 Found 2385 hits with Last Name = 'laird' and Initial = 'er'
Found 2385 hits with Last Name = 'laird' and Initial = 'er' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203205
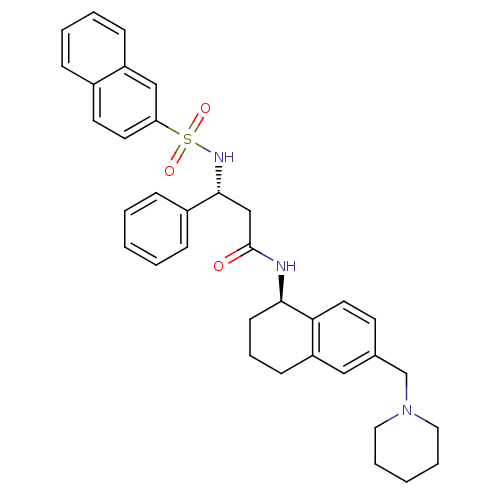
((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C35H39N3O3S/c39-35(36-33-15-9-14-30-22-26(16-19-32(30)33)25-38-20-7-2-8-21-38)24-34(28-11-3-1-4-12-28)37-42(40,41)31-18-17-27-10-5-6-13-29(27)23-31/h1,3-6,10-13,16-19,22-23,33-34,37H,2,7-9,14-15,20-21,24-25H2,(H,36,39)/t33-,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203200
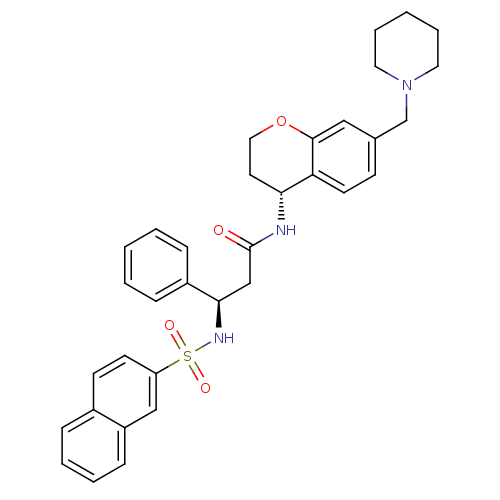
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203199
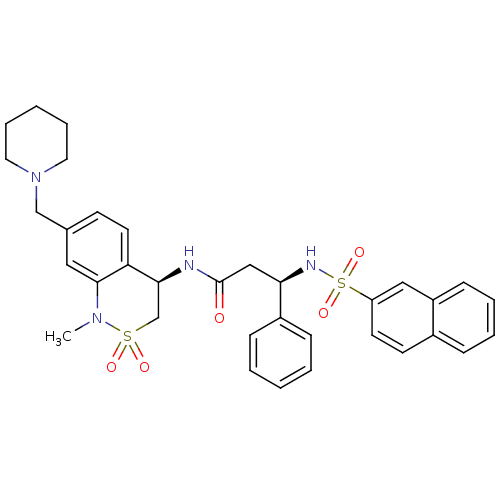
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES CN1c2cc(CN3CCCCC3)ccc2[C@H](CS1(=O)=O)NC(=O)C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C34H38N4O5S2/c1-37-33-20-25(23-38-18-8-3-9-19-38)14-17-30(33)32(24-44(37,40)41)35-34(39)22-31(27-11-4-2-5-12-27)36-45(42,43)29-16-15-26-10-6-7-13-28(26)21-29/h2,4-7,10-17,20-21,31-32,36H,3,8-9,18-19,22-24H2,1H3,(H,35,39)/t31-,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073839
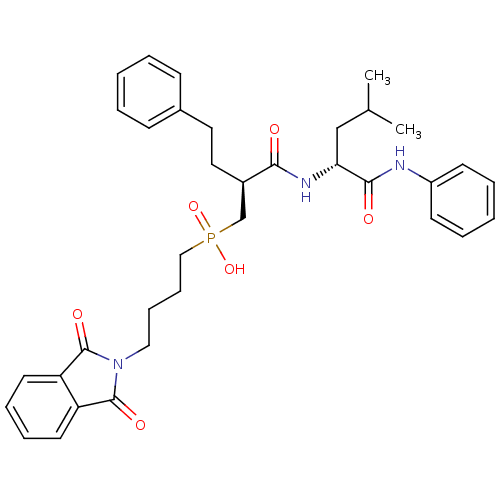
(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203211
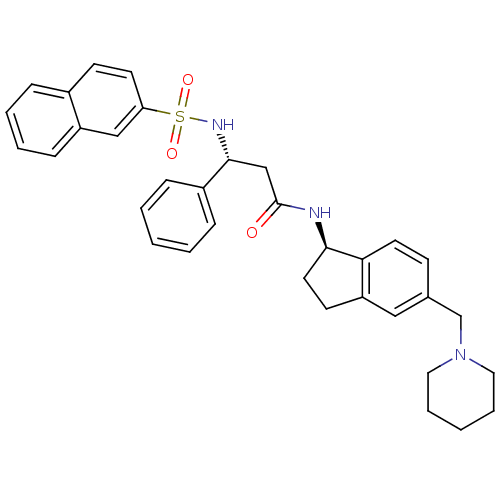
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O3S/c38-34(35-32-18-15-29-21-25(13-17-31(29)32)24-37-19-7-2-8-20-37)23-33(27-10-3-1-4-11-27)36-41(39,40)30-16-14-26-9-5-6-12-28(26)22-30/h1,3-6,9-14,16-17,21-22,32-33,36H,2,7-8,15,18-20,23-24H2,(H,35,38)/t32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50073823

(CHEMBL24871 | {4-[((S)-1-Acetyl-pyrrolidine-2-carb...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-2 (MMP-2) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203210
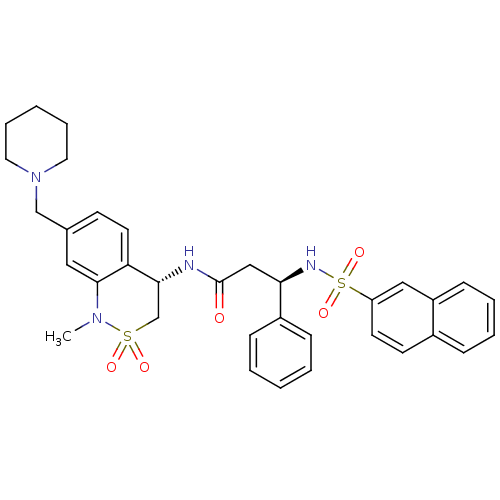
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES CN1c2cc(CN3CCCCC3)ccc2[C@@H](CS1(=O)=O)NC(=O)C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C34H38N4O5S2/c1-37-33-20-25(23-38-18-8-3-9-19-38)14-17-30(33)32(24-44(37,40)41)35-34(39)22-31(27-11-4-2-5-12-27)36-45(42,43)29-16-15-26-10-6-7-13-28(26)21-29/h2,4-7,10-17,20-21,31-32,36H,3,8-9,18-19,22-24H2,1H3,(H,35,39)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203197
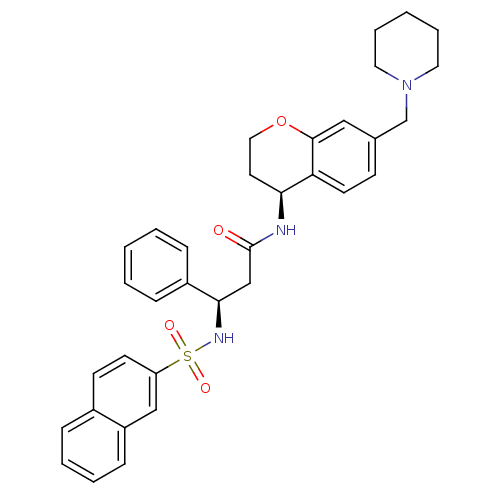
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203206

((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES C[C@@H](NC(=O)C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)c1ccc(CN2CCCCC2)cc1 Show InChI InChI=1S/C33H37N3O3S/c1-25(27-16-14-26(15-17-27)24-36-20-8-3-9-21-36)34-33(37)23-32(29-11-4-2-5-12-29)35-40(38,39)31-19-18-28-10-6-7-13-30(28)22-31/h2,4-7,10-19,22,25,32,35H,3,8-9,20-21,23-24H2,1H3,(H,34,37)/t25-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50073839

(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-2 (MMP-2) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203208
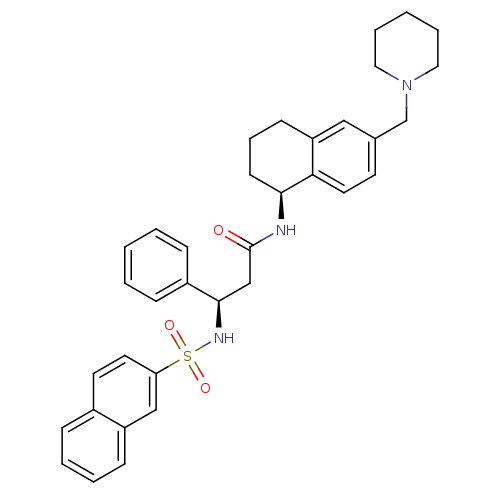
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCCc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C35H39N3O3S/c39-35(36-33-15-9-14-30-22-26(16-19-32(30)33)25-38-20-7-2-8-21-38)24-34(28-11-3-1-4-12-28)37-42(40,41)31-18-17-27-10-5-6-13-29(27)23-31/h1,3-6,10-13,16-19,22-23,33-34,37H,2,7-9,14-15,20-21,24-25H2,(H,36,39)/t33-,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073823

(CHEMBL24871 | {4-[((S)-1-Acetyl-pyrrolidine-2-carb...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203202
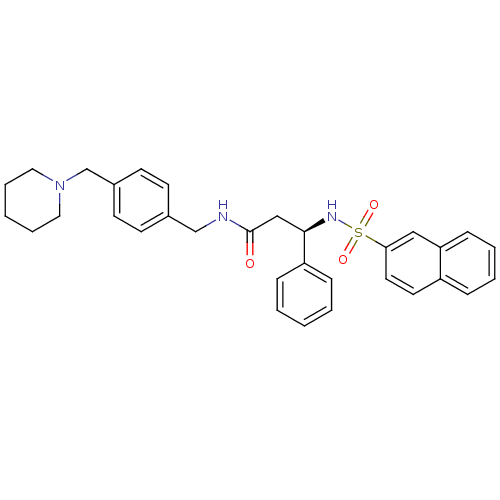
((R)-N-(4-(piperidin-1-ylmethyl)benzyl)-3-(naphthal...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)NCc1ccc(CN2CCCCC2)cc1 Show InChI InChI=1S/C32H35N3O3S/c36-32(33-23-25-13-15-26(16-14-25)24-35-19-7-2-8-20-35)22-31(28-10-3-1-4-11-28)34-39(37,38)30-18-17-27-9-5-6-12-29(27)21-30/h1,3-6,9-18,21,31,34H,2,7-8,19-20,22-24H2,(H,33,36)/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203203
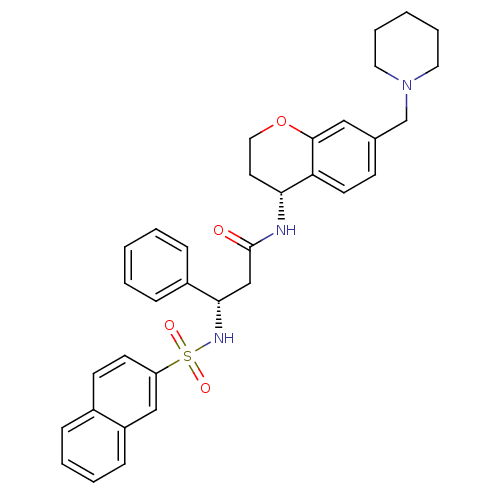
((S)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203207
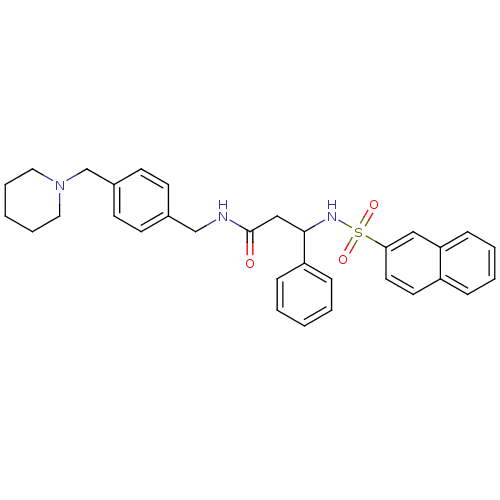
(3-(RS)-N-(4-(piperidin-1-ylmethyl)benzyl)-3-(napht...)Show SMILES O=C(CC(NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)NCc1ccc(CN2CCCCC2)cc1 Show InChI InChI=1S/C32H35N3O3S/c36-32(33-23-25-13-15-26(16-14-25)24-35-19-7-2-8-20-35)22-31(28-10-3-1-4-11-28)34-39(37,38)30-18-17-27-9-5-6-12-29(27)21-30/h1,3-6,9-18,21,31,34H,2,7-8,19-20,22-24H2,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203204
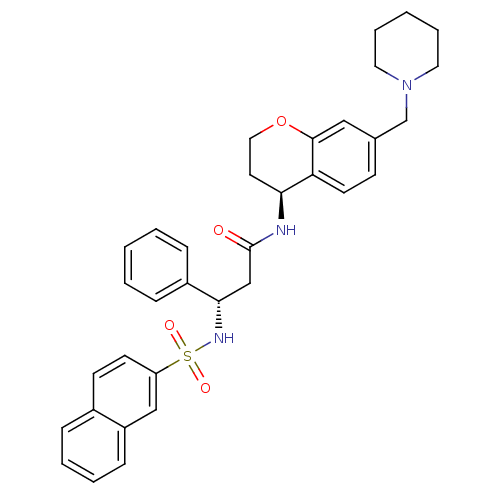
((S)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203198
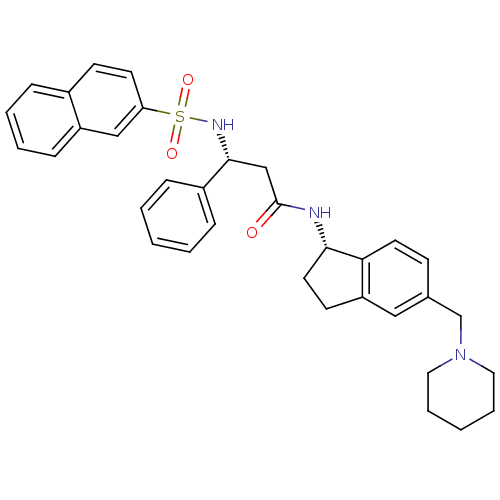
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O3S/c38-34(35-32-18-15-29-21-25(13-17-31(29)32)24-37-19-7-2-8-20-37)23-33(27-10-3-1-4-11-27)36-41(39,40)30-16-14-26-9-5-6-12-28(26)22-30/h1,3-6,9-14,16-17,21-22,32-33,36H,2,7-8,15,18-20,23-24H2,(H,35,38)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203208

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCCc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C35H39N3O3S/c39-35(36-33-15-9-14-30-22-26(16-19-32(30)33)25-38-20-7-2-8-21-38)24-34(28-11-3-1-4-12-28)37-42(40,41)31-18-17-27-10-5-6-13-29(27)23-31/h1,3-6,10-13,16-19,22-23,33-34,37H,2,7-9,14-15,20-21,24-25H2,(H,36,39)/t33-,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203210

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES CN1c2cc(CN3CCCCC3)ccc2[C@@H](CS1(=O)=O)NC(=O)C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C34H38N4O5S2/c1-37-33-20-25(23-38-18-8-3-9-19-38)14-17-30(33)32(24-44(37,40)41)35-34(39)22-31(27-11-4-2-5-12-27)36-45(42,43)29-16-15-26-10-6-7-13-28(26)21-29/h2,4-7,10-17,20-21,31-32,36H,3,8-9,18-19,22-24H2,1H3,(H,35,39)/t31-,32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203197

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203199

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES CN1c2cc(CN3CCCCC3)ccc2[C@H](CS1(=O)=O)NC(=O)C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C34H38N4O5S2/c1-37-33-20-25(23-38-18-8-3-9-19-38)14-17-30(33)32(24-44(37,40)41)35-34(39)22-31(27-11-4-2-5-12-27)36-45(42,43)29-16-15-26-10-6-7-13-28(26)21-29/h2,4-7,10-17,20-21,31-32,36H,3,8-9,18-19,22-24H2,1H3,(H,35,39)/t31-,32+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203211

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O3S/c38-34(35-32-18-15-29-21-25(13-17-31(29)32)24-37-19-7-2-8-20-37)23-33(27-10-3-1-4-11-27)36-41(39,40)30-16-14-26-9-5-6-12-28(26)22-30/h1,3-6,9-14,16-17,21-22,32-33,36H,2,7-8,15,18-20,23-24H2,(H,35,38)/t32-,33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203200

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203207

(3-(RS)-N-(4-(piperidin-1-ylmethyl)benzyl)-3-(napht...)Show SMILES O=C(CC(NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)NCc1ccc(CN2CCCCC2)cc1 Show InChI InChI=1S/C32H35N3O3S/c36-32(33-23-25-13-15-26(16-14-25)24-35-19-7-2-8-20-35)22-31(28-10-3-1-4-11-28)34-39(37,38)30-18-17-27-9-5-6-12-29(27)21-30/h1,3-6,9-18,21,31,34H,2,7-8,19-20,22-24H2,(H,33,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203205

((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C35H39N3O3S/c39-35(36-33-15-9-14-30-22-26(16-19-32(30)33)25-38-20-7-2-8-21-38)24-34(28-11-3-1-4-12-28)37-42(40,41)31-18-17-27-10-5-6-13-29(27)23-31/h1,3-6,10-13,16-19,22-23,33-34,37H,2,7-9,14-15,20-21,24-25H2,(H,36,39)/t33-,34-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203198

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@H]1CCc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O3S/c38-34(35-32-18-15-29-21-25(13-17-31(29)32)24-37-19-7-2-8-20-37)23-33(27-10-3-1-4-11-27)36-41(39,40)30-16-14-26-9-5-6-12-28(26)22-30/h1,3-6,9-14,16-17,21-22,32-33,36H,2,7-8,15,18-20,23-24H2,(H,35,38)/t32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224846
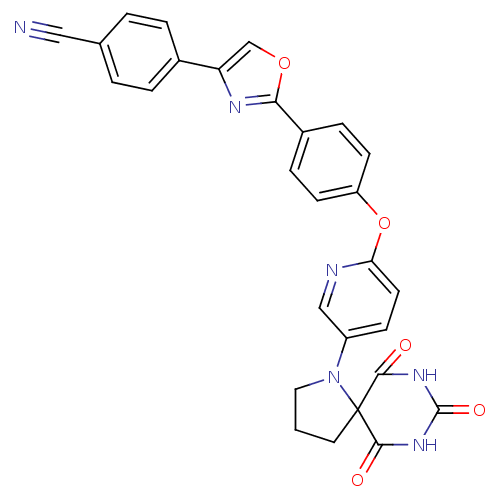
(4-(2-{4-[5-(6,8,10-trioxo-1,7,9-triaza-spiro[4.5]d...)Show SMILES O=C1NC(=O)C2(CCCN2c2ccc(Oc3ccc(cc3)-c3nc(co3)-c3ccc(cc3)C#N)nc2)C(=O)N1 Show InChI InChI=1S/C28H20N6O5/c29-14-17-2-4-18(5-3-17)22-16-38-24(31-22)19-6-9-21(10-7-19)39-23-11-8-20(15-30-23)34-13-1-12-28(34)25(35)32-27(37)33-26(28)36/h2-11,15-16H,1,12-13H2,(H2,32,33,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224838
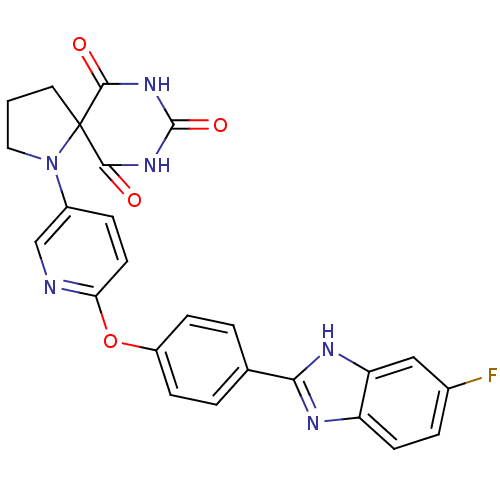
(1-{6-[4-(6-fluoro-1H-benzoimidazol-2-yl)-phenoxy]-...)Show SMILES Fc1ccc2nc([nH]c2c1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C25H19FN6O4/c26-15-4-8-18-19(12-15)29-21(28-18)14-2-6-17(7-3-14)36-20-9-5-16(13-27-20)32-11-1-10-25(32)22(33)30-24(35)31-23(25)34/h2-9,12-13H,1,10-11H2,(H,28,29)(H2,30,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224842

(1-(6-{4-[4-(3-fluoro-phenyl)-oxazol-2-yl]-phenoxy}...)Show SMILES Fc1cccc(c1)-c1coc(n1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C27H20FN5O5/c28-18-4-1-3-17(13-18)21-15-37-23(30-21)16-5-8-20(9-6-16)38-22-10-7-19(14-29-22)33-12-2-11-27(33)24(34)31-26(36)32-25(27)35/h1,3-10,13-15H,2,11-12H2,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224852
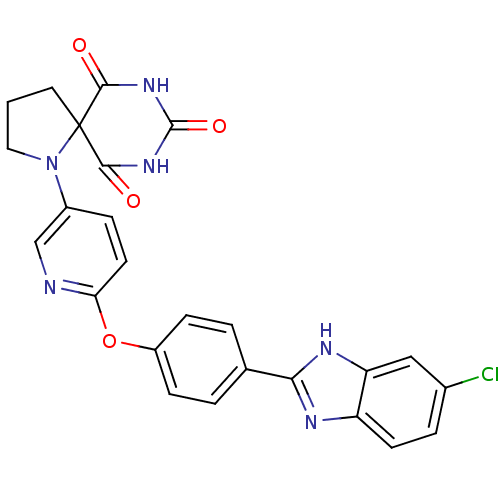
(1-{6-[4-(6-chloro-1H-benzoimidazol-2-yl)-phenoxy]-...)Show SMILES Clc1ccc2nc([nH]c2c1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C25H19ClN6O4/c26-15-4-8-18-19(12-15)29-21(28-18)14-2-6-17(7-3-14)36-20-9-5-16(13-27-20)32-11-1-10-25(32)22(33)30-24(35)31-23(25)34/h2-9,12-13H,1,10-11H2,(H,28,29)(H2,30,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224856

(1-{6-[4-(1H-benzoimidazol-2-yl)-phenoxy]-pyridin-3...)Show SMILES O=C1NC(=O)C2(CCCN2c2ccc(Oc3ccc(cc3)-c3nc4ccccc4[nH]3)nc2)C(=O)N1 Show InChI InChI=1S/C25H20N6O4/c32-22-25(23(33)30-24(34)29-22)12-3-13-31(25)16-8-11-20(26-14-16)35-17-9-6-15(7-10-17)21-27-18-4-1-2-5-19(18)28-21/h1-2,4-11,14H,3,12-13H2,(H,27,28)(H2,29,30,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224847
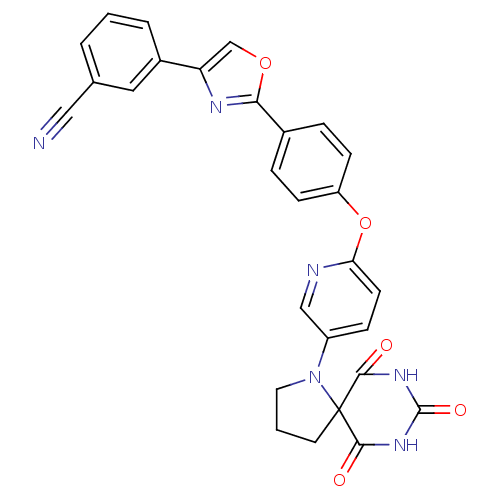
(3-(2-{4-[5-(6,8,10-trioxo-1,7,9-triaza-spiro[4.5]d...)Show SMILES O=C1NC(=O)C2(CCCN2c2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccc(c3)C#N)nc2)C(=O)N1 Show InChI InChI=1S/C28H20N6O5/c29-14-17-3-1-4-19(13-17)22-16-38-24(31-22)18-5-8-21(9-6-18)39-23-10-7-20(15-30-23)34-12-2-11-28(34)25(35)32-27(37)33-26(28)36/h1,3-10,13,15-16H,2,11-12H2,(H2,32,33,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224837

(1-(6-{4-[4-(4-fluoro-phenyl)-oxazol-2-yl]-phenoxy}...)Show SMILES Fc1ccc(cc1)-c1coc(n1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C27H20FN5O5/c28-18-6-2-16(3-7-18)21-15-37-23(30-21)17-4-9-20(10-5-17)38-22-11-8-19(14-29-22)33-13-1-12-27(33)24(34)31-26(36)32-25(27)35/h2-11,14-15H,1,12-13H2,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224857
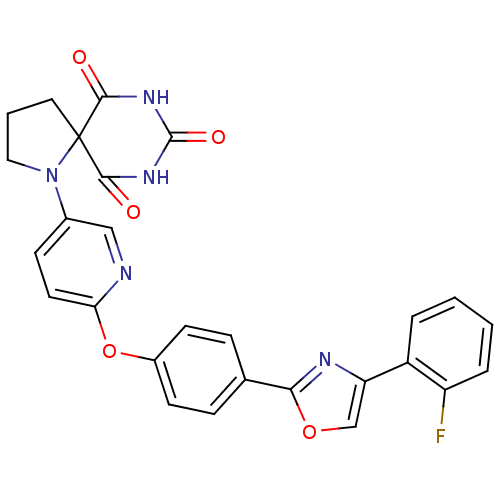
(1-(6-{4-[4-(2-fluoro-phenyl)-oxazol-2-yl]-phenoxy}...)Show SMILES Fc1ccccc1-c1coc(n1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C27H20FN5O5/c28-20-5-2-1-4-19(20)21-15-37-23(30-21)16-6-9-18(10-7-16)38-22-11-8-17(14-29-22)33-13-3-12-27(33)24(34)31-26(36)32-25(27)35/h1-2,4-11,14-15H,3,12-13H2,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50484212
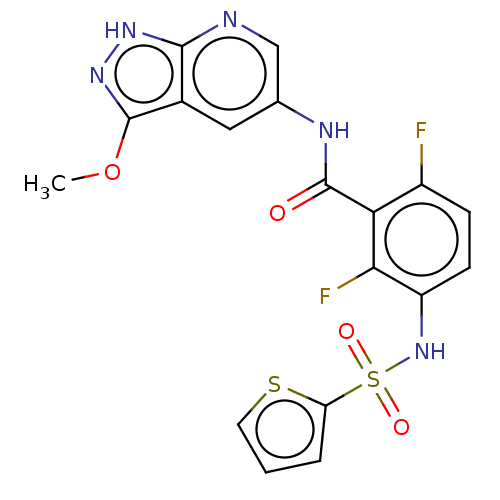
(CHEMBL1822264)Show SMILES COc1n[nH]c2ncc(NC(=O)c3c(F)ccc(NS(=O)(=O)c4cccs4)c3F)cc12 Show InChI InChI=1S/C18H13F2N5O4S2/c1-29-18-10-7-9(8-21-16(10)23-24-18)22-17(26)14-11(19)4-5-12(15(14)20)25-31(27,28)13-3-2-6-30-13/h2-8,25H,1H3,(H,22,26)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ArrayBioPharma
Curated by ChEMBL
| Assay Description
Inhibition of full length B-Raf V600E mutant |
Bioorg Med Chem Lett 21: 5533-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.097
BindingDB Entry DOI: 10.7270/Q28K7CX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50496943
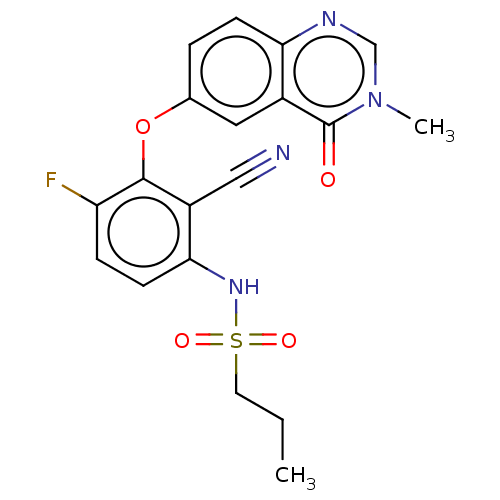
(CHEMBL3236471)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(Oc2ccc3ncn(C)c(=O)c3c2)c1C#N Show InChI InChI=1S/C19H17FN4O4S/c1-3-8-29(26,27)23-17-7-5-15(20)18(14(17)10-21)28-12-4-6-16-13(9-12)19(25)24(2)11-22-16/h4-7,9,11,23H,3,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length B-Raf V600E mutant (unknown origin) |
Bioorg Med Chem Lett 24: 1923-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.007
BindingDB Entry DOI: 10.7270/Q2Q24368 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50496941
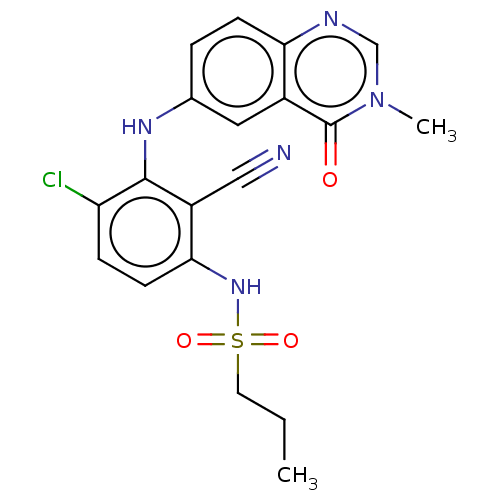
(CHEMBL3236463)Show SMILES CCCS(=O)(=O)Nc1ccc(Cl)c(Nc2ccc3ncn(C)c(=O)c3c2)c1C#N Show InChI InChI=1S/C19H18ClN5O3S/c1-3-8-29(27,28)24-17-7-5-15(20)18(14(17)10-21)23-12-4-6-16-13(9-12)19(26)25(2)11-22-16/h4-7,9,11,23-24H,3,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length B-Raf V600E mutant (unknown origin) |
Bioorg Med Chem Lett 24: 1923-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.007
BindingDB Entry DOI: 10.7270/Q2Q24368 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50496954
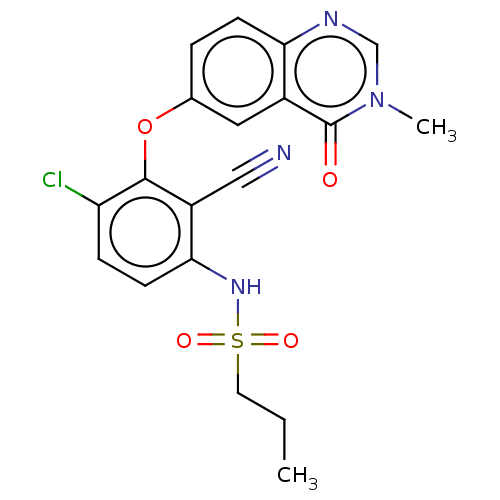
(CHEMBL3236470)Show SMILES CCCS(=O)(=O)Nc1ccc(Cl)c(Oc2ccc3ncn(C)c(=O)c3c2)c1C#N Show InChI InChI=1S/C19H17ClN4O4S/c1-3-8-29(26,27)23-17-7-5-15(20)18(14(17)10-21)28-12-4-6-16-13(9-12)19(25)24(2)11-22-16/h4-7,9,11,23H,3,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length B-Raf V600E mutant (unknown origin) |
Bioorg Med Chem Lett 24: 1923-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.007
BindingDB Entry DOI: 10.7270/Q2Q24368 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50496946
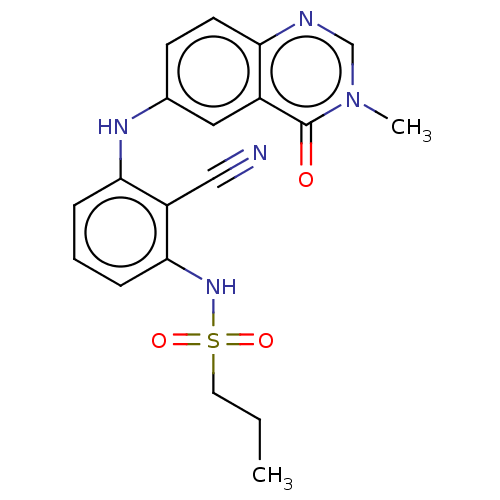
(CHEMBL3236464)Show SMILES CCCS(=O)(=O)Nc1cccc(Nc2ccc3ncn(C)c(=O)c3c2)c1C#N Show InChI InChI=1S/C19H19N5O3S/c1-3-9-28(26,27)23-18-6-4-5-17(15(18)11-20)22-13-7-8-16-14(10-13)19(25)24(2)12-21-16/h4-8,10,12,22-23H,3,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length B-Raf V600E mutant (unknown origin) |
Bioorg Med Chem Lett 24: 1923-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.007
BindingDB Entry DOI: 10.7270/Q2Q24368 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50496948
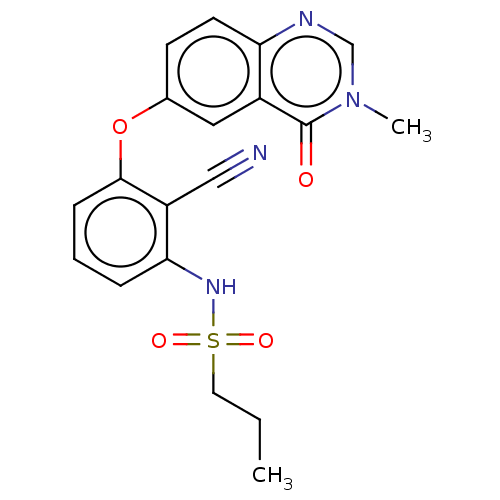
(CHEMBL3236472)Show SMILES CCCS(=O)(=O)Nc1cccc(Oc2ccc3ncn(C)c(=O)c3c2)c1C#N Show InChI InChI=1S/C19H18N4O4S/c1-3-9-28(25,26)22-17-5-4-6-18(15(17)11-20)27-13-7-8-16-14(10-13)19(24)23(2)12-21-16/h4-8,10,12,22H,3,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length B-Raf V600E mutant (unknown origin) |
Bioorg Med Chem Lett 24: 1923-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.007
BindingDB Entry DOI: 10.7270/Q2Q24368 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM565148

(US11414404, Ex. # 25 | US11634409, Example 25)Show SMILES Cc1c(Nc2c(F)ccc(NS(=O)(=O)c3cc(F)ccc3F)c2Cl)ccc2ncn(C)c(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A competitive displacement assay was configured for B-Raf that monitors the amount of a fluorescently-tagged “tracer” bound to B-Raf via TR-FRET from... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JD511G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM565148

(US11414404, Ex. # 25 | US11634409, Example 25)Show SMILES Cc1c(Nc2c(F)ccc(NS(=O)(=O)c3cc(F)ccc3F)c2Cl)ccc2ncn(C)c(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FT8R02 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194286
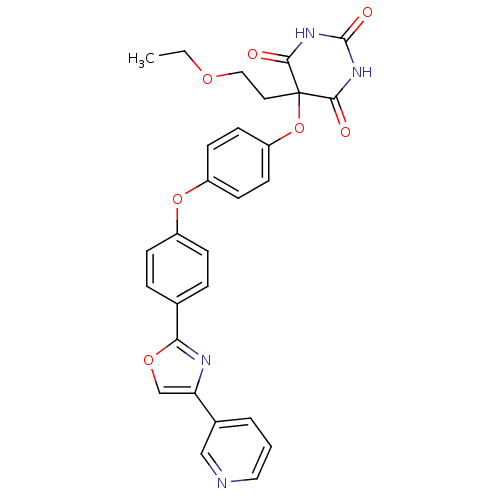
(5-(2-ethoxyethyl)-5-(4-(4-(4-(pyridin-3-yl)oxazol-...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccnc3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C28H24N4O7/c1-2-36-15-13-28(25(33)31-27(35)32-26(28)34)39-22-11-9-21(10-12-22)38-20-7-5-18(6-8-20)24-30-23(17-37-24)19-4-3-14-29-16-19/h3-12,14,16-17H,2,13,15H2,1H3,(H2,31,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 5822-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.066
BindingDB Entry DOI: 10.7270/Q27P8Z1R |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194306

(5-(2-ethoxyethyl)-5-(4-(4-(4-(3-fluorophenyl)oxazo...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccc(F)c3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C29H24FN3O7/c1-2-37-15-14-29(26(34)32-28(36)33-27(29)35)40-23-12-10-22(11-13-23)39-21-8-6-18(7-9-21)25-31-24(17-38-25)19-4-3-5-20(30)16-19/h3-13,16-17H,2,14-15H2,1H3,(H2,32,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194306

(5-(2-ethoxyethyl)-5-(4-(4-(4-(3-fluorophenyl)oxazo...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccc(F)c3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C29H24FN3O7/c1-2-37-15-14-29(26(34)32-28(36)33-27(29)35)40-23-12-10-22(11-13-23)39-21-8-6-18(7-9-21)25-31-24(17-38-25)19-4-3-5-20(30)16-19/h3-13,16-17H,2,14-15H2,1H3,(H2,32,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 5822-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.066
BindingDB Entry DOI: 10.7270/Q27P8Z1R |
More data for this
Ligand-Target Pair | |
Collagenase 3
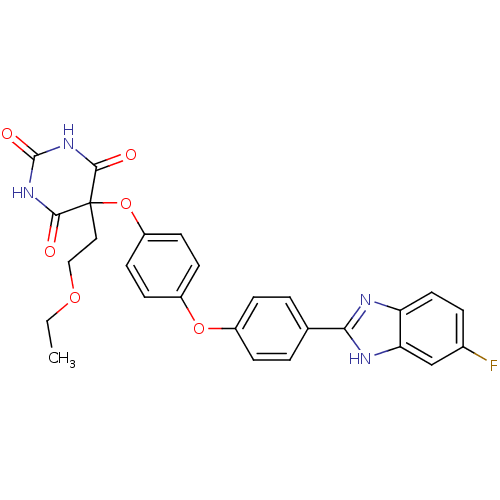
(Homo sapiens (Human)) | BDBM50224845
(5-(2-ethoxyethyl)-5-(4-(4-(6-fluoro-1H-benzo[d]imi...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc4ccc(F)cc4[nH]3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C27H23FN4O6/c1-2-36-14-13-27(24(33)31-26(35)32-25(27)34)38-20-10-8-19(9-11-20)37-18-6-3-16(4-7-18)23-29-21-12-5-17(28)15-22(21)30-23/h3-12,15H,2,13-14H2,1H3,(H,29,30)(H2,31,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
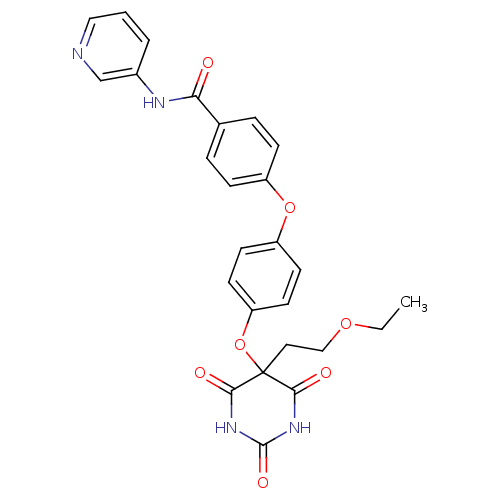
(Homo sapiens (Human)) | BDBM50194278
(4-(4-(5-(2-ethoxyethyl)-2,4,6-trioxo-hexahydropyri...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)C(=O)Nc3cccnc3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C26H24N4O7/c1-2-35-15-13-26(23(32)29-25(34)30-24(26)33)37-21-11-9-20(10-12-21)36-19-7-5-17(6-8-19)22(31)28-18-4-3-14-27-16-18/h3-12,14,16H,2,13,15H2,1H3,(H,28,31)(H2,29,30,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 5822-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.066
BindingDB Entry DOI: 10.7270/Q27P8Z1R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
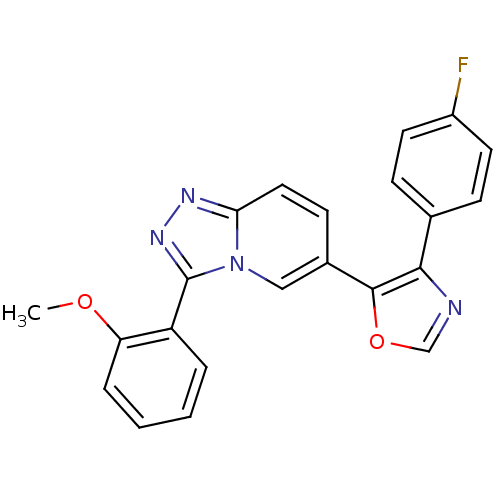
(Homo sapiens (Human)) | BDBM16384
(4-(4-fluorophenyl)-5-[3-(2-methoxyphenyl)-[1,2,4]t...)Show SMILES COc1ccccc1-c1nnc2ccc(cn12)-c1ocnc1-c1ccc(F)cc1 Show InChI InChI=1S/C22H15FN4O2/c1-28-18-5-3-2-4-17(18)22-26-25-19-11-8-15(12-27(19)22)21-20(24-13-29-21)14-6-9-16(23)10-7-14/h2-13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... |
Bioorg Med Chem Lett 16: 4339-44 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.056
BindingDB Entry DOI: 10.7270/Q2251GFZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16385
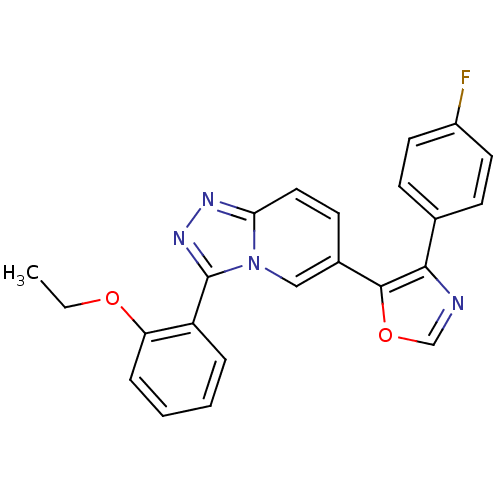
(5-[3-(2-ethoxyphenyl)-[1,2,4]triazolo[3,4-a]pyridi...)Show SMILES CCOc1ccccc1-c1nnc2ccc(cn12)-c1ocnc1-c1ccc(F)cc1 Show InChI InChI=1S/C23H17FN4O2/c1-2-29-19-6-4-3-5-18(19)23-27-26-20-12-9-16(13-28(20)23)22-21(25-14-30-22)15-7-10-17(24)11-8-15/h3-14H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... |
Bioorg Med Chem Lett 16: 4339-44 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.056
BindingDB Entry DOI: 10.7270/Q2251GFZ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203200

((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity at African green monkey bradykinin B1 receptor assessed as effect on DAK-mediated calcium mobilization |
J Med Chem 50: 607-10 (2007)
Article DOI: 10.1021/jm061224g
BindingDB Entry DOI: 10.7270/Q2KP81T0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data