

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human DOT1L using oligo-nucleosome/[3H]-SAM as substrate preincubated for 30 mins followed by substrate addition measured after 120 min... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023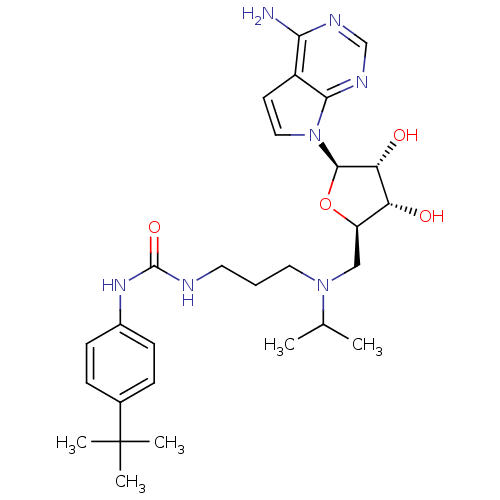 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Binding affinity to human DOT1L after 120 mins | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human DOT1L using oligo-nucleosome/[3H]-SAM as substrate preincubated for 30 mins followed by substrate addition measured after 120 min... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50075073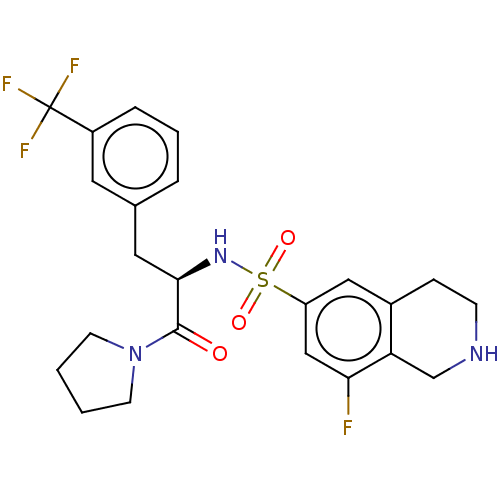 (CHEMBL3414622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of full-length human SETD7 expressed in Escherichia coli BL21 (DE3) using biotinylated histone H3 (1 to 25) as substrate after 1 hr by Fla... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 A677G mutant assessed as H3K27me1 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641F mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 A677G mutant assessed as H3K27me0 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human wild-type EZH2 assessed as H3K27me0 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641H mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641N mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641S mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 Y641C mutant assessed as H3K27me2 level after 30 mins by scintillation counting analysis in presence of [3H]-SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172038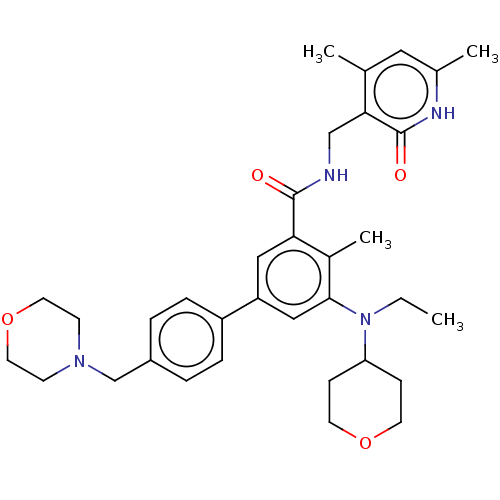 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of wild-type human EZH2 by flash plate assay | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178102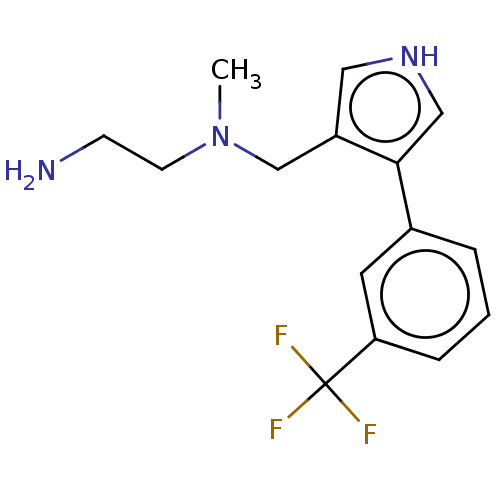 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075071 (CHEMBL3414619) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of EZH2 (unknown origin) using biotinylated-histone H3 (1 to 24) as substrate by Lineweaver-Burk plot analysis in presence of ... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 11 | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075054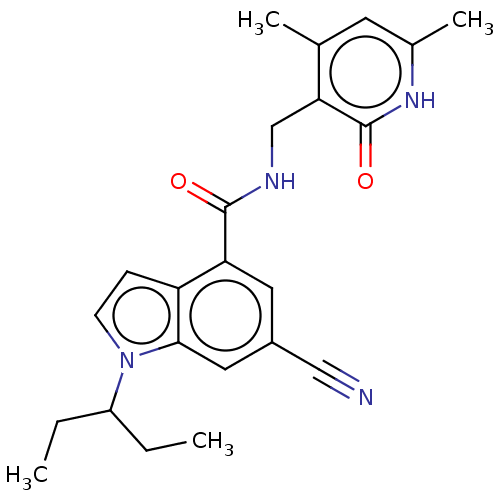 (CHEMBL3414574) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG tagged full-length human EZH2 expressed in baculovirus infected Sf9 cells using H3K27 as substrate in presence of SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 23 | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051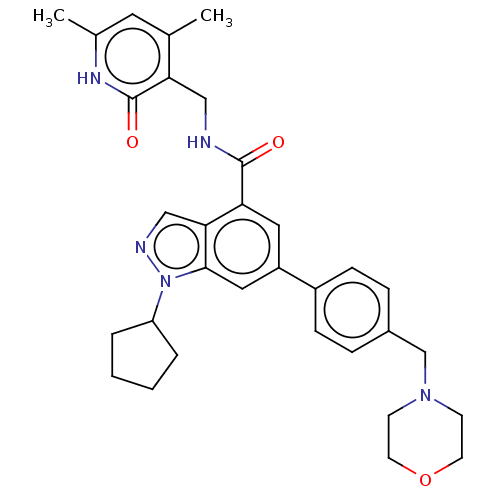 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of human EZH2 using SAM and histone H3 (16 to 30) as substrate preincubated for 30 mins followed by substrate addition measure... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 55 | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50194756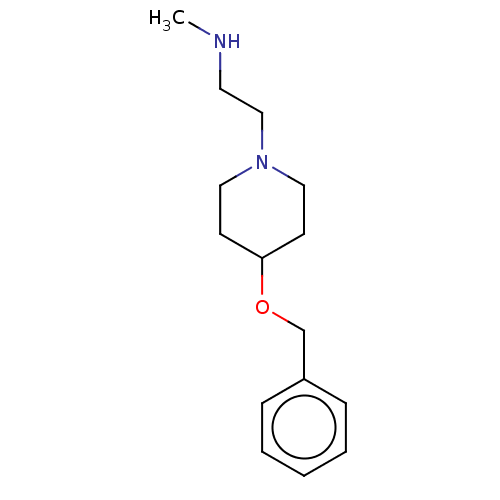 (CHEMBL3961701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Displacement of [3H]pentazocine from guinea pig sigma1 receptor | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194756 (CHEMBL3961701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Displacement of [3H]alpha-methylhistamine from human histamine H3 receptor expressed HEK Flp-In cell membranes | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178101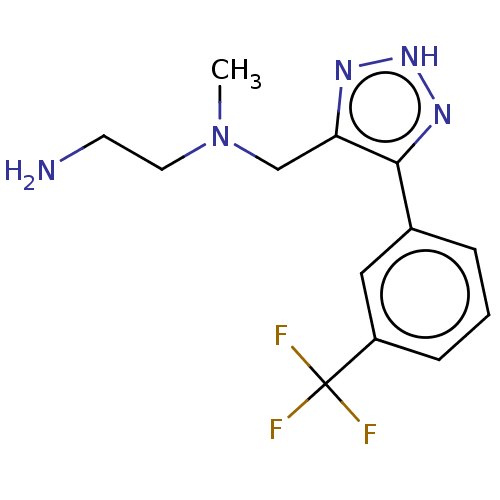 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396981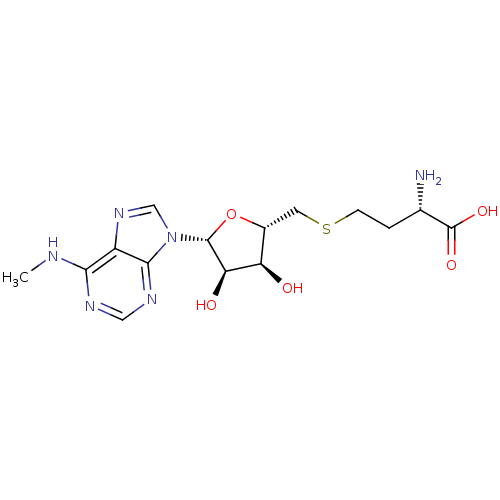 (CHEMBL2171174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of human DOT1L (1 to 472) using oligo-nucleosome as substrate preincubated for 10 mins followed by substrate addition measured... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400783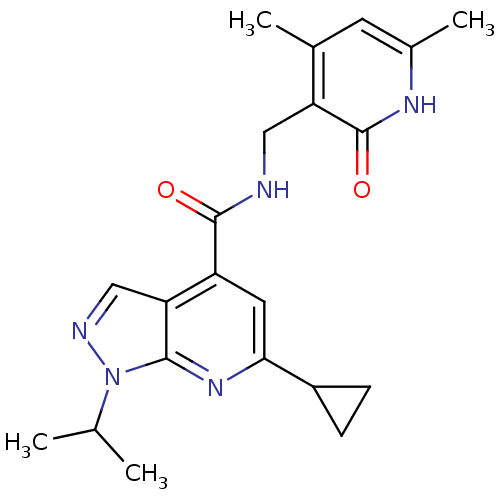 (CHEMBL1608462) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Non-competitive inhibition of human EZH2 using [3H]-SAM as substrate in presence of H3K27A | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400783 (CHEMBL1608462) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of human EZH2 using H3K27A as substrate in presence of SAM | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116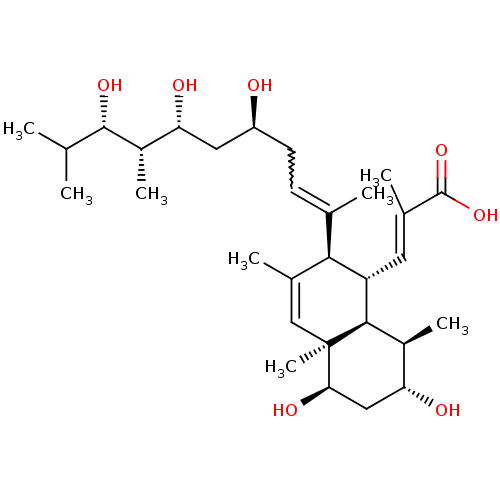 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of SETD8 (unknown origin) using H4 (1 to 24) as substrate assessed as incorporation of [3H]-methyl group from [3H-Me]-SAM to p... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50396981 (CHEMBL2171174) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of PRMT1 (unknown origin) using histone-H4 as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50396981 (CHEMBL2171174) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of human CARM1 using oligo-nucleosome as substrate preincubated for 10 mins followed by substrate addition measured after 30 m... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H1 (Homo sapiens (Human)) | BDBM50396981 (CHEMBL2171174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of SUV39H1 (unknown origin) using histone-H3 (1 to 21) as substrate preincubated for 10 mins followed by substrate addition me... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50396981 (CHEMBL2171174) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of G9a (unknown origin) using histone-H3 (1 to 21) as substrate preincubated for 10 mins followed by substrate addition measur... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.75E+4 | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of recombinant human DOT1L (1 to 416) using [3H]-SAM, SAM and nucleosome as substrate assessed as incorporation of radioactivity into nucl... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50075073 (CHEMBL3414622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of full-length human SETD7 expressed in Escherichia coli BL21 (DE3) using biotinylated histone H3 (1 to 25) as substrate after 1 hr by Fla... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of DOT1L in human MV4-11 cells expressing MLL-AF4 assessed as reduction of H3K79me2 level after 4 days by ELISA method | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of DOT1L in human MV4-11 cells expressing MLL-AF4 assessed as cell growth inhibition after 14 days by Guava Viacount assay | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT1 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT6 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT3 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT8 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT4 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 370 total ) | Next | Last >> |