

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031962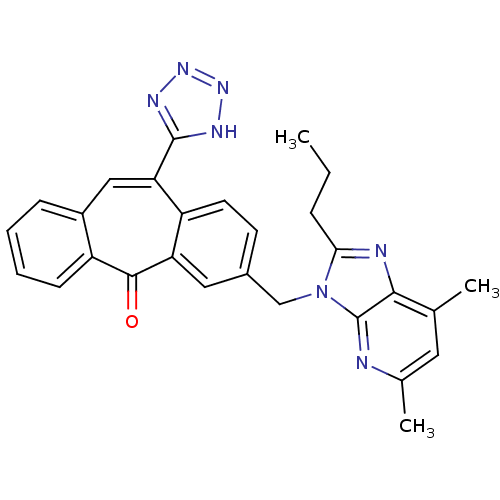 (3-(5,7-Dimethyl-2-propyl-imidazo[4,5-b]pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031954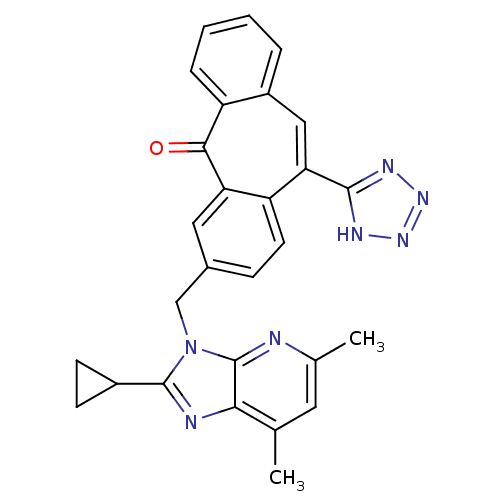 (3-(2-Cyclopropyl-5,7-dimethyl-imidazo[4,5-b]pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031953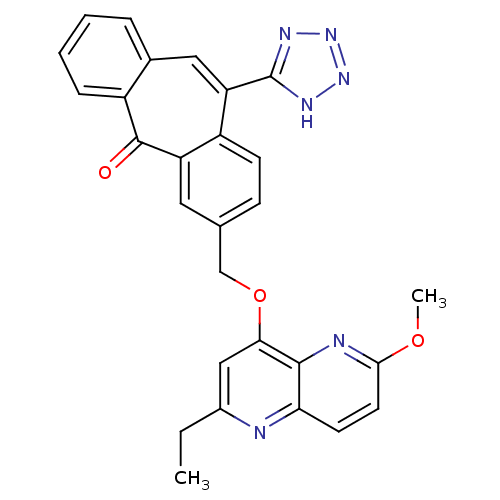 (3-(2-Ethyl-6-methoxy-[1,5]naphthyridin-4-yloxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031957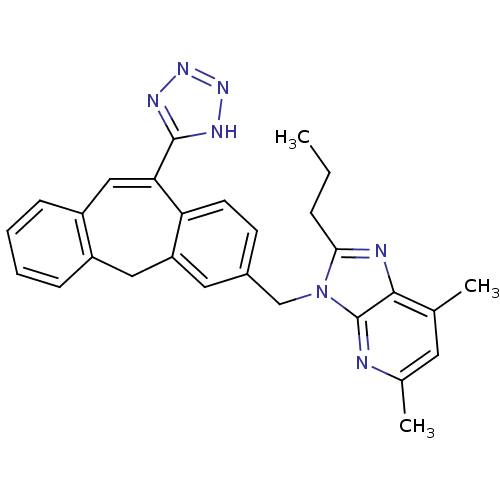 (5,7-Dimethyl-2-propyl-3-[11-(1H-tetrazol-5-yl)-5H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031960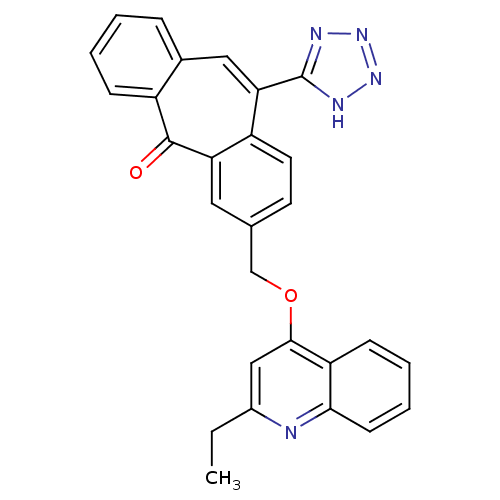 (3-(2-Ethyl-quinolin-4-yloxymethyl)-11-(1H-tetrazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031961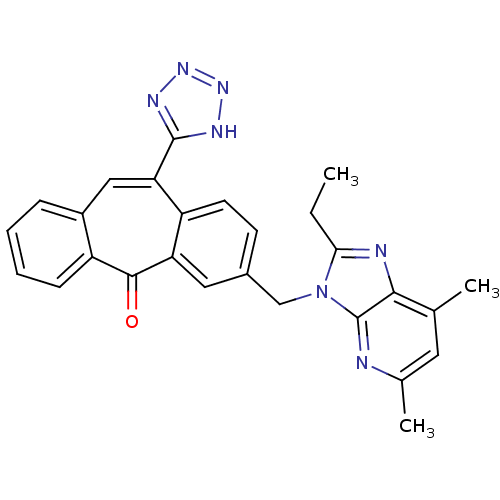 (3-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031955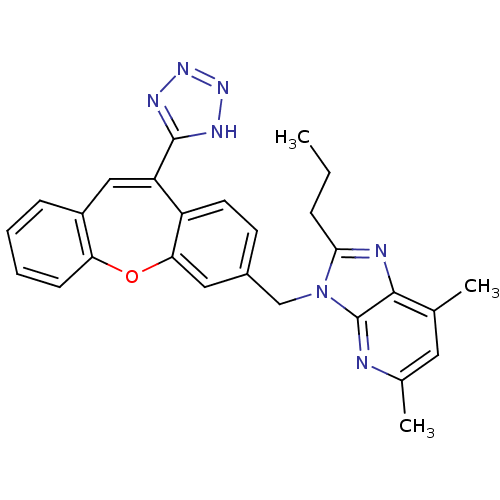 (5,7-Dimethyl-2-propyl-3-[11-(1H-tetrazol-5-yl)-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031964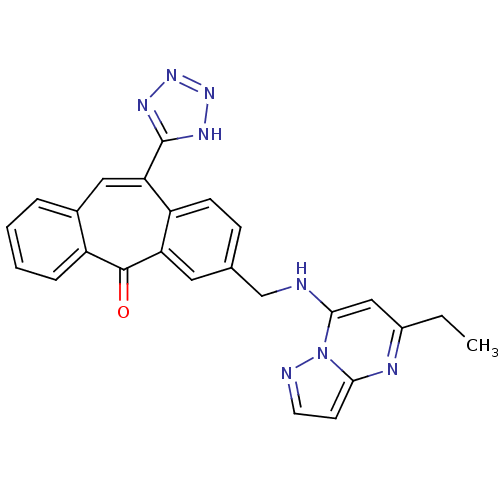 (3-[(5-Ethyl-pyrazolo[1,5-a]pyrimidin-7-ylamino)-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031956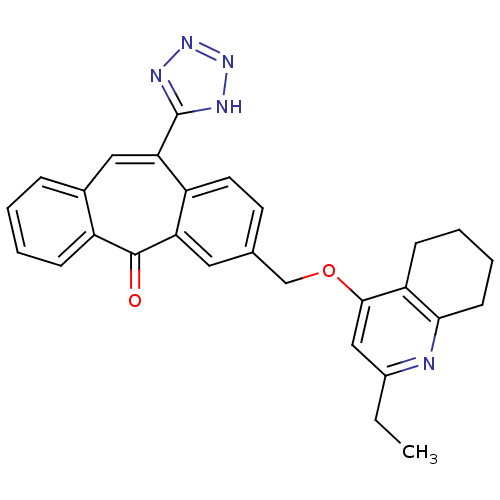 (3-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031959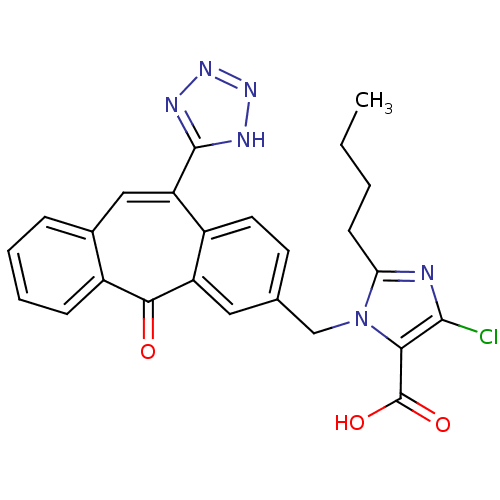 (2-Butyl-5-chloro-3-[5-oxo-11-(1H-tetrazol-5-yl)-5H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031966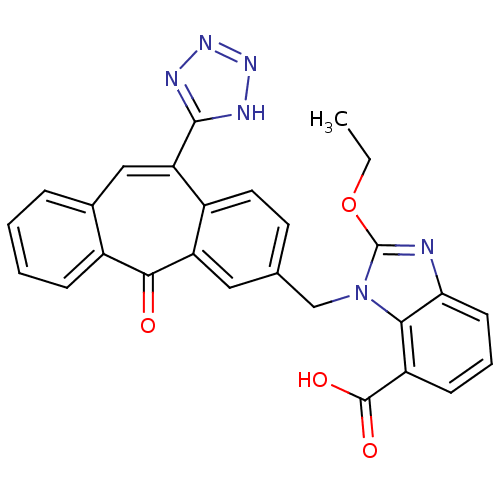 (2-Ethoxy-3-[5-oxo-11-(1H-tetrazol-5-yl)-5H-dibenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031965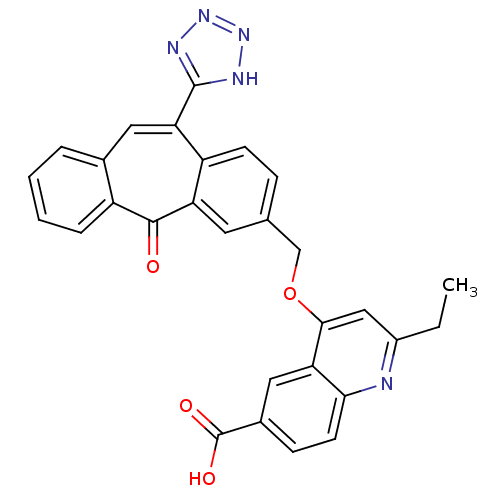 (2-Ethyl-4-[5-oxo-11-(1H-tetrazol-5-yl)-5H-dibenzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031950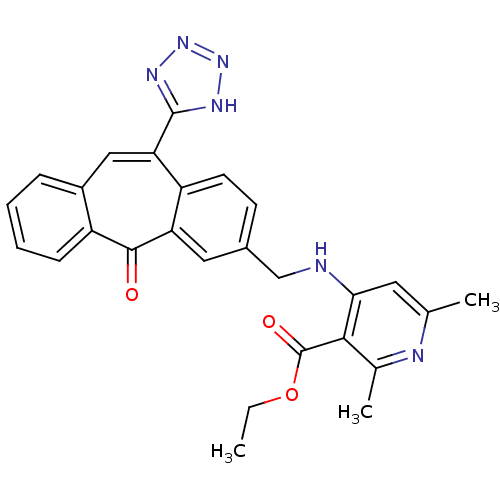 (2,6-Dimethyl-4-{[5-oxo-11-(1H-tetrazol-5-yl)-5H-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031951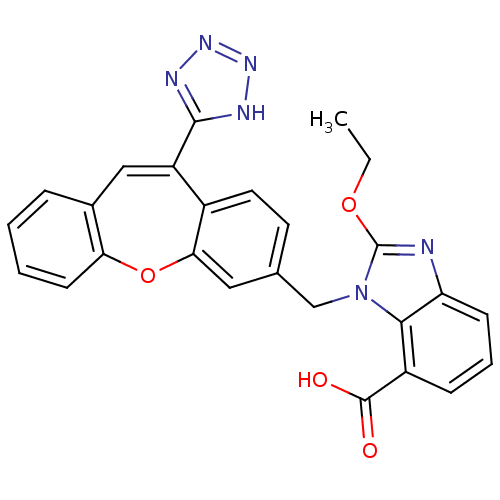 (2-Ethoxy-3-[11-(1H-tetrazol-5-yl)-dibenzo[b,f]oxep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031949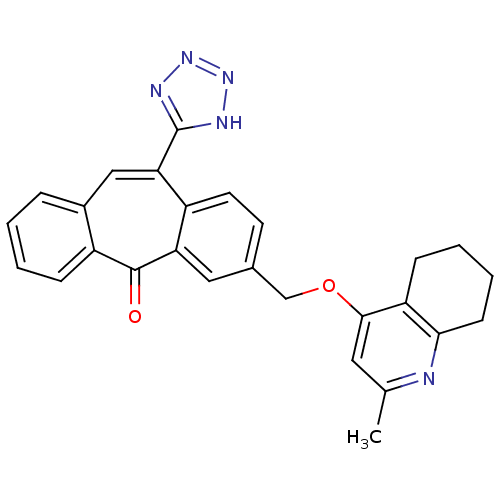 (3-(2-Methyl-5,6,7,8-tetrahydro-quinolin-4-yloxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031963 (2,6-Dimethyl-4-[5-oxo-11-(1H-tetrazol-5-yl)-5H-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031952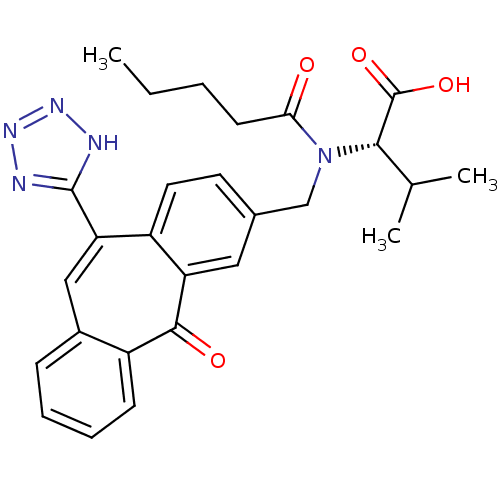 ((S)-3-Methyl-2-{[5-oxo-11-(1H-tetrazol-5-yl)-5H-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031958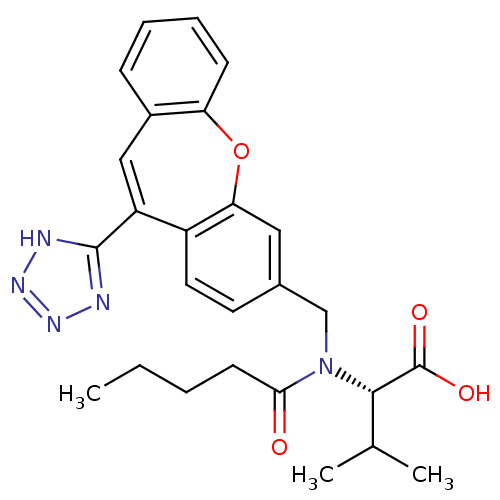 ((S)-3-Methyl-2-{pentanoyl-[11-(1H-tetrazol-5-yl)-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50031948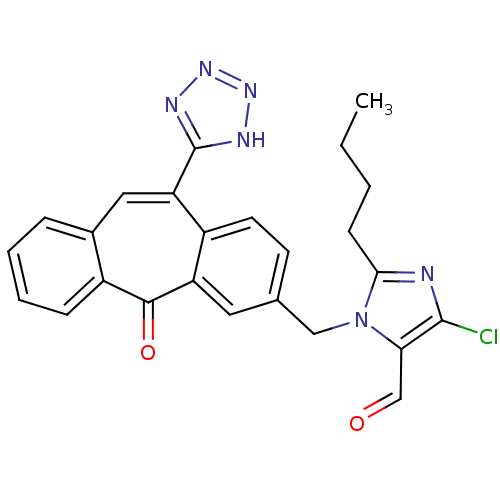 (2-Butyl-5-chloro-3-[5-oxo-11-(1H-tetrazol-5-yl)-5H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Ability to displace [125I]-AII binding to COS cells transfected with a cDNA encoding human Angiotensin II receptor, type 1 | J Med Chem 38: 2728-41 (1995) BindingDB Entry DOI: 10.7270/Q23B5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077162 ((R)-3-(1H-Indol-3-yl)-2-[4-(4-methoxy-phenylethyny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077157 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063129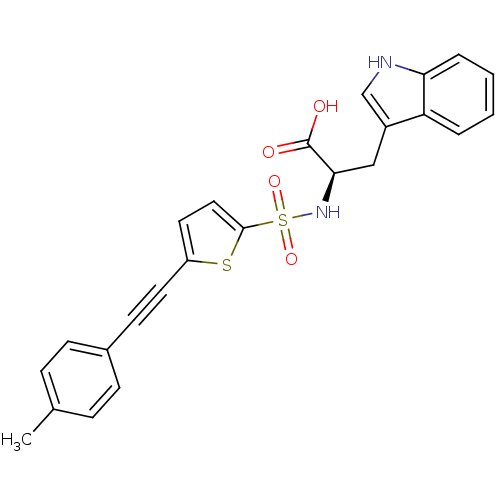 ((R)-3-(1H-Indol-3-yl)-2-(5-p-tolylethynyl-thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50062551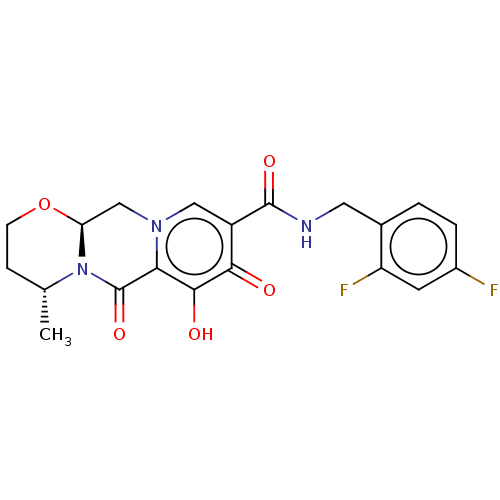 (CHEBI:76010 | Dolutegravir | GSK1349572 | S-349572) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... | J Med Chem 56: 5901-16 (2013) Article DOI: 10.1021/jm400645w BindingDB Entry DOI: 10.7270/Q21G0Q64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492496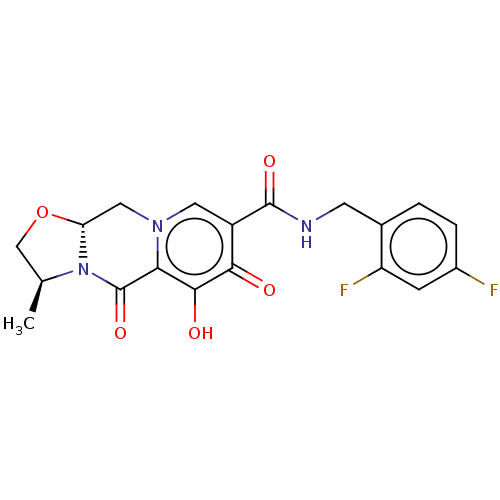 (Cabotegravir | GSK-1265744A | GSK1265744 | GSK1265...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... | J Med Chem 56: 5901-16 (2013) Article DOI: 10.1021/jm400645w BindingDB Entry DOI: 10.7270/Q21G0Q64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077161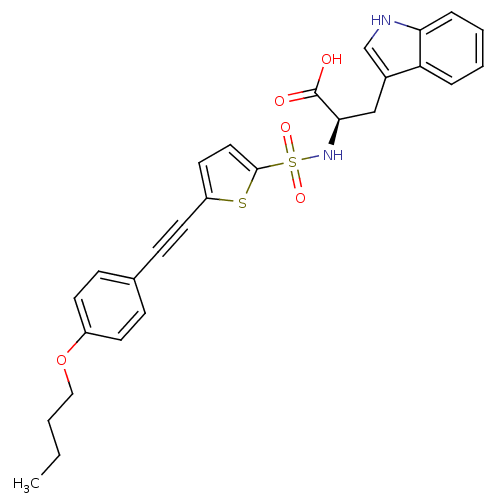 ((R)-2-[5-(4-Butoxy-phenylethynyl)-thiophene-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077151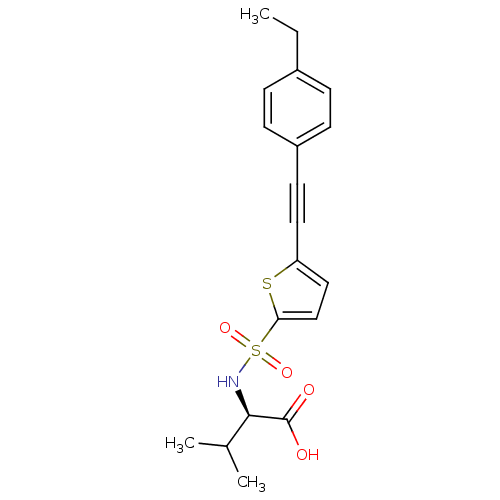 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077155 ((R)-2-[5-(4-Butyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077152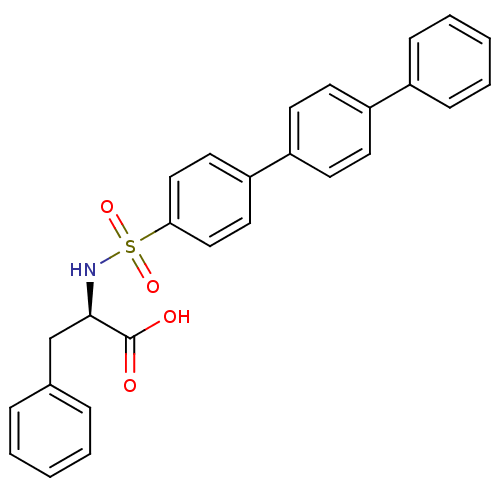 ((R)-3-Phenyl-2-([1,1';4',1'']terphenyl-4-sulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077163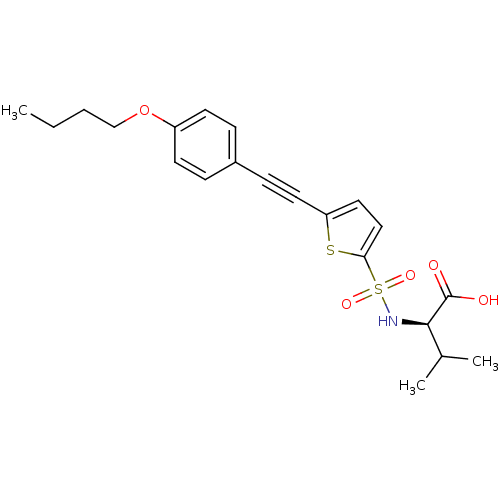 ((R)-2-[5-(4-Butoxy-phenylethynyl)-thiophene-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077151 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492497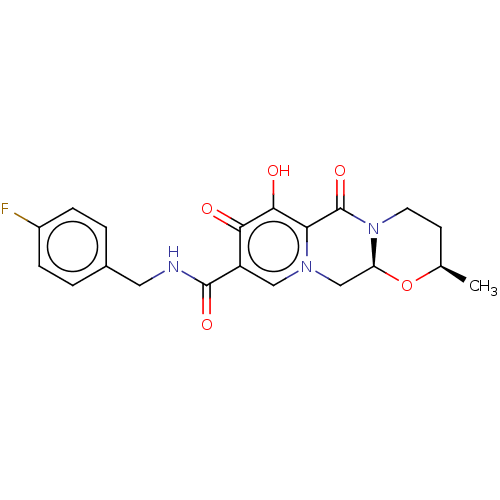 (CHEMBL2403118) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity using [3H]-DNA as substrate preincubated for 60 mins prior to substrate addition measured afte... | J Med Chem 56: 5901-16 (2013) Article DOI: 10.1021/jm400645w BindingDB Entry DOI: 10.7270/Q21G0Q64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077158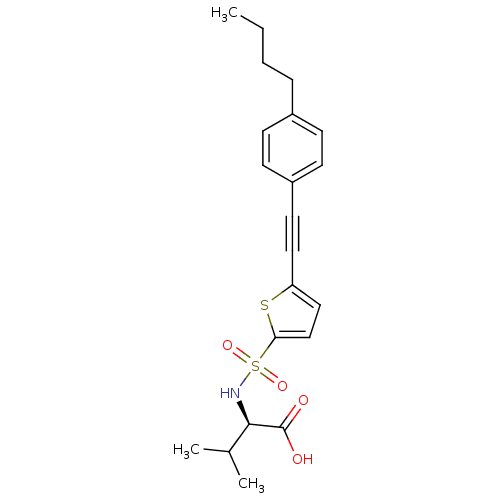 ((R)-2-[5-(4-Butyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063164 ((R)-3-Methyl-2-(5-p-tolylethynyl-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077157 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077162 ((R)-3-(1H-Indol-3-yl)-2-[4-(4-methoxy-phenylethyny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063129 ((R)-3-(1H-Indol-3-yl)-2-(5-p-tolylethynyl-thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077164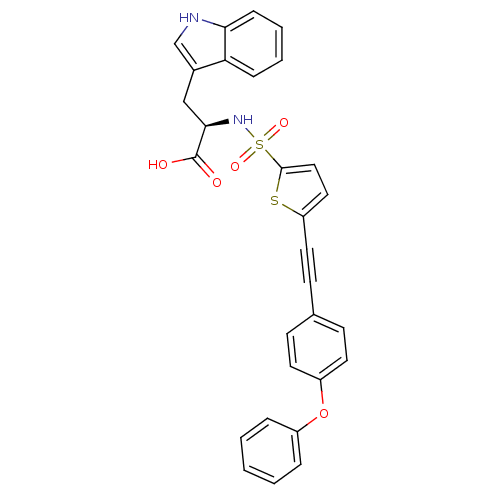 ((R)-3-(1H-Indol-3-yl)-2-[5-(4-phenoxy-phenylethyny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485874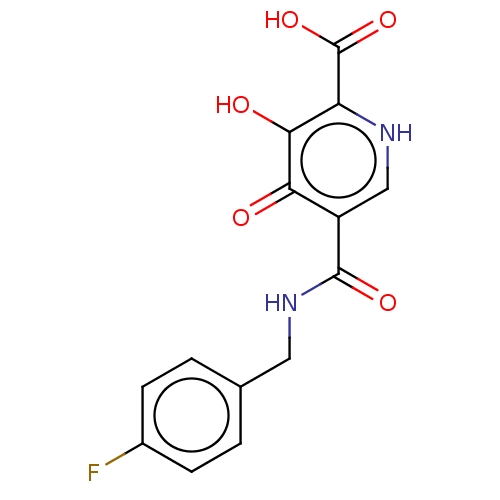 (CHEMBL2171671) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using pre-incubation and wash with Mg2+ cofactor by ELISA based microtiter plate integration as... | J Med Chem 55: 8735-44 (2012) Article DOI: 10.1021/jm3010459 BindingDB Entry DOI: 10.7270/Q27M0BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077152 ((R)-3-Phenyl-2-([1,1';4',1'']terphenyl-4-sulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077149 ((R)-3-Methyl-2-[5-(4-phenoxy-phenylethynyl)-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485875 (CHEMBL2171670) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using pre-incubation and wash with Mg2+ cofactor by ELISA based microtiter plate integration as... | J Med Chem 55: 8735-44 (2012) Article DOI: 10.1021/jm3010459 BindingDB Entry DOI: 10.7270/Q27M0BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077153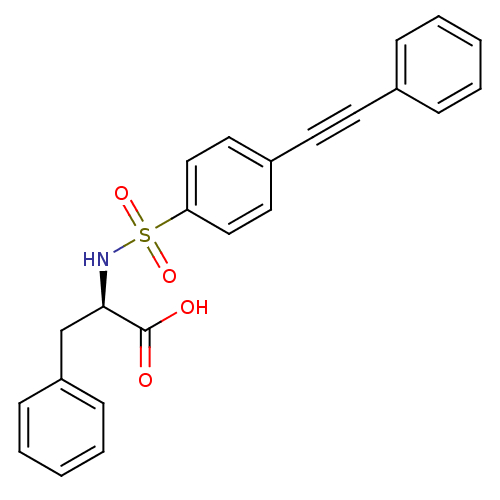 ((R)-3-Phenyl-2-(4-phenylethynyl-benzenesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063164 ((R)-3-Methyl-2-(5-p-tolylethynyl-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077150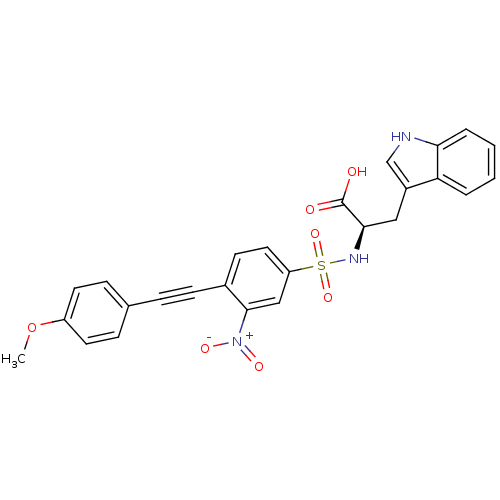 ((R)-3-(1H-Indol-3-yl)-2-[4-(4-methoxy-phenylethyny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063133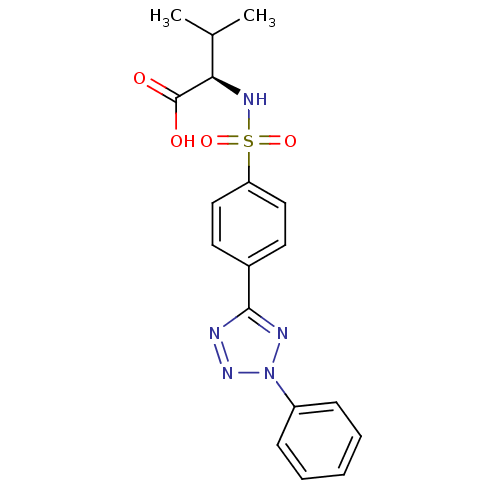 ((R)-3-Methyl-2-[4-(2-phenyl-2H-tetrazol-5-yl)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077159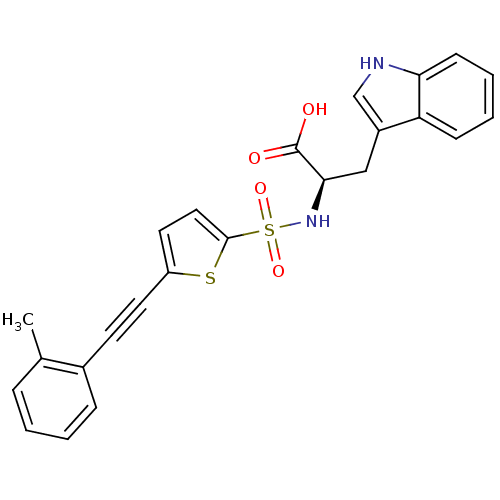 ((R)-3-(1H-Indol-3-yl)-2-(5-o-tolylethynyl-thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485873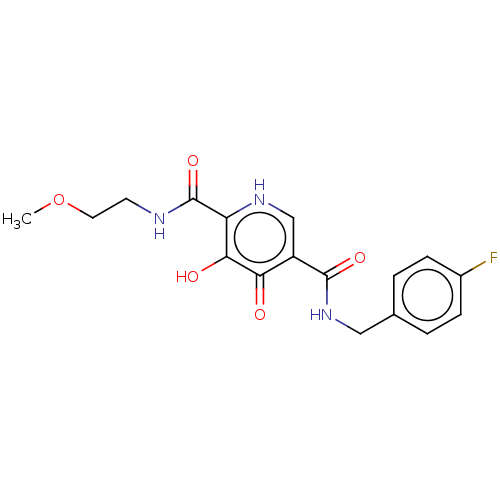 (CHEMBL2171665) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using pre-incubation and wash with Mg2+ cofactor by ELISA based microtiter plate integration as... | J Med Chem 55: 8735-44 (2012) Article DOI: 10.1021/jm3010459 BindingDB Entry DOI: 10.7270/Q27M0BTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077158 ((R)-2-[5-(4-Butyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077163 ((R)-2-[5-(4-Butoxy-phenylethynyl)-thiophene-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077149 ((R)-3-Methyl-2-[5-(4-phenoxy-phenylethynyl)-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |