 Found 1016 hits with Last Name = 'keddy' and Initial = 'r'
Found 1016 hits with Last Name = 'keddy' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143965
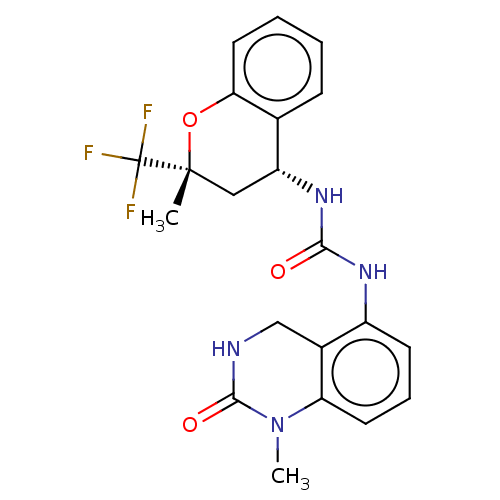
(US8969325, 166)Show SMILES CN1C(=O)NCc2c(NC(=O)N[C@@H]3C[C@@](C)(Oc4ccccc34)C(F)(F)F)cccc12 Show InChI InChI=1S/C21H21F3N4O3/c1-20(21(22,23)24)10-15(12-6-3-4-9-17(12)31-20)27-18(29)26-14-7-5-8-16-13(14)11-25-19(30)28(16)2/h3-9,15H,10-11H2,1-2H3,(H,25,30)(H2,26,27,29)/t15-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143964
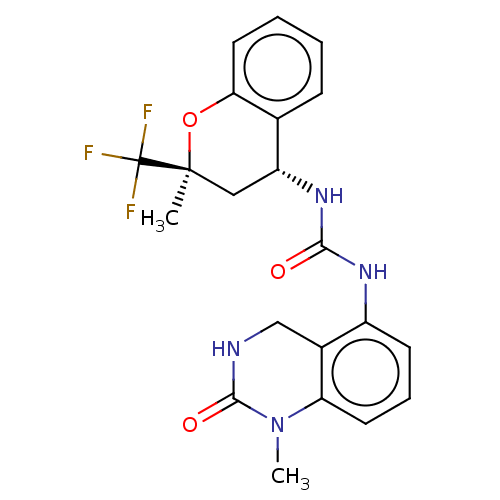
(US8969325, 165)Show SMILES CN1C(=O)NCc2c(NC(=O)N[C@@H]3C[C@](C)(Oc4ccccc34)C(F)(F)F)cccc12 Show InChI InChI=1S/C21H21F3N4O3/c1-20(21(22,23)24)10-15(12-6-3-4-9-17(12)31-20)27-18(29)26-14-7-5-8-16-13(14)11-25-19(30)28(16)2/h3-9,15H,10-11H2,1-2H3,(H,25,30)(H2,26,27,29)/t15-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319456
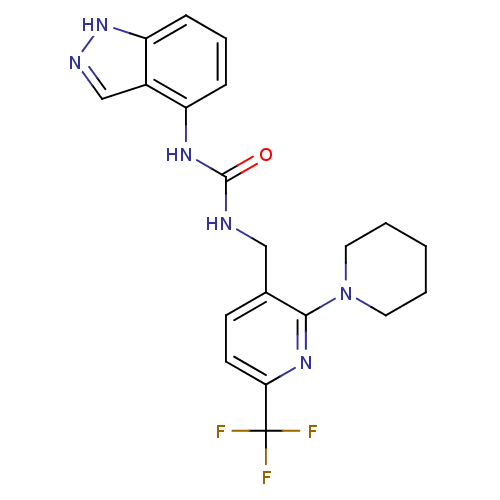
(1-(1H-indazol-4-yl)-3-((2-(piperidin-1-yl)-6-(trif...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(n1)N1CCCCC1 Show InChI InChI=1S/C20H21F3N6O/c21-20(22,23)17-8-7-13(18(27-17)29-9-2-1-3-10-29)11-24-19(30)26-15-5-4-6-16-14(15)12-25-28-16/h4-8,12H,1-3,9-11H2,(H,25,28)(H2,24,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143829

(US8969325, 30)Show SMILES C[C@@]1(C[C@@H](NC(=O)Nc2ccc3CCC(=O)Nc3c2)c2ccc(Cl)cc2O1)C(F)F Show InChI InChI=1S/C21H20ClF2N3O3/c1-21(19(23)24)10-16(14-6-4-12(22)8-17(14)30-21)27-20(29)25-13-5-2-11-3-7-18(28)26-15(11)9-13/h2,4-6,8-9,16,19H,3,7,10H2,1H3,(H,26,28)(H2,25,27,29)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143834

(US8969325, 35)Show SMILES FC(F)(F)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1ccc2CCC(=O)Nc2c1 Show InChI InChI=1S/C20H18F3N3O2/c21-20(22,23)13-4-6-15-12(9-13)2-7-16(15)26-19(28)24-14-5-1-11-3-8-18(27)25-17(11)10-14/h1,4-6,9-10,16H,2-3,7-8H2,(H,25,27)(H2,24,26,28)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319471

(1-(2-(3,3-dimethylbutyl)-4-(trifluoromethyl)benzyl...)Show SMILES CC(C)(C)CCc1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O/c1-21(2,3)10-9-14-11-16(22(23,24)25)8-7-15(14)12-26-20(30)28-18-5-4-6-19-17(18)13-27-29-19/h4-8,11,13H,9-10,12H2,1-3H3,(H,27,29)(H2,26,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM144122
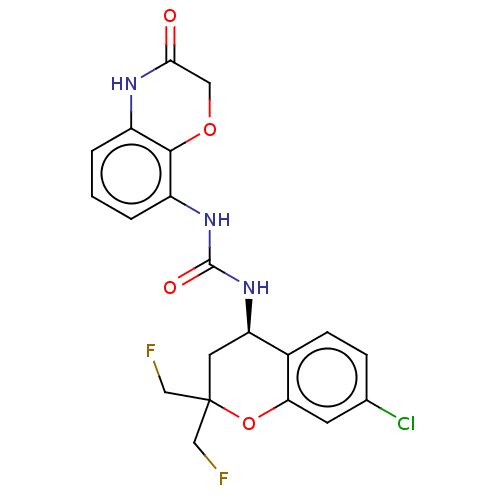
(US8969325, 323)Show SMILES FCC1(CF)C[C@@H](NC(=O)Nc2cccc3NC(=O)COc23)c2ccc(Cl)cc2O1 Show InChI InChI=1S/C20H18ClF2N3O4/c21-11-4-5-12-15(7-20(9-22,10-23)30-16(12)6-11)26-19(28)25-14-3-1-2-13-18(14)29-8-17(27)24-13/h1-6,15H,7-10H2,(H,24,27)(H2,25,26,28)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143966
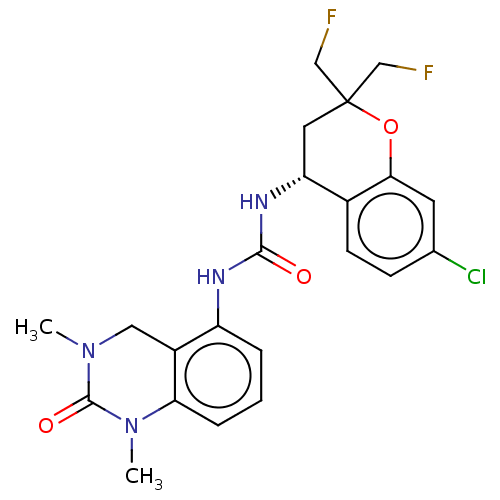
(US8969325, 167)Show SMILES CN1Cc2c(NC(=O)N[C@@H]3CC(CF)(CF)Oc4cc(Cl)ccc34)cccc2N(C)C1=O Show InChI InChI=1S/C22H23ClF2N4O3/c1-28-10-15-16(4-3-5-18(15)29(2)21(28)31)26-20(30)27-17-9-22(11-24,12-25)32-19-8-13(23)6-7-14(17)19/h3-8,17H,9-12H2,1-2H3,(H2,26,27,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50133817
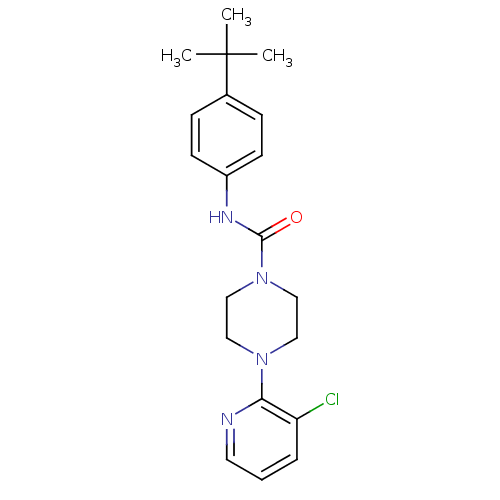
(4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...)Show SMILES CC(C)(C)c1ccc(NC(=O)N2CCN(CC2)c2ncccc2Cl)cc1 Show InChI InChI=1S/C20H25ClN4O/c1-20(2,3)15-6-8-16(9-7-15)23-19(26)25-13-11-24(12-14-25)18-17(21)5-4-10-22-18/h4-10H,11-14H2,1-3H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in human 1321N1 cells assessed as inhibition of capsaicin-induced calcium influx by FLIPR as... |
Bioorg Med Chem 16: 8516-25 (2008)
Article DOI: 10.1016/j.bmc.2008.08.005
BindingDB Entry DOI: 10.7270/Q2FX798X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319465
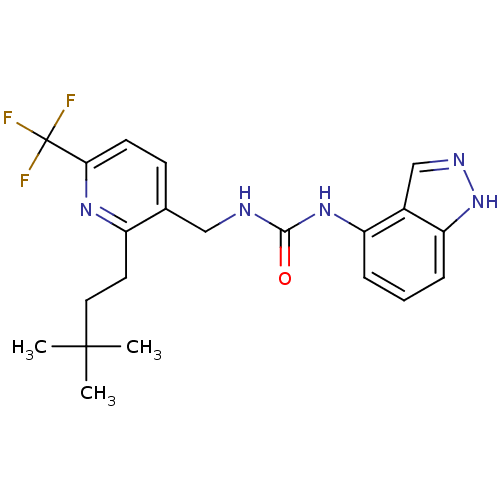
(1-((2-(3,3-dimethylbutyl)-6-(trifluoromethyl)pyrid...)Show SMILES CC(C)(C)CCc1nc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N5O/c1-20(2,3)10-9-15-13(7-8-18(27-15)21(22,23)24)11-25-19(30)28-16-5-4-6-17-14(16)12-26-29-17/h4-8,12H,9-11H2,1-3H3,(H,26,29)(H2,25,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186332

(US9163017, 6)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)-c3ccc(cc3)-c3c[nH]c(n3)[C@@H]3CCCN3C(=O)[C@H](N(C)C)c3ccccc3)ncnc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186333
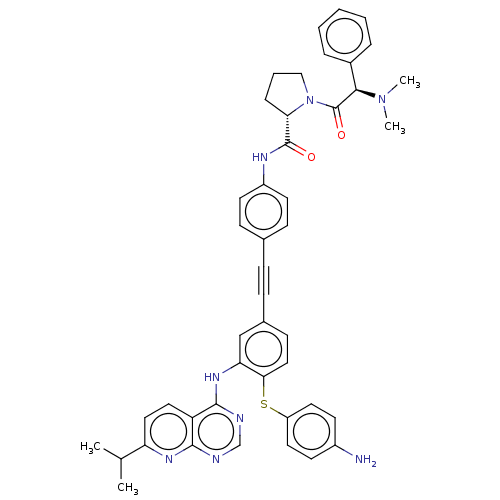
(US9163017, 7)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C#Cc3ccc(NC(=O)[C@@H]4CCCN4C(=O)[C@H](N(C)C)c4ccccc4)cc3)ncnc2n1 |r| Show InChI InChI=1S/C45H44N8O2S/c1-29(2)37-24-23-36-42(50-37)47-28-48-43(36)51-38-27-31(16-25-40(38)56-35-21-17-33(46)18-22-35)13-12-30-14-19-34(20-15-30)49-44(54)39-11-8-26-53(39)45(55)41(52(3)4)32-9-6-5-7-10-32/h5-7,9-10,14-25,27-29,39,41H,8,11,26,46H2,1-4H3,(H,49,54)(H,47,48,50,51)/t39-,41+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186335

(US9163017, 9)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C#Cc3ccc(cc3)-c3c[nH]c(n3)[C@@H]3CCCN3C(=O)[C@H](N(C)C)c3ccccc3)ncnc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186336
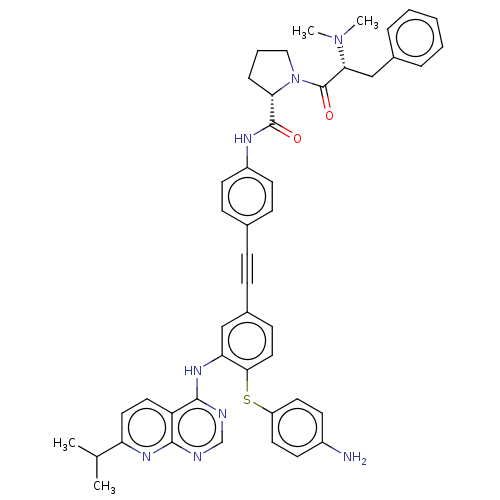
(US9163017, 10)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C#Cc3ccc(NC(=O)[C@@H]4CCCN4C(=O)[C@@H](Cc4ccccc4)N(C)C)cc3)ncnc2n1 |r| Show InChI InChI=1S/C46H46N8O2S/c1-30(2)38-24-23-37-43(51-38)48-29-49-44(37)52-39-27-33(16-25-42(39)57-36-21-17-34(47)18-22-36)13-12-31-14-19-35(20-15-31)50-45(55)40-11-8-26-54(40)46(56)41(53(3)4)28-32-9-6-5-7-10-32/h5-7,9-10,14-25,27,29-30,40-41H,8,11,26,28,47H2,1-4H3,(H,50,55)(H,48,49,51,52)/t40-,41+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186337

(US9163017, 11)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)Nc1ccc(cc1)C#Cc1ccc(Sc2ccc(N)cc2)c(Nc2ncnc3nc(ccc23)C(C)C)c1 |r| Show InChI InChI=1S/C42H44N8O4S/c1-25(2)33-20-19-32-38(47-33)44-24-45-39(32)48-34-23-28(12-21-36(34)55-31-17-13-29(43)14-18-31)9-8-27-10-15-30(16-11-27)46-40(51)35-7-6-22-50(35)41(52)37(26(3)4)49-42(53)54-5/h10-21,23-26,35,37H,6-7,22,43H2,1-5H3,(H,46,51)(H,49,53)(H,44,45,47,48)/t35-,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186338
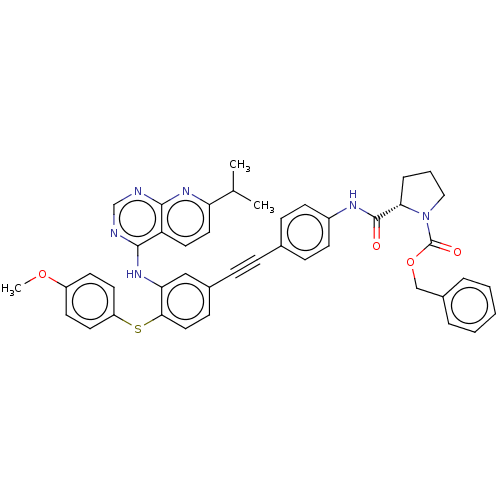
(US9163017, 12)Show SMILES COc1ccc(Sc2ccc(cc2Nc2ncnc3nc(ccc23)C(C)C)C#Cc2ccc(NC(=O)[C@@H]3CCCN3C(=O)OCc3ccccc3)cc2)cc1 |r| Show InChI InChI=1S/C44H40N6O4S/c1-29(2)37-23-22-36-41(48-37)45-28-46-42(36)49-38-26-31(15-24-40(38)55-35-20-18-34(53-3)19-21-35)12-11-30-13-16-33(17-14-30)47-43(51)39-10-7-25-50(39)44(52)54-27-32-8-5-4-6-9-32/h4-6,8-9,13-24,26,28-29,39H,7,10,25,27H2,1-3H3,(H,47,51)(H,45,46,48,49)/t39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186339

(US9163017, 13)Show SMILES COc1ccc(Sc2ccc(cc2Nc2ncnc3nc(ccc23)C(C)C)C#Cc2ccc(cc2)-c2c[nH]c(n2)[C@@H]2CCCN2C(=O)[C@H](N(C)C)c2ccccc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186342

(US9163017, 16)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C(=O)Nc3cccc(NC(=O)[C@@H]4CCCN4C(=O)OCc4ccccc4)c3)ncnc2n1 |r| Show InChI InChI=1S/C42H40N8O4S/c1-26(2)34-19-18-33-38(48-34)44-25-45-39(33)49-35-22-28(13-20-37(35)55-32-16-14-29(43)15-17-32)40(51)46-30-10-6-11-31(23-30)47-41(52)36-12-7-21-50(36)42(53)54-24-27-8-4-3-5-9-27/h3-6,8-11,13-20,22-23,25-26,36H,7,12,21,24,43H2,1-2H3,(H,46,51)(H,47,52)(H,44,45,48,49)/t36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186343

(US9163017, 17)Show SMILES CC(C)c1ccc2c(Nc3cc(NC(=O)[C@@H]4CCCN4C(=O)OCc4ccccc4)ccc3Sc3ccc(N)cc3)ncnc2n1 |r| Show InChI InChI=1S/C35H35N7O3S/c1-22(2)28-16-15-27-32(40-28)37-21-38-33(27)41-29-19-25(12-17-31(29)46-26-13-10-24(36)11-14-26)39-34(43)30-9-6-18-42(30)35(44)45-20-23-7-4-3-5-8-23/h3-5,7-8,10-17,19,21-22,30H,6,9,18,20,36H2,1-2H3,(H,39,43)(H,37,38,40,41)/t30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186331

(US9163017, 5)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C#Cc3ccc(NC(=O)[C@@H]4CCCN4C(=O)OCc4ccccc4)cc3)ncnc2n1 |r| Show InChI InChI=1S/C43H39N7O3S/c1-28(2)36-22-21-35-40(48-36)45-27-46-41(35)49-37-25-30(14-23-39(37)54-34-19-15-32(44)16-20-34)11-10-29-12-17-33(18-13-29)47-42(51)38-9-6-24-50(38)43(52)53-26-31-7-4-3-5-8-31/h3-5,7-8,12-23,25,27-28,38H,6,9,24,26,44H2,1-2H3,(H,47,51)(H,45,46,48,49)/t38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186324

(US9163017, 3)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C(=O)Nc3ccc(NC(=O)C4CCCN4C(=O)OCc4ccccc4)cc3)ncnc2n1 Show InChI InChI=1S/C42H40N8O4S/c1-26(2)34-20-19-33-38(48-34)44-25-45-39(33)49-35-23-28(10-21-37(35)55-32-17-11-29(43)12-18-32)40(51)46-30-13-15-31(16-14-30)47-41(52)36-9-6-22-50(36)42(53)54-24-27-7-4-3-5-8-27/h3-5,7-8,10-21,23,25-26,36H,6,9,22,24,43H2,1-2H3,(H,46,51)(H,47,52)(H,44,45,48,49) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186318
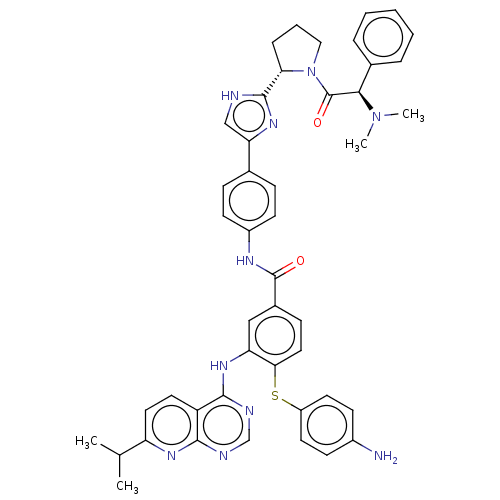
(US9163017, 2)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)C(=O)Nc3ccc(cc3)-c3c[nH]c(n3)[C@@H]3CCCN3C(=O)[C@H](N(C)C)c3ccccc3)ncnc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186334

(US9163017, 8)Show SMILES CC(C)c1ccc2c(Nc3cc(ccc3Sc3ccc(N)cc3)-c3ccc(cc3)-c3ccc(cc3)-c3c[nH]c(n3)[C@@H]3CCCN3C(=O)[C@H](N(C)C)c3ccccc3)ncnc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC.
US Patent
| Assay Description
The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... |
US Patent US9163017 (2015)
BindingDB Entry DOI: 10.7270/Q2F47MX7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50264620
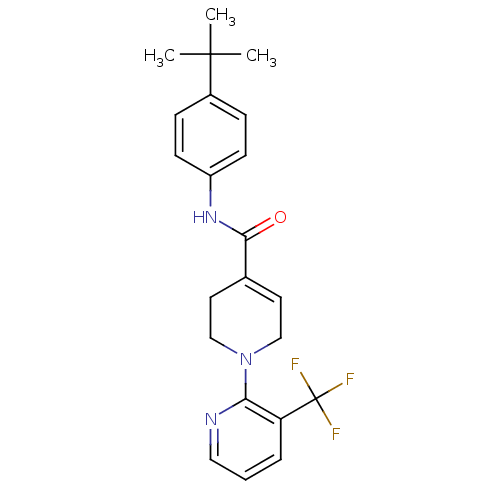
(CHEMBL518979 | N-(4-tert-Butylphenyl)-1-(3-(triflu...)Show SMILES CC(C)(C)c1ccc(NC(=O)C2=CCN(CC2)c2ncccc2C(F)(F)F)cc1 |t:11| Show InChI InChI=1S/C22H24F3N3O/c1-21(2,3)16-6-8-17(9-7-16)27-20(29)15-10-13-28(14-11-15)19-18(22(23,24)25)5-4-12-26-19/h4-10,12H,11,13-14H2,1-3H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in human 1321N1 cells assessed as inhibition of capsaicin-induced calcium influx by FLIPR as... |
Bioorg Med Chem 16: 8516-25 (2008)
Article DOI: 10.1016/j.bmc.2008.08.005
BindingDB Entry DOI: 10.7270/Q2FX798X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319464

(1-(1H-indazol-4-yl)-3-((2-(neopentyloxy)-6-(triflu...)Show SMILES CC(C)(C)COc1nc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C20H22F3N5O2/c1-19(2,3)11-30-17-12(7-8-16(27-17)20(21,22)23)9-24-18(29)26-14-5-4-6-15-13(14)10-25-28-15/h4-8,10H,9,11H2,1-3H3,(H,25,28)(H2,24,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319470
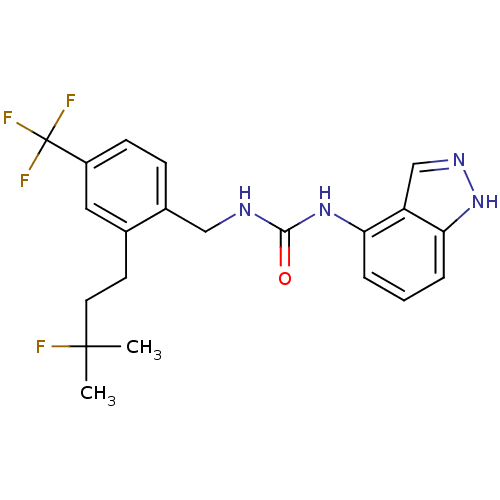
(1-(2-(3-fluoro-3-methylbutyl)-4-(trifluoromethyl)b...)Show SMILES CC(C)(F)CCc1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C21H22F4N4O/c1-20(2,22)9-8-13-10-15(21(23,24)25)7-6-14(13)11-26-19(30)28-17-4-3-5-18-16(17)12-27-29-18/h3-7,10,12H,8-9,11H2,1-2H3,(H,27,29)(H2,26,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143833
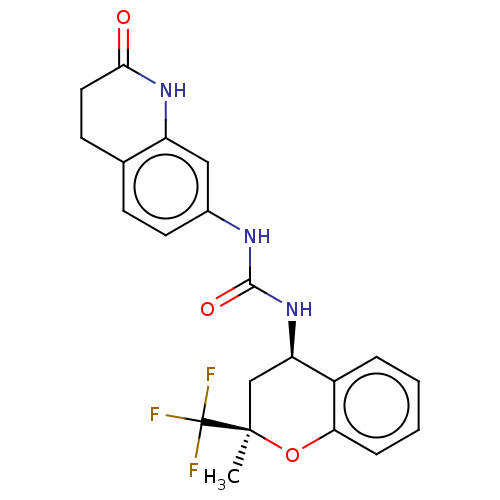
(US8969325, 34)Show SMILES C[C@@]1(C[C@@H](NC(=O)Nc2ccc3CCC(=O)Nc3c2)c2ccccc2O1)C(F)(F)F Show InChI InChI=1S/C21H20F3N3O3/c1-20(21(22,23)24)11-16(14-4-2-3-5-17(14)30-20)27-19(29)25-13-8-6-12-7-9-18(28)26-15(12)10-13/h2-6,8,10,16H,7,9,11H2,1H3,(H,26,28)(H2,25,27,29)/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319454
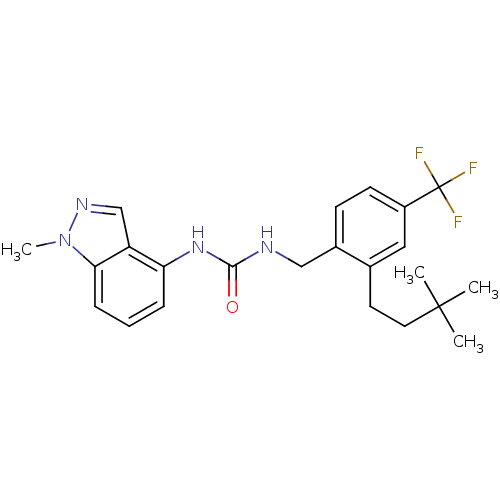
(1-(2-(3,3-dimethylbutyl)-4-(trifluoromethyl)benzyl...)Show SMILES Cn1ncc2c(NC(=O)NCc3ccc(cc3CCC(C)(C)C)C(F)(F)F)cccc12 Show InChI InChI=1S/C23H27F3N4O/c1-22(2,3)11-10-15-12-17(23(24,25)26)9-8-16(15)13-27-21(31)29-19-6-5-7-20-18(19)14-28-30(20)4/h5-9,12,14H,10-11,13H2,1-4H3,(H2,27,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced effect at pH 5.5 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319467
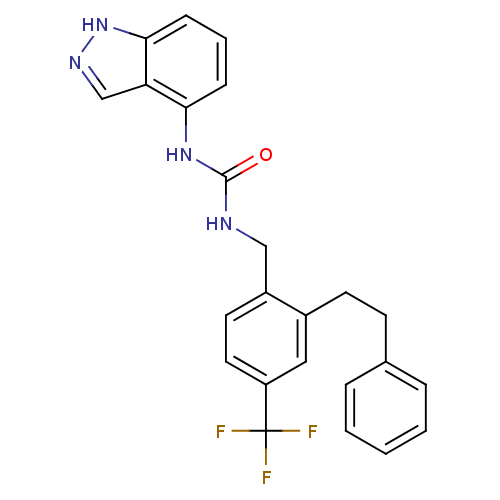
(1-(1H-indazol-4-yl)-3-(2-phenethyl-4-(trifluoromet...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(CCc2ccccc2)c1 Show InChI InChI=1S/C24H21F3N4O/c25-24(26,27)19-12-11-18(17(13-19)10-9-16-5-2-1-3-6-16)14-28-23(32)30-21-7-4-8-22-20(21)15-29-31-22/h1-8,11-13,15H,9-10,14H2,(H,29,31)(H2,28,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319466
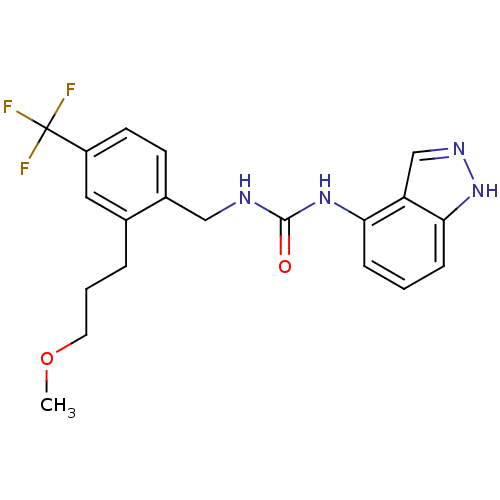
(1-(1H-indazol-4-yl)-3-(2-(3-methoxypropyl)-4-(trif...)Show SMILES COCCCc1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C20H21F3N4O2/c1-29-9-3-4-13-10-15(20(21,22)23)8-7-14(13)11-24-19(28)26-17-5-2-6-18-16(17)12-25-27-18/h2,5-8,10,12H,3-4,9,11H2,1H3,(H,25,27)(H2,24,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143895

(US8969325, 96)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3CCc4cc(Cl)ccc34)c2ccc1=O Show InChI InChI=1S/C20H18ClN3O2/c1-24-18-4-2-3-16(15(18)8-10-19(24)25)22-20(26)23-17-9-5-12-11-13(21)6-7-14(12)17/h2-4,6-8,10-11,17H,5,9H2,1H3,(H2,22,23,26)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50264703
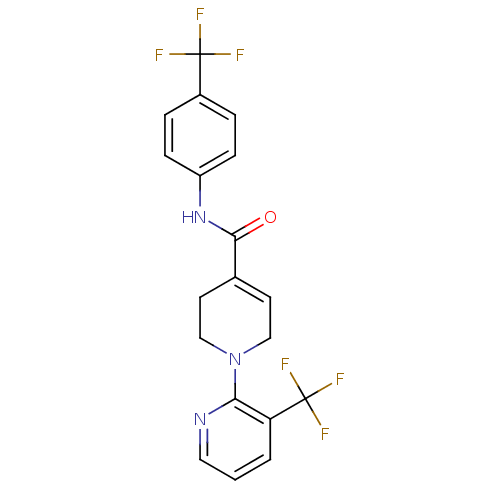
(CHEMBL522425 | N-(4-(Trifluoromethyl)phenyl)-1-(3-...)Show SMILES FC(F)(F)c1ccc(NC(=O)C2=CCN(CC2)c2ncccc2C(F)(F)F)cc1 |t:11| Show InChI InChI=1S/C19H15F6N3O/c20-18(21,22)13-3-5-14(6-4-13)27-17(29)12-7-10-28(11-8-12)16-15(19(23,24)25)2-1-9-26-16/h1-7,9H,8,10-11H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in human 1321N1 cells assessed as inhibition of capsaicin-induced calcium influx by FLIPR as... |
Bioorg Med Chem 16: 8516-25 (2008)
Article DOI: 10.1016/j.bmc.2008.08.005
BindingDB Entry DOI: 10.7270/Q2FX798X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143933

(US8969325, 134)Show SMILES C[C@H]1Oc2c(NC(=O)N[C@@H]3CC(CF)(CF)Oc4cc(Cl)ccc34)cccc2NC1=O Show InChI InChI=1S/C21H20ClF2N3O4/c1-11-19(28)25-14-3-2-4-15(18(14)30-11)26-20(29)27-16-8-21(9-23,10-24)31-17-7-12(22)5-6-13(16)17/h2-7,11,16H,8-10H2,1H3,(H,25,28)(H2,26,27,29)/t11-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319468
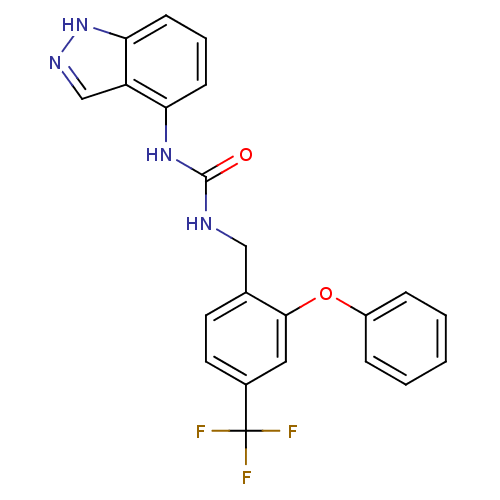
(1-(1H-indazol-4-yl)-3-(2-phenoxy-4-(trifluoromethy...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(Oc2ccccc2)c1 Show InChI InChI=1S/C22H17F3N4O2/c23-22(24,25)15-10-9-14(20(11-15)31-16-5-2-1-3-6-16)12-26-21(30)28-18-7-4-8-19-17(18)13-27-29-19/h1-11,13H,12H2,(H,27,29)(H2,26,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319469
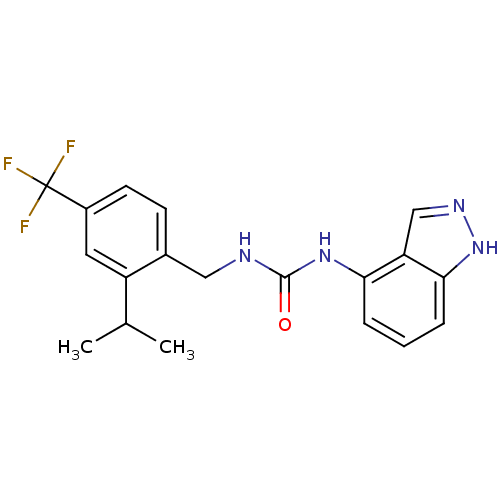
(1-(1H-indazol-4-yl)-3-(2-isopropyl-4-(trifluoromet...)Show SMILES CC(C)c1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C19H19F3N4O/c1-11(2)14-8-13(19(20,21)22)7-6-12(14)9-23-18(27)25-16-4-3-5-17-15(16)10-24-26-17/h3-8,10-11H,9H2,1-2H3,(H,24,26)(H2,23,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319461
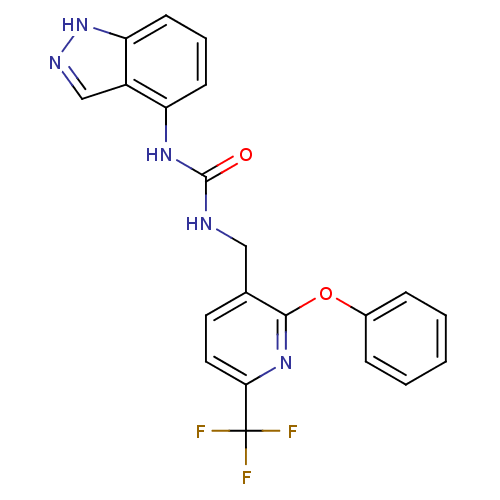
(1-(1H-indazol-4-yl)-3-((2-phenoxy-6-(trifluorometh...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(Oc2ccccc2)n1 Show InChI InChI=1S/C21H16F3N5O2/c22-21(23,24)18-10-9-13(19(28-18)31-14-5-2-1-3-6-14)11-25-20(30)27-16-7-4-8-17-15(16)12-26-29-17/h1-10,12H,11H2,(H,26,29)(H2,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50264621
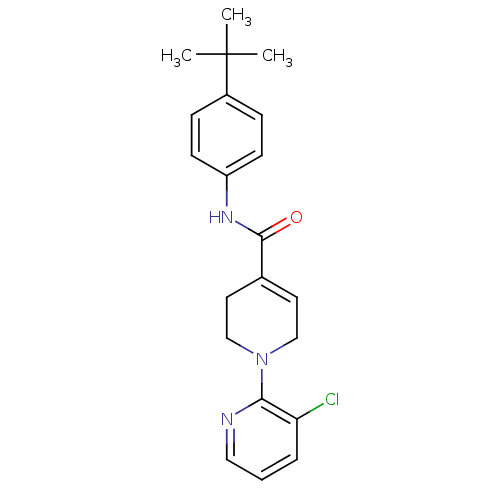
(CHEMBL482835 | N-(4-tert-Butylphenyl)-1-(3-chlorop...)Show SMILES CC(C)(C)c1ccc(NC(=O)C2=CCN(CC2)c2ncccc2Cl)cc1 |t:11| Show InChI InChI=1S/C21H24ClN3O/c1-21(2,3)16-6-8-17(9-7-16)24-20(26)15-10-13-25(14-11-15)19-18(22)5-4-12-23-19/h4-10,12H,11,13-14H2,1-3H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in human 1321N1 cells assessed as inhibition of capsaicin-induced calcium influx by FLIPR as... |
Bioorg Med Chem 16: 8516-25 (2008)
Article DOI: 10.1016/j.bmc.2008.08.005
BindingDB Entry DOI: 10.7270/Q2FX798X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50264668
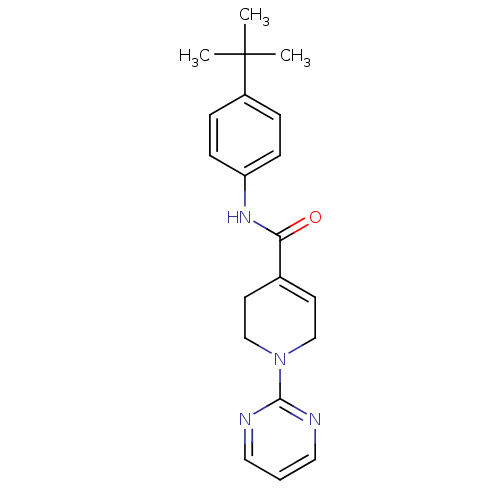
(CHEMBL491234 | N-(4-tert-Butylphenyl)-1-(pyrimidin...)Show SMILES CC(C)(C)c1ccc(NC(=O)C2=CCN(CC2)c2ncccn2)cc1 |t:11| Show InChI InChI=1S/C20H24N4O/c1-20(2,3)16-5-7-17(8-6-16)23-18(25)15-9-13-24(14-10-15)19-21-11-4-12-22-19/h4-9,11-12H,10,13-14H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in human 1321N1 cells assessed as inhibition of capsaicin-induced calcium influx by FLIPR as... |
Bioorg Med Chem 16: 8516-25 (2008)
Article DOI: 10.1016/j.bmc.2008.08.005
BindingDB Entry DOI: 10.7270/Q2FX798X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143800
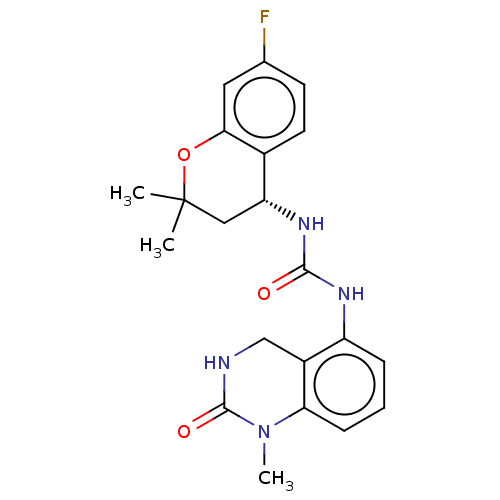
(US8969325, 1)Show SMILES CN1C(=O)NCc2c(NC(=O)N[C@@H]3CC(C)(C)Oc4cc(F)ccc34)cccc12 Show InChI InChI=1S/C21H23FN4O3/c1-21(2)10-16(13-8-7-12(22)9-18(13)29-21)25-19(27)24-15-5-4-6-17-14(15)11-23-20(28)26(17)3/h4-9,16H,10-11H2,1-3H3,(H,23,28)(H2,24,25,27)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143885
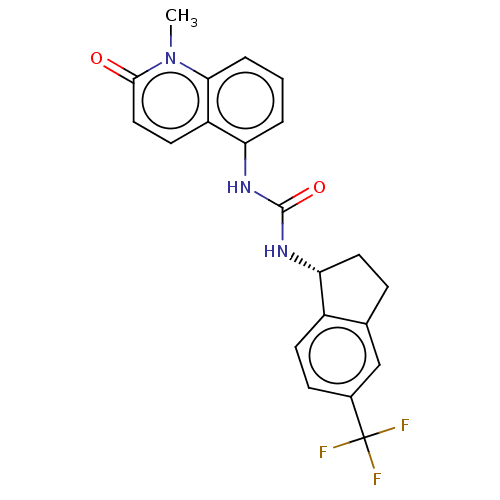
(US8969325, 86)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3CCc4cc(ccc34)C(F)(F)F)c2ccc1=O Show InChI InChI=1S/C21H18F3N3O2/c1-27-18-4-2-3-16(15(18)8-10-19(27)28)25-20(29)26-17-9-5-12-11-13(21(22,23)24)6-7-14(12)17/h2-4,6-8,10-11,17H,5,9H2,1H3,(H2,25,26,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143818

(US8969325, 19)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3CC(C)(C)Oc4cc(F)ccc34)c2ccc1=O Show InChI InChI=1S/C22H22FN3O3/c1-22(2)12-17(15-8-7-13(23)11-19(15)29-22)25-21(28)24-16-5-4-6-18-14(16)9-10-20(27)26(18)3/h4-11,17H,12H2,1-3H3,(H2,24,25,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143889
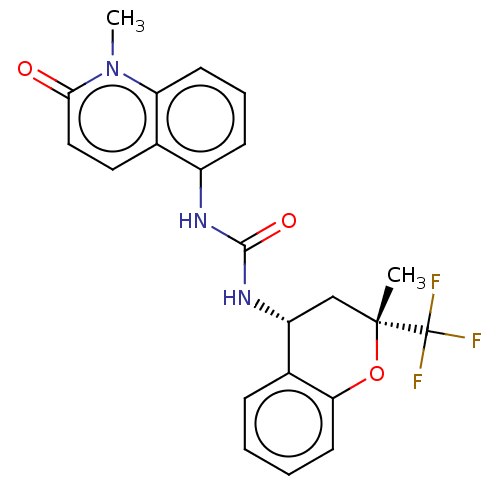
(US8969325, 90)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3C[C@@](C)(Oc4ccccc34)C(F)(F)F)c2ccc1=O Show InChI InChI=1S/C22H20F3N3O3/c1-21(22(23,24)25)12-16(14-6-3-4-9-18(14)31-21)27-20(30)26-15-7-5-8-17-13(15)10-11-19(29)28(17)2/h3-11,16H,12H2,1-2H3,(H2,26,27,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143939
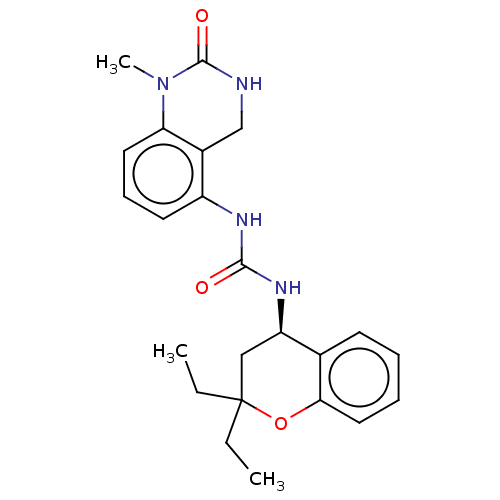
(US8969325, 140)Show SMILES CCC1(CC)C[C@@H](NC(=O)Nc2cccc3N(C)C(=O)NCc23)c2ccccc2O1 Show InChI InChI=1S/C23H28N4O3/c1-4-23(5-2)13-18(15-9-6-7-12-20(15)30-23)26-21(28)25-17-10-8-11-19-16(17)14-24-22(29)27(19)3/h6-12,18H,4-5,13-14H2,1-3H3,(H,24,29)(H2,25,26,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319459
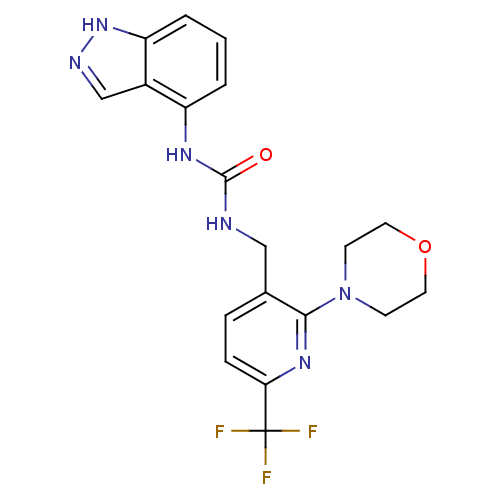
(1-(1H-indazol-4-yl)-3-((2-morpholino-6-(trifluorom...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(n1)N1CCOCC1 Show InChI InChI=1S/C19H19F3N6O2/c20-19(21,22)16-5-4-12(17(26-16)28-6-8-30-9-7-28)10-23-18(29)25-14-2-1-3-15-13(14)11-24-27-15/h1-5,11H,6-10H2,(H,24,27)(H2,23,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319463
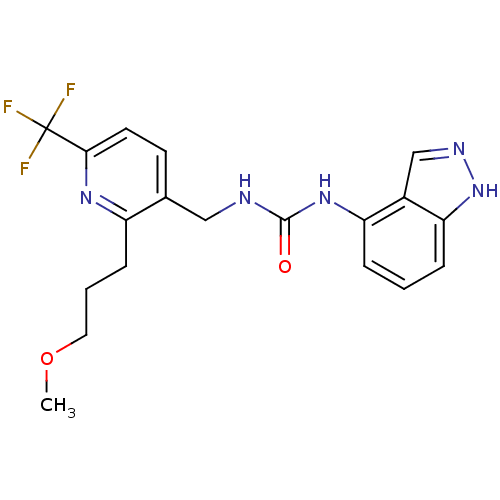
(1-(1H-indazol-4-yl)-3-((2-(3-methoxypropyl)-6-(tri...)Show SMILES COCCCc1nc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C19H20F3N5O2/c1-29-9-3-6-14-12(7-8-17(25-14)19(20,21)22)10-23-18(28)26-15-4-2-5-16-13(15)11-24-27-16/h2,4-5,7-8,11H,3,6,9-10H2,1H3,(H,24,27)(H2,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
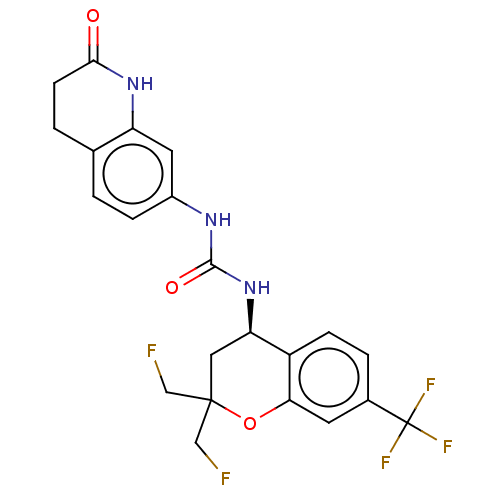
(Homo sapiens (Human)) | BDBM144119
(US8969325, 320)Show SMILES FCC1(CF)C[C@@H](NC(=O)Nc2ccc3CCC(=O)Nc3c2)c2ccc(cc2O1)C(F)(F)F Show InChI InChI=1S/C22H20F5N3O3/c23-10-21(11-24)9-17(15-5-3-13(22(25,26)27)7-18(15)33-21)30-20(32)28-14-4-1-12-2-6-19(31)29-16(12)8-14/h1,3-5,7-8,17H,2,6,9-11H2,(H,29,31)(H2,28,30,32)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
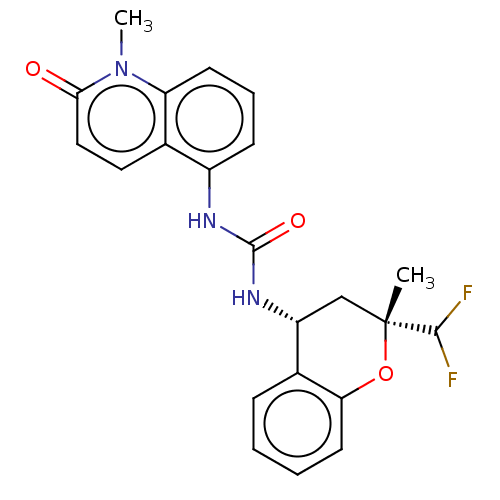
(Homo sapiens (Human)) | BDBM143827
(US8969325, 28)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3C[C@@](C)(Oc4ccccc34)C(F)F)c2ccc1=O Show InChI InChI=1S/C22H21F2N3O3/c1-22(20(23)24)12-16(14-6-3-4-9-18(14)30-22)26-21(29)25-15-7-5-8-17-13(15)10-11-19(28)27(17)2/h3-11,16,20H,12H2,1-2H3,(H2,25,26,29)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
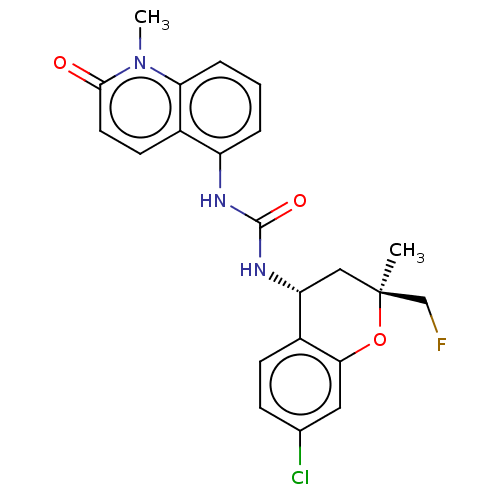
(Homo sapiens (Human)) | BDBM143840
(US8969325, 41)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3C[C@@](C)(CF)Oc4cc(Cl)ccc34)c2ccc1=O Show InChI InChI=1S/C22H21ClFN3O3/c1-22(12-24)11-17(15-7-6-13(23)10-19(15)30-22)26-21(29)25-16-4-3-5-18-14(16)8-9-20(28)27(18)2/h3-10,17H,11-12H2,1-2H3,(H2,25,26,29)/t17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM143938
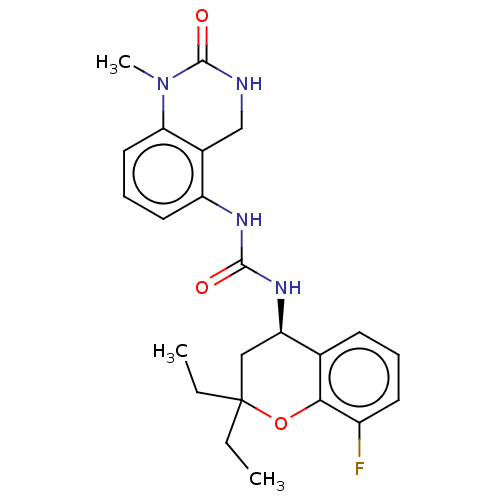
(US8969325, 139)Show SMILES CCC1(CC)C[C@@H](NC(=O)Nc2cccc3N(C)C(=O)NCc23)c2cccc(F)c2O1 Show InChI InChI=1S/C23H27FN4O3/c1-4-23(5-2)12-18(14-8-6-9-16(24)20(14)31-23)27-21(29)26-17-10-7-11-19-15(17)13-25-22(30)28(19)3/h6-11,18H,4-5,12-13H2,1-3H3,(H,25,30)(H2,26,27,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM144051
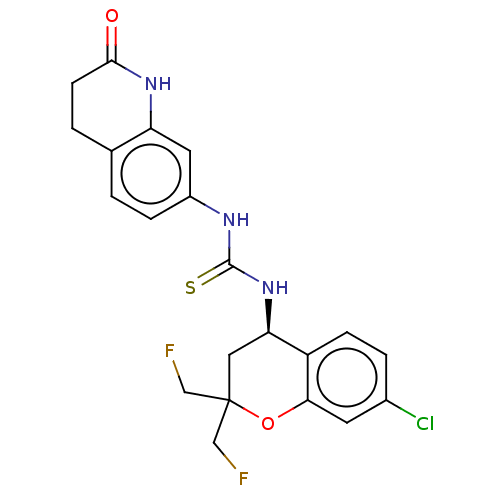
(US8969325, 252)Show SMILES FCC1(CF)C[C@@H](NC(=S)Nc2ccc3CCC(=O)Nc3c2)c2ccc(Cl)cc2O1 Show InChI InChI=1S/C21H20ClF2N3O2S/c22-13-3-5-15-17(9-21(10-23,11-24)29-18(15)7-13)27-20(30)25-14-4-1-12-2-6-19(28)26-16(12)8-14/h1,3-5,7-8,17H,2,6,9-11H2,(H,26,28)(H2,25,27,30)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup... |
US Patent US8969325 (2015)
BindingDB Entry DOI: 10.7270/Q29P30BX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data