

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Escherichia coli) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077837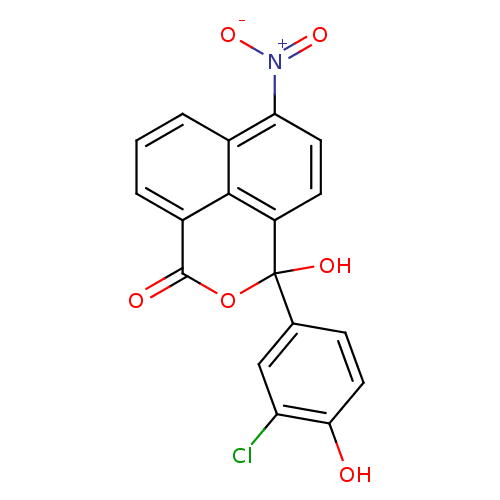 (3-(3-Chloro-4-hydroxy-phenyl)-3-hydroxy-6-nitro-3H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18763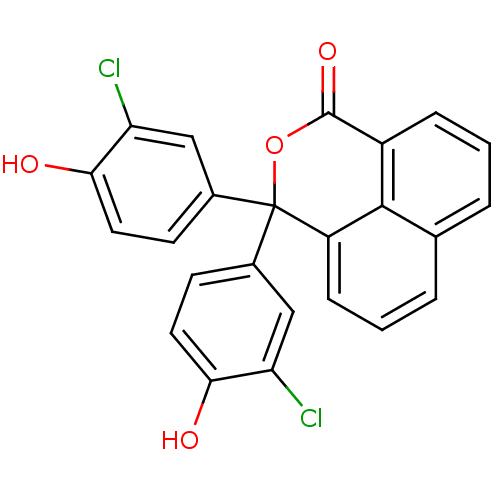 (1,8-naphthalein derivative, 10 | 4,4-bis(3-chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077852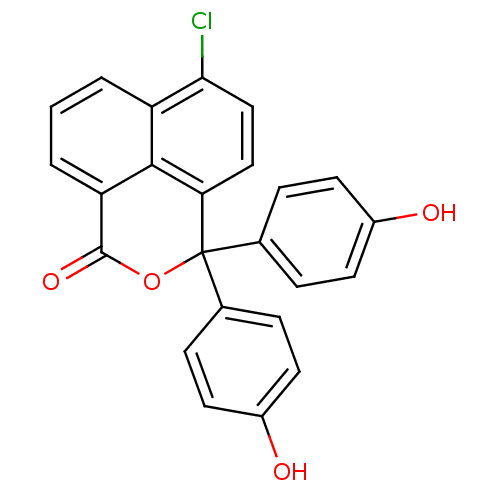 (6-Chloro-3,3-bis-(4-hydroxy-phenyl)-3H-benzo[de]is...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18764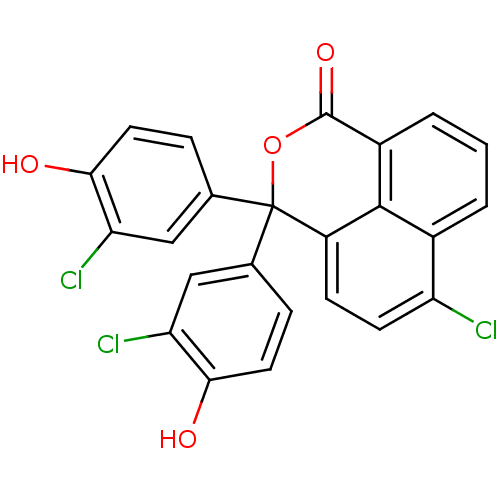 (1,8-naphthalein derivative, 11 | 8-chloro-4,4-bis(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077849 (3,3-Bis-(4-hydroxy-phenyl)-6-nitro-3H-benzo[de]iso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077838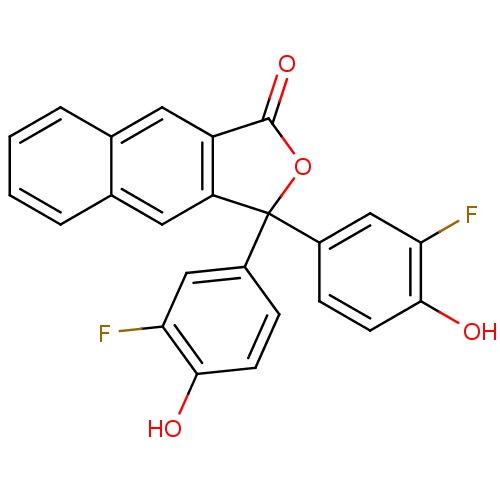 (3,3-Bis-(3-fluoro-4-hydroxy-phenyl)-3H-naphtho[2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077836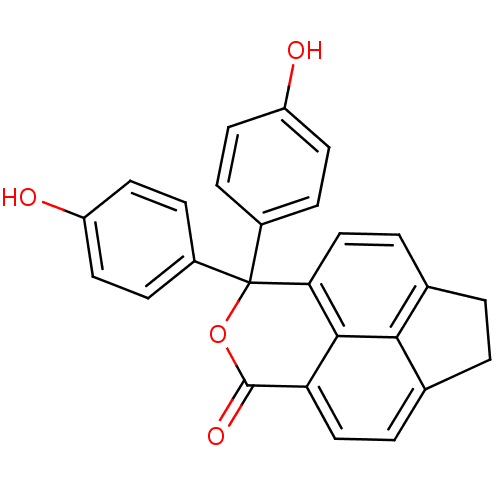 (7,7-Bis-(4-hydroxy-phenyl)-2,7-dihydro-1H-6-oxa-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077847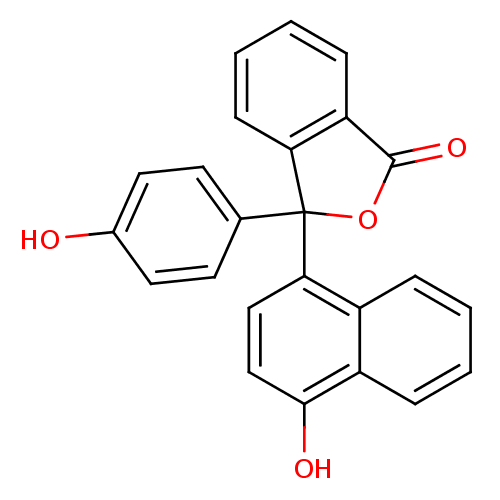 (3-(4-Hydroxy-naphthalen-1-yl)-3-(4-hydroxy-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077850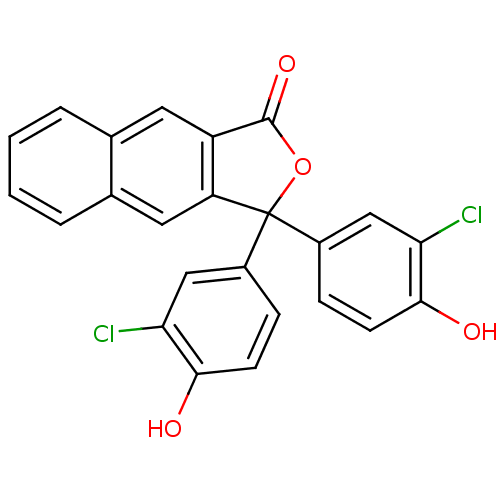 (3,3-Bis-(3-chloro-4-hydroxy-phenyl)-3H-naphtho[2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18769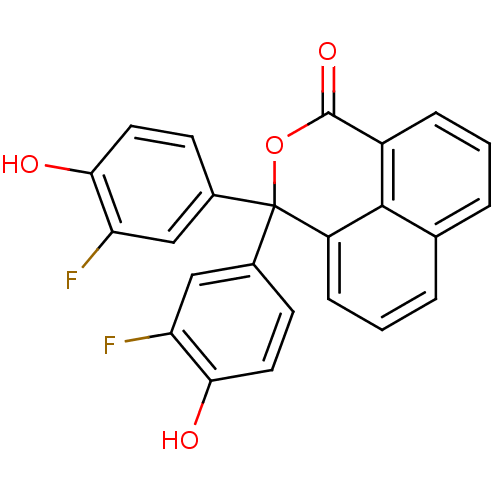 (1,8-naphthalein derivative, 17 | 4,4-bis(3-fluoro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077841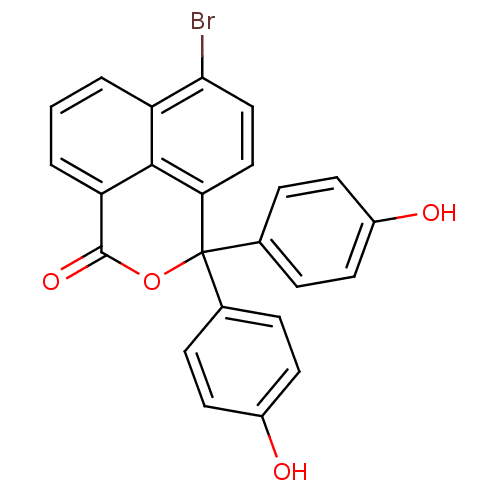 (6-Bromo-3,3-bis-(4-hydroxy-phenyl)-3H-benzo[de]iso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077845 (3,3-Bis-(3-chloro-4-hydroxy-phenyl)-3H-isobenzofur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077848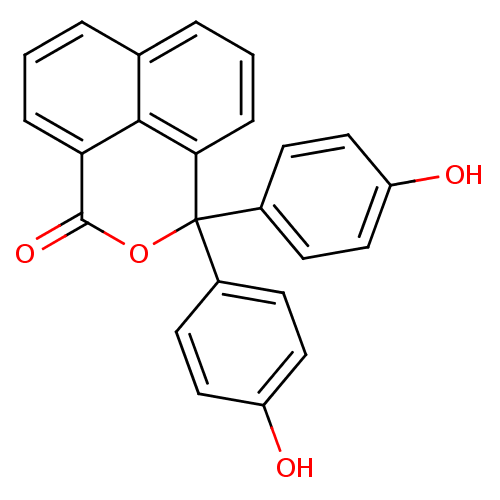 (3,3-Bis-(4-hydroxy-phenyl)-3H-benzo[de]isochromen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077844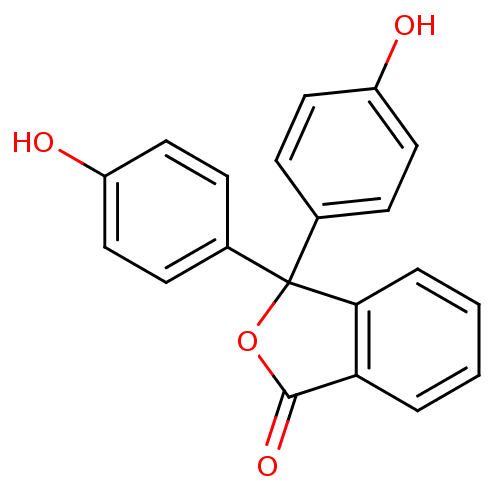 (3,3-Bis-(4-hydroxy-phenyl)-3H-isobenzofuran-1-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077846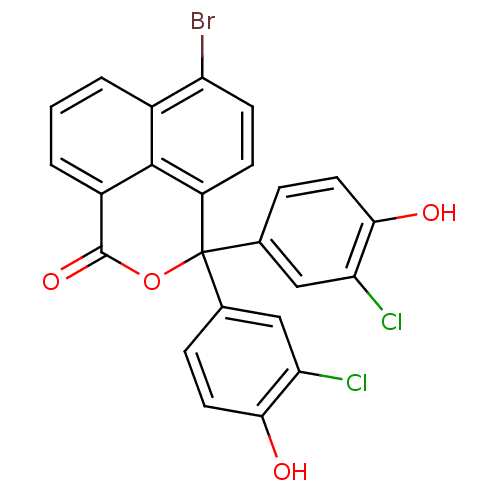 (6-Bromo-3,3-bis-(3-chloro-4-hydroxy-phenyl)-3H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077851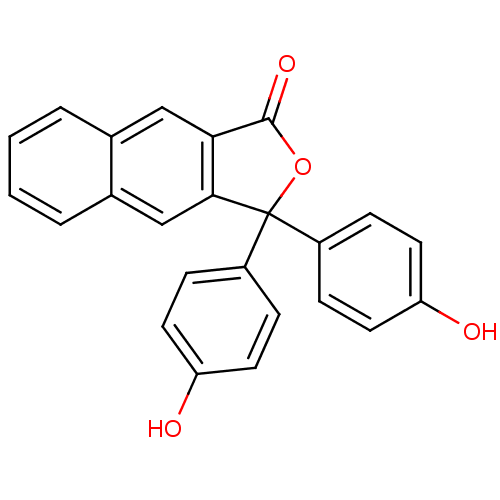 (3,3-Bis-(4-hydroxy-phenyl)-3H-naphtho[2,3-c]furan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077839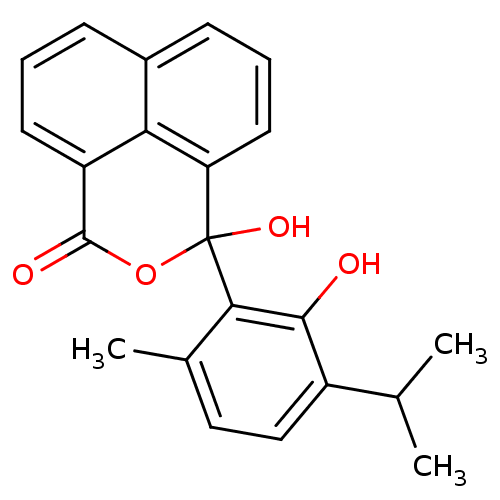 (3-Hydroxy-3-(2-hydroxy-3-isopropyl-6-methyl-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077843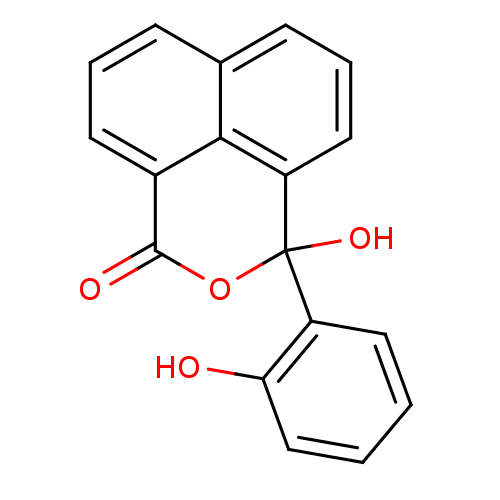 (3-Hydroxy-3-(2-hydroxy-phenyl)-3H-benzo[de]isochro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50077840 (3-Hydroxy-3-(2-propoxy-phenyl)-3H-benzo[de]isochro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description In vitro inhibition of Lactobacillus casei Thymidylate synthase. | J Med Chem 42: 2112-24 (1999) Article DOI: 10.1021/jm9900016 BindingDB Entry DOI: 10.7270/Q23F4NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2325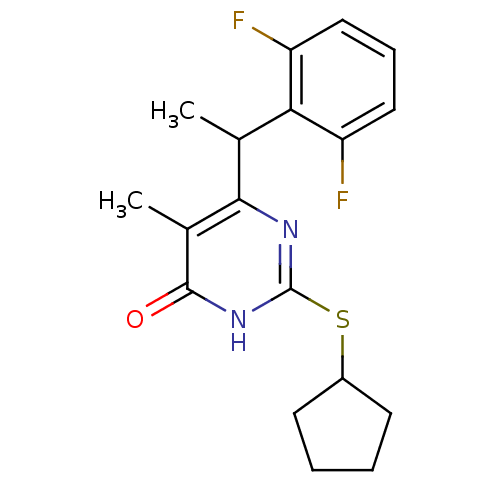 (2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2325 (2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase | Bioorg Med Chem 16: 4173-85 (2008) Article DOI: 10.1016/j.bmc.2007.12.046 BindingDB Entry DOI: 10.7270/Q2WW7MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2323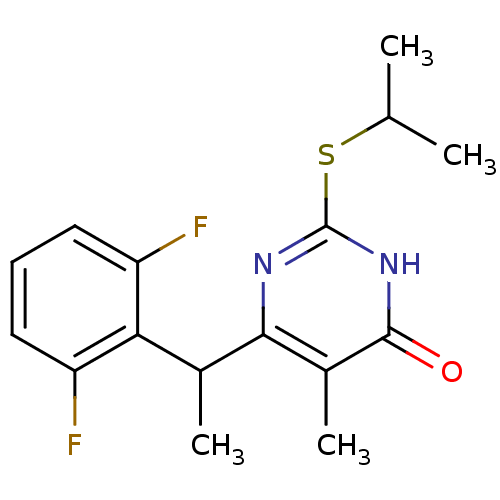 (6-[1-(2,6-difluorophenyl)ethyl]-5-methyl-2-(propan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2324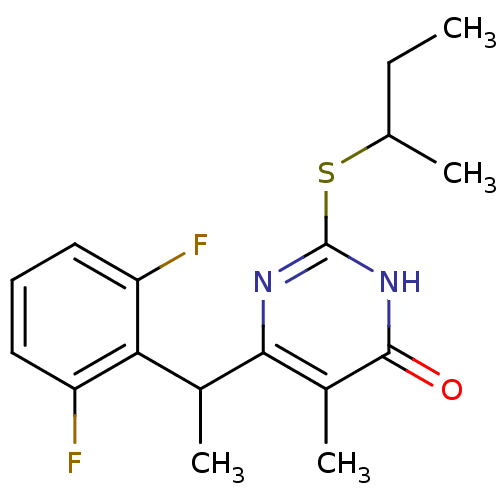 (2-(butan-2-ylsulfanyl)-6-[1-(2,6-difluorophenyl)et...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2322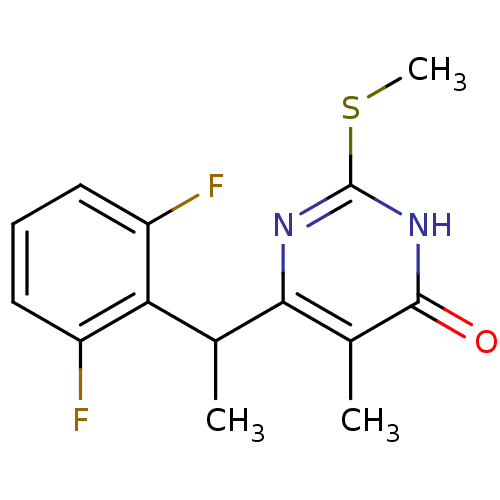 (6-[1-(2,6-difluorophenyl)ethyl]-5-methyl-2-(methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2278 (3-(benzenesulfonyl)-5-chloro-1H-indole-2-carboxami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 46: 2482-93 (2003) Article DOI: 10.1021/jm0211063 BindingDB Entry DOI: 10.7270/Q2BP0101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2321 (2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2486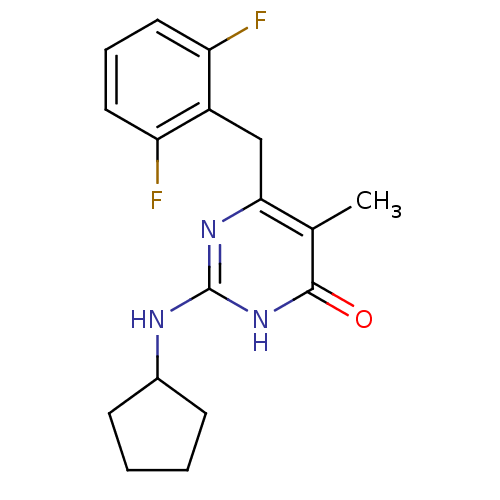 (2-(cyclopentylamino)-6-[(2,6-difluorophenyl)methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2486 (2-(cyclopentylamino)-6-[(2,6-difluorophenyl)methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2482 (5-Chloro-3-[(3,5-dimethylphenyl)sulfonyl]-1H-indol...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 46: 2482-93 (2003) Article DOI: 10.1021/jm0211063 BindingDB Entry DOI: 10.7270/Q2BP0101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 46: 2482-93 (2003) Article DOI: 10.1021/jm0211063 BindingDB Entry DOI: 10.7270/Q2BP0101 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2488 (2-(cyclopentylamino)-6-[1-(2,6-difluorophenyl)ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2488 (2-(cyclopentylamino)-6-[1-(2,6-difluorophenyl)ethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2487 (2-(cyclopentylamino)-6-[1-(2,6-difluorophenyl)ethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337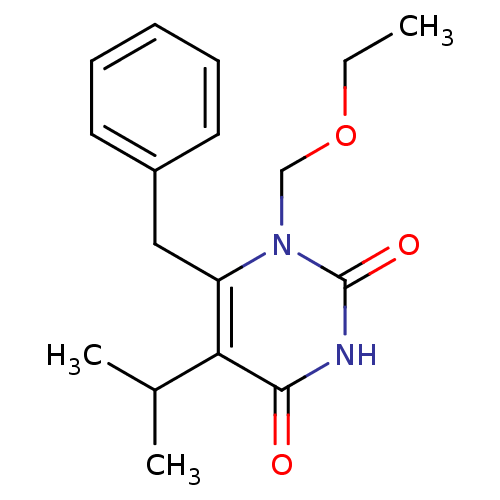 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2487 (2-(cyclopentylamino)-6-[1-(2,6-difluorophenyl)ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2334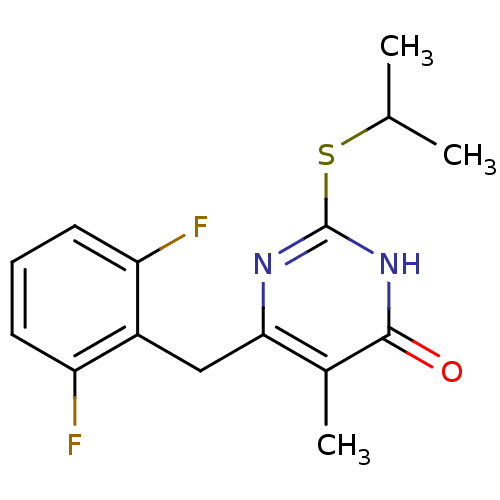 (6-[(2,6-difluorophenyl)methyl]-5-methyl-2-(propan-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2321 (2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2332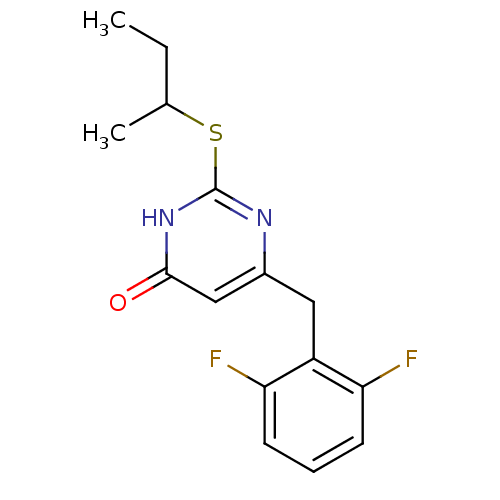 (2-(butan-2-ylsulfanyl)-6-[(2,6-difluorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2331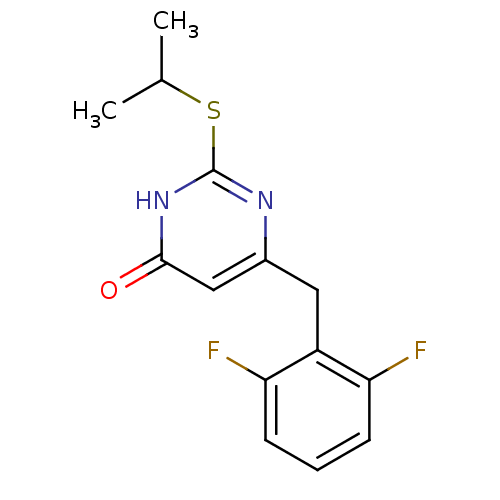 (6-[(2,6-difluorophenyl)methyl]-2-(propan-2-ylsulfa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2335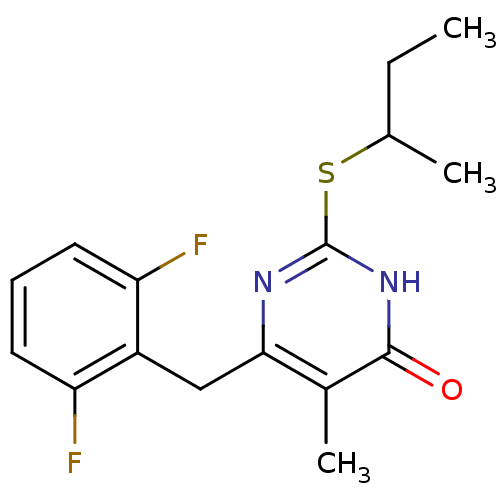 (2-(butan-2-ylsulfanyl)-6-[(2,6-difluorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2332 (2-(butan-2-ylsulfanyl)-6-[(2,6-difluorophenyl)meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 398 total ) | Next | Last >> |