 Found 710 hits with Last Name = 'lamsal' and Initial = 'a'
Found 710 hits with Last Name = 'lamsal' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic acetylcholine receptor subtype 5 expressed in CHO cell membranes by scintillation counting method |
J Med Chem 57: 7804-10 (2014)
Article DOI: 10.1021/jm500995y
BindingDB Entry DOI: 10.7270/Q2ST7RFM |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant human DAT expressed in CHO-S cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant human DAT expressed in CHO-S cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249435
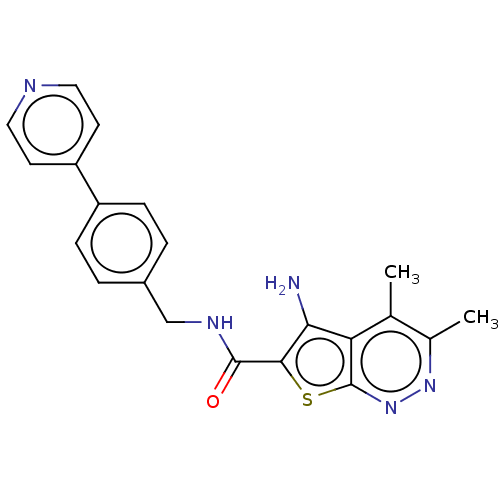
(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Induction of CYP3A4 in cryopreserved human hepatocytes measured after 48 hrs |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50253092
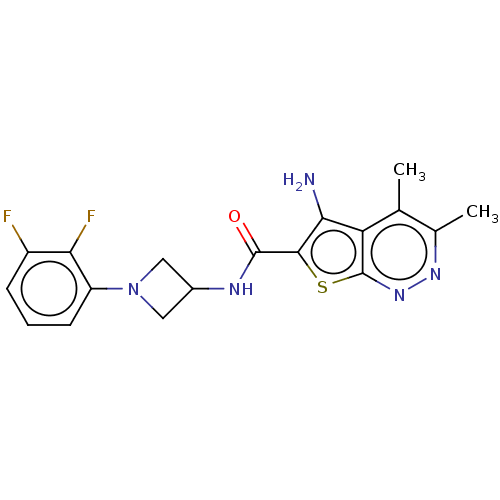
(CHEMBL4062319)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C Show InChI InChI=1S/C18H17F2N5OS/c1-8-9(2)23-24-18-13(8)15(21)16(27-18)17(26)22-10-6-25(7-10)12-5-3-4-11(19)14(12)20/h3-5,10H,6-7,21H2,1-2H3,(H,22,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from human recombinant dopamine transporter expressed in CHO cells |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Paroxetine from recombinant human SERT expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Paroxetine from recombinant human SERT expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249405

(CHEMBL4102040)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccc(F)nc3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-9-13-3-5-14(6-4-13)15-7-8-16(22)24-10-15/h3-8,10H,9,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50249435

(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50174739
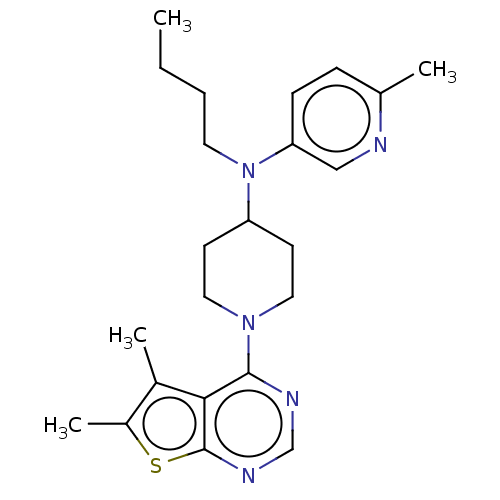
(CHEMBL3808906)Show SMILES CCCCN(C1CCN(CC1)c1ncnc2sc(C)c(C)c12)c1ccc(C)nc1 Show InChI InChI=1S/C23H31N5S/c1-5-6-11-28(20-8-7-16(2)24-14-20)19-9-12-27(13-10-19)22-21-17(3)18(4)29-23(21)26-15-25-22/h7-8,14-15,19H,5-6,9-13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human M4 receptor expressed in CHO cells coexpressing Gqi5 by calcium mobilization assay |
Bioorg Med Chem Lett 26: 3029-33 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.010
BindingDB Entry DOI: 10.7270/Q2DV1MTJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H] GR-65630 from recombinant human 5-HT3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H] GR-65630 from recombinant human 5-HT3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50249435

(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50174738
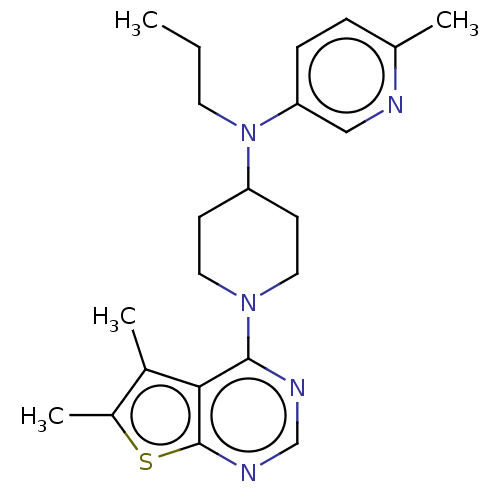
(CHEMBL3808947)Show SMILES CCCN(C1CCN(CC1)c1ncnc2sc(C)c(C)c12)c1ccc(C)nc1 Show InChI InChI=1S/C22H29N5S/c1-5-10-27(19-7-6-15(2)23-13-19)18-8-11-26(12-9-18)21-20-16(3)17(4)28-22(20)25-14-24-21/h6-7,13-14,18H,5,8-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human M4 receptor expressed in CHO cells coexpressing Gqi5 by calcium mobilization assay |
Bioorg Med Chem Lett 26: 3029-33 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.010
BindingDB Entry DOI: 10.7270/Q2DV1MTJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249434
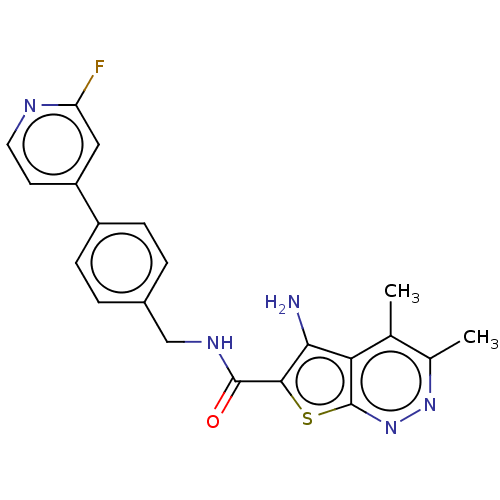
(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50174737

(CHEMBL1357034)Show SMILES Cc1sc2ncnc(N3CCC(CC3)C(=O)Nc3ccc(cc3)S(N)(=O)=O)c2c1C Show InChI InChI=1S/C20H23N5O3S2/c1-12-13(2)29-20-17(12)18(22-11-23-20)25-9-7-14(8-10-25)19(26)24-15-3-5-16(6-4-15)30(21,27)28/h3-6,11,14H,7-10H2,1-2H3,(H,24,26)(H2,21,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 26: 3029-33 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.010
BindingDB Entry DOI: 10.7270/Q2DV1MTJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50253092

(CHEMBL4062319)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C Show InChI InChI=1S/C18H17F2N5OS/c1-8-9(2)23-24-18-13(8)15(21)16(27-18)17(26)22-10-6-25(7-10)12-5-3-4-11(19)14(12)20/h3-5,10H,6-7,21H2,1-2H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human Cav1.2 by radio-ligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50174737

(CHEMBL1357034)Show SMILES Cc1sc2ncnc(N3CCC(CC3)C(=O)Nc3ccc(cc3)S(N)(=O)=O)c2c1C Show InChI InChI=1S/C20H23N5O3S2/c1-12-13(2)29-20-17(12)18(22-11-23-20)25-9-7-14(8-10-25)19(26)24-15-3-5-16(6-4-15)30(21,27)28/h3-6,11,14H,7-10H2,1-2H3,(H,24,26)(H2,21,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 26: 3029-33 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.010
BindingDB Entry DOI: 10.7270/Q2DV1MTJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50253092

(CHEMBL4062319)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C Show InChI InChI=1S/C18H17F2N5OS/c1-8-9(2)23-24-18-13(8)15(21)16(27-18)17(26)22-10-6-25(7-10)12-5-3-4-11(19)14(12)20/h3-5,10H,6-7,21H2,1-2H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of ghrelin receptor (unknown origin) by radioligand binding assay |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249435

(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249403
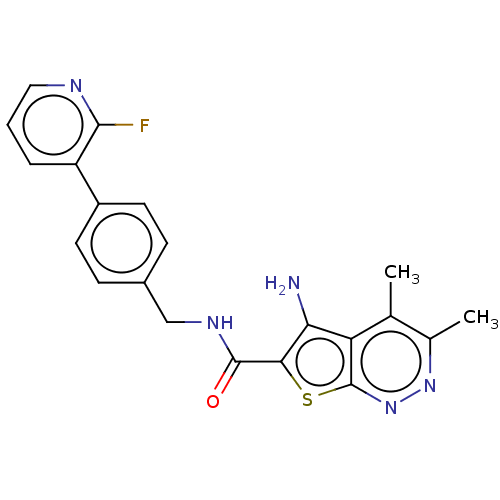
(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50253092

(CHEMBL4062319)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C Show InChI InChI=1S/C18H17F2N5OS/c1-8-9(2)23-24-18-13(8)15(21)16(27-18)17(26)22-10-6-25(7-10)12-5-3-4-11(19)14(12)20/h3-5,10H,6-7,21H2,1-2H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50253092

(CHEMBL4062319)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C Show InChI InChI=1S/C18H17F2N5OS/c1-8-9(2)23-24-18-13(8)15(21)16(27-18)17(26)22-10-6-25(7-10)12-5-3-4-11(19)14(12)20/h3-5,10H,6-7,21H2,1-2H3,(H,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50249403

(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50174737

(CHEMBL1357034)Show SMILES Cc1sc2ncnc(N3CCC(CC3)C(=O)Nc3ccc(cc3)S(N)(=O)=O)c2c1C Show InChI InChI=1S/C20H23N5O3S2/c1-12-13(2)29-20-17(12)18(22-11-23-20)25-9-7-14(8-10-25)19(26)24-15-3-5-16(6-4-15)30(21,27)28/h3-6,11,14H,7-10H2,1-2H3,(H,24,26)(H2,21,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 26: 3029-33 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.010
BindingDB Entry DOI: 10.7270/Q2DV1MTJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50174737

(CHEMBL1357034)Show SMILES Cc1sc2ncnc(N3CCC(CC3)C(=O)Nc3ccc(cc3)S(N)(=O)=O)c2c1C Show InChI InChI=1S/C20H23N5O3S2/c1-12-13(2)29-20-17(12)18(22-11-23-20)25-9-7-14(8-10-25)19(26)24-15-3-5-16(6-4-15)30(21,27)28/h3-6,11,14H,7-10H2,1-2H3,(H,24,26)(H2,21,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 26: 3029-33 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.010
BindingDB Entry DOI: 10.7270/Q2DV1MTJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249434

(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Induction of CYP3A4 in cryopreserved human hepatocytes measured after 48 hrs |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249405

(CHEMBL4102040)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccc(F)nc3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-9-13-3-5-14(6-4-13)15-7-8-16(22)24-10-15/h3-8,10H,9,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50249434

(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50253092

(CHEMBL4062319)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3cccc(F)c3F)c(N)c2c1C Show InChI InChI=1S/C18H17F2N5OS/c1-8-9(2)23-24-18-13(8)15(21)16(27-18)17(26)22-10-6-25(7-10)12-5-3-4-11(19)14(12)20/h3-5,10H,6-7,21H2,1-2H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50249405

(CHEMBL4102040)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccc(F)nc3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-9-13-3-5-14(6-4-13)15-7-8-16(22)24-10-15/h3-8,10H,9,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50207517
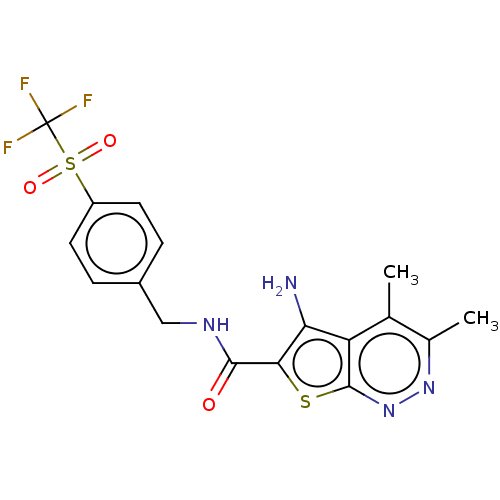
(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50249405

(CHEMBL4102040)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccc(F)nc3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-9-13-3-5-14(6-4-13)15-7-8-16(22)24-10-15/h3-8,10H,9,23H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50249434

(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249403

(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50207463

(CHEMBL3963788)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)F)c(N)c2c1C Show InChI InChI=1S/C17H16F2N4O3S2/c1-8-9(2)22-23-16-12(8)13(20)14(27-16)15(24)21-7-10-3-5-11(6-4-10)28(25,26)17(18)19/h3-6,17H,7,20H2,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50451549
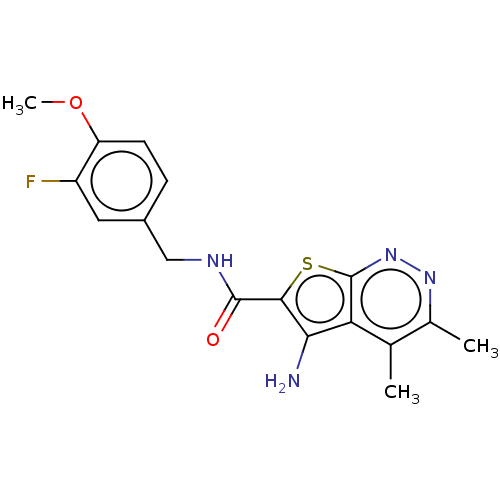
(CHEMBL4207737)Show InChI InChI=1S/C17H17FN4O2S/c1-8-9(2)21-22-17-13(8)14(19)15(25-17)16(23)20-7-10-4-5-12(24-3)11(18)6-10/h4-6H,7,19H2,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50249403

(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50207517

(CHEMBL3915634)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(=O)(=O)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C17H15F3N4O3S2/c1-8-9(2)23-24-16-12(8)13(21)14(28-16)15(25)22-7-10-3-5-11(6-4-10)29(26,27)17(18,19)20/h3-6H,7,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50207516

(CHEMBL3942511)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)S(F)(F)(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C16H15F5N4OS2/c1-8-9(2)24-25-16-12(8)13(22)14(27-16)15(26)23-7-10-3-5-11(6-4-10)28(17,18,19,20)21/h3-6H,7,22H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 171-175 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.086
BindingDB Entry DOI: 10.7270/Q2WM1GC0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50253091

(CHEMBL4083081)Show SMILES Cc1nnc2sc(C(=O)NC3CN(C3)c3ccc(nc3)C(F)(F)F)c(N)c2c1C Show InChI InChI=1S/C18H17F3N6OS/c1-8-9(2)25-26-17-13(8)14(22)15(29-17)16(28)24-10-6-27(7-10)11-3-4-12(23-5-11)18(19,20)21/h3-5,10H,6-7,22H2,1-2H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2990-2995 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.014
BindingDB Entry DOI: 10.7270/Q2MS3W6Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data