-10-21(28)16-33-24,35-25(36)34-23-4-2-3-5-23)19-12-20(27(30,31)32)14-22(29)13-19/h6-14,16,23H,2-5,15H2,1H3,(H2,34,35,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50394939
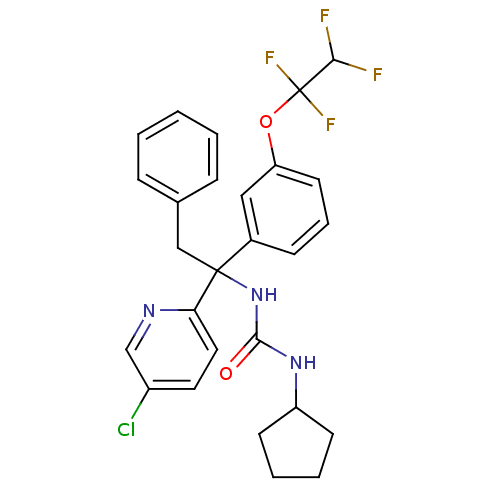
(CHEMBL2165681)Show SMILES FC(F)C(F)(F)Oc1cccc(c1)C(Cc1ccccc1)(NC(=O)NC1CCCC1)c1ccc(Cl)cn1 Show InChI InChI=1S/C27H26ClF4N3O2/c28-20-13-14-23(33-17-20)26(16-18-7-2-1-3-8-18,35-25(36)34-21-10-4-5-11-21)19-9-6-12-22(15-19)37-27(31,32)24(29)30/h1-3,6-9,12-15,17,21,24H,4-5,10-11,16H2,(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50394943
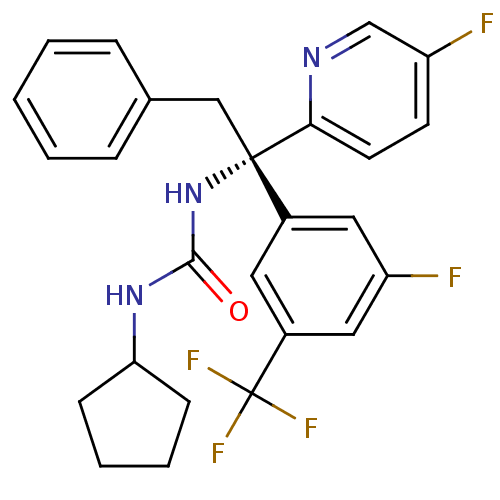
(CHEMBL2165717)Show SMILES Fc1ccc(nc1)[C@@](Cc1ccccc1)(NC(=O)NC1CCCC1)c1cc(F)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H24F5N3O/c27-20-10-11-23(32-16-20)25(15-17-6-2-1-3-7-17,34-24(35)33-22-8-4-5-9-22)18-12-19(26(29,30)31)14-21(28)13-18/h1-3,6-7,10-14,16,22H,4-5,8-9,15H2,(H2,33,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay |
J Med Chem 55: 6162-75 (2012)
Article DOI: 10.1021/jm300611v
BindingDB Entry DOI: 10.7270/Q2KH0PF5 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50516408
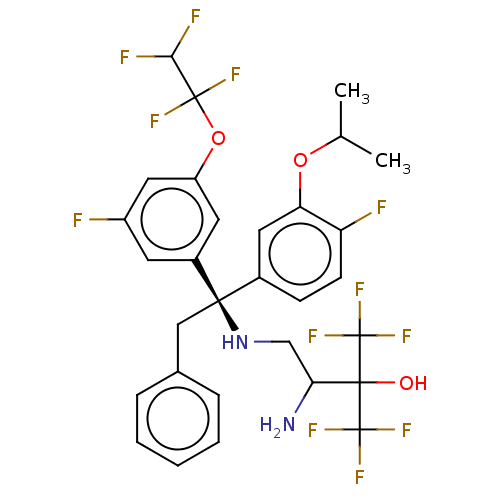
(CHEMBL4468692)Show SMILES CC(C)Oc1cc(ccc1F)[C@@](Cc1ccccc1)(NCC(N)C(O)(C(F)(F)F)C(F)(F)F)c1cc(F)cc(OC(F)(F)C(F)F)c1 |r| Show InChI InChI=1S/C30H28F12N2O3/c1-16(2)46-23-12-18(8-9-22(23)32)26(14-17-6-4-3-5-7-17,44-15-24(43)27(45,29(37,38)39)30(40,41)42)19-10-20(31)13-21(11-19)47-28(35,36)25(33)34/h3-13,16,24-25,44-45H,14-15,43H2,1-2H3/t24?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay |
ACS Med Chem Lett 10: 911-916 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00086
BindingDB Entry DOI: 10.7270/Q2TX3JQJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data