

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50375511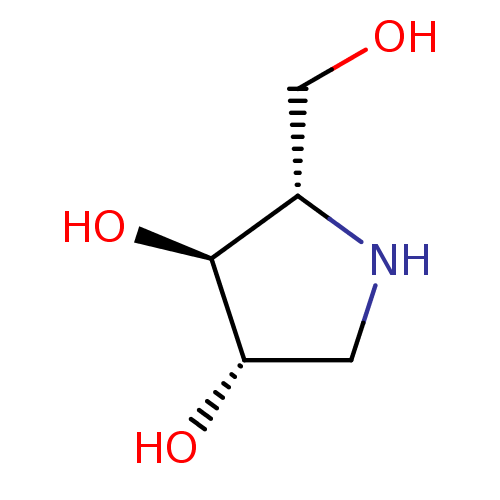 (CHEMBL406973) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50375510 (CHEMBL405957) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50016703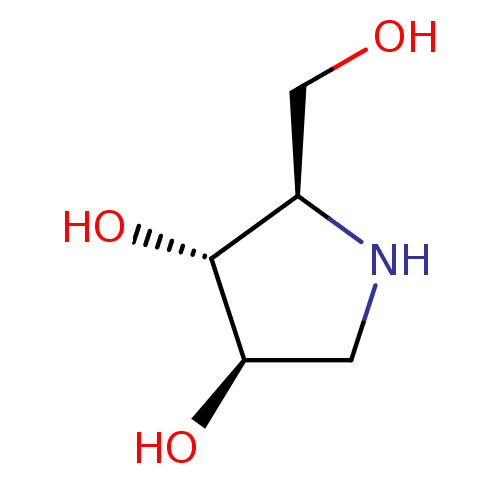 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Competitive inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed b... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320036 (CHEMBL1086235 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM248156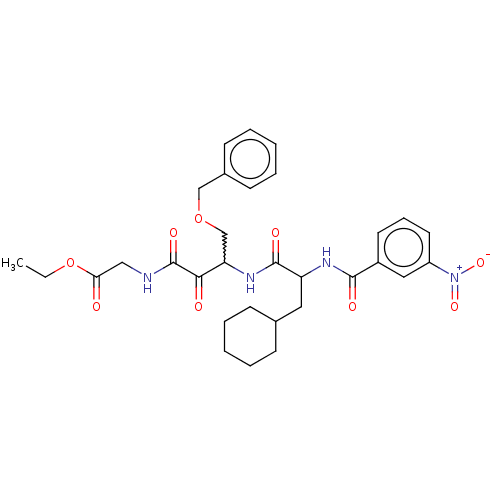 (Cathepsin S inhibitor alpha-ketoamide, 6b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University | Assay Description A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... | J Enzyme Inhib Med Chem 29: 538-46 (2014) Article DOI: 10.3109/14756366.2013.823957 BindingDB Entry DOI: 10.7270/Q2RN36RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320040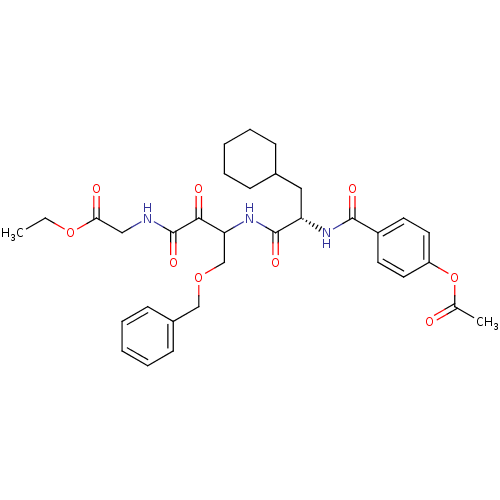 (CHEMBL1085728 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320065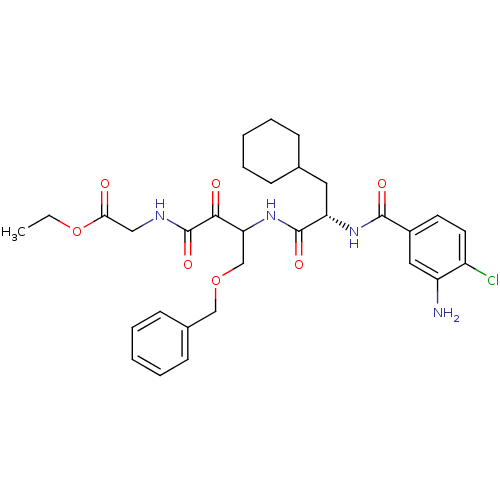 (CHEMBL1085970 | Glycine, N-[(3S)-4-Benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320057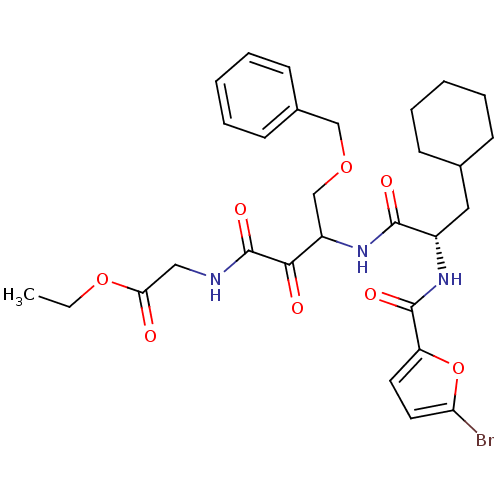 (CHEMBL1083620 | ethyl 2-(4-(benzyloxy)-3-((S)-2-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206326 (CHEMBL3973923) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320039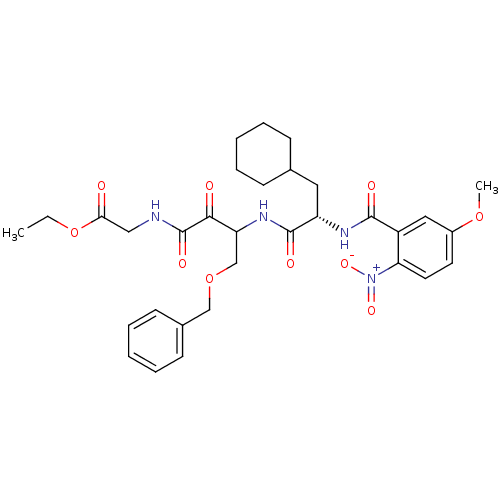 (CHEMBL1085726 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM248161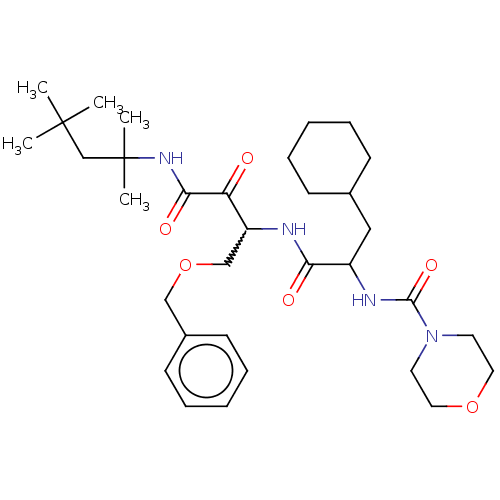 (Cathepsin S inhibitor alpha-ketoamide, 6w) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University | Assay Description A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... | J Enzyme Inhib Med Chem 29: 538-46 (2014) Article DOI: 10.3109/14756366.2013.823957 BindingDB Entry DOI: 10.7270/Q2RN36RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM248157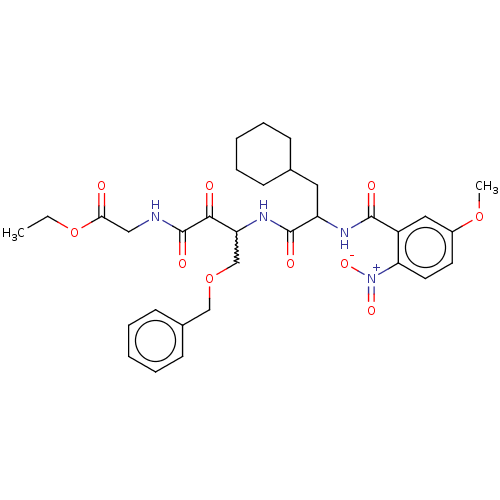 (Cathepsin S inhibitor alpha-ketoamide, 6e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University | Assay Description A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... | J Enzyme Inhib Med Chem 29: 538-46 (2014) Article DOI: 10.3109/14756366.2013.823957 BindingDB Entry DOI: 10.7270/Q2RN36RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320046 (CHEMBL1083930 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320041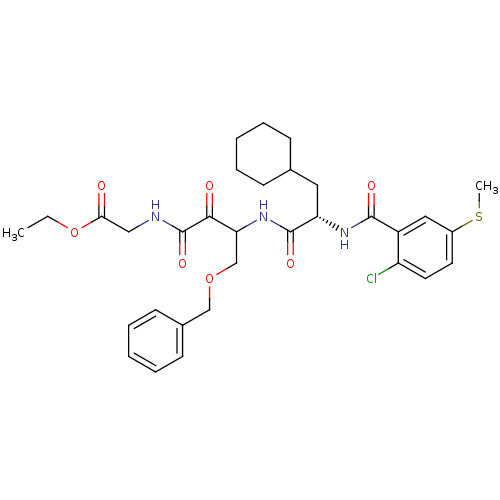 (CHEMBL1083919 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320042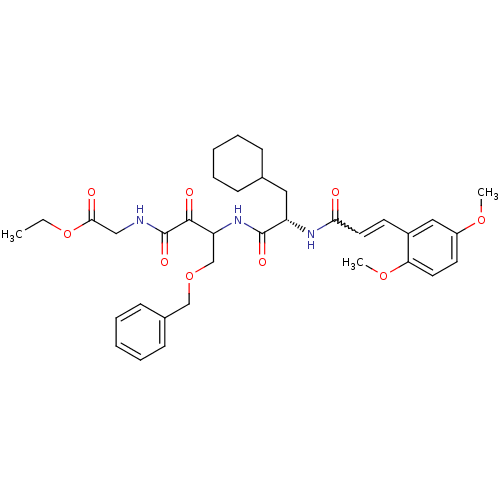 (CHEMBL1085729 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320047 (CHEMBL1085966 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320035 (CHEMBL1083318 | Glycine, N-[(3S)-4-Benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM248155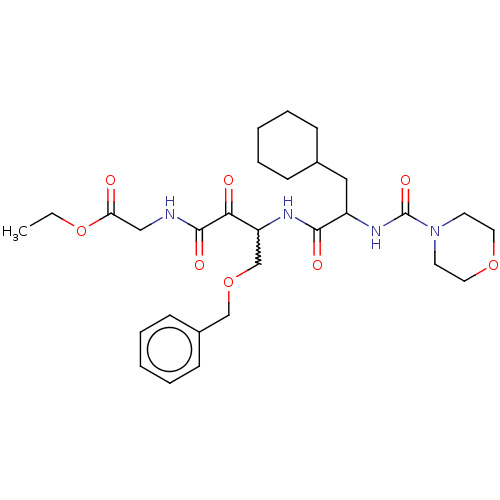 (Cathepsin S inhibitor alpha-ketoamide, 6a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University | Assay Description A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... | J Enzyme Inhib Med Chem 29: 538-46 (2014) Article DOI: 10.3109/14756366.2013.823957 BindingDB Entry DOI: 10.7270/Q2RN36RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121549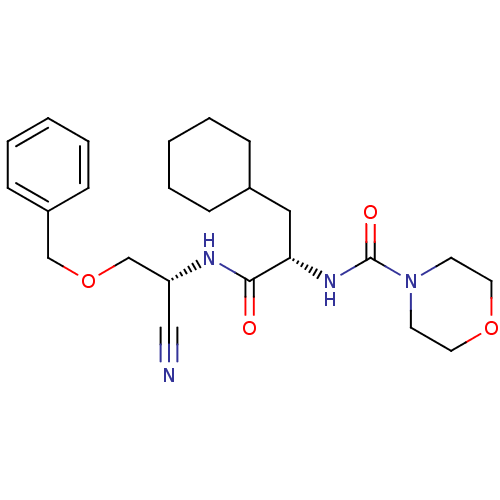 (CHEMBL347111 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320051 (CHEMBL1085974 | ethyl 2-(4-(benzyloxy)-3-((S)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320060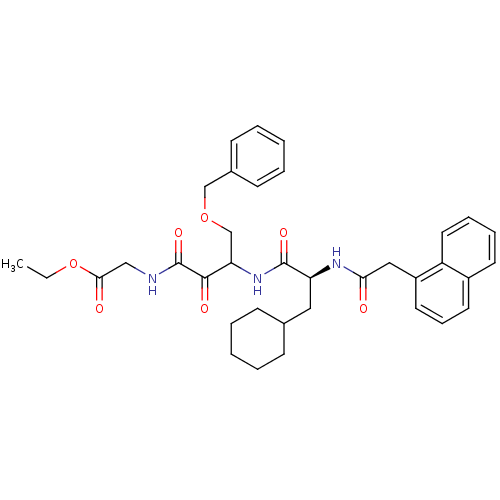 (CHEMBL1085982 | ethyl 2-(4-(benzyloxy)-3-((S)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM248159 (Cathepsin S inhibitor alpha-ketoamide, 6p) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University | Assay Description A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... | J Enzyme Inhib Med Chem 29: 538-46 (2014) Article DOI: 10.3109/14756366.2013.823957 BindingDB Entry DOI: 10.7270/Q2RN36RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320064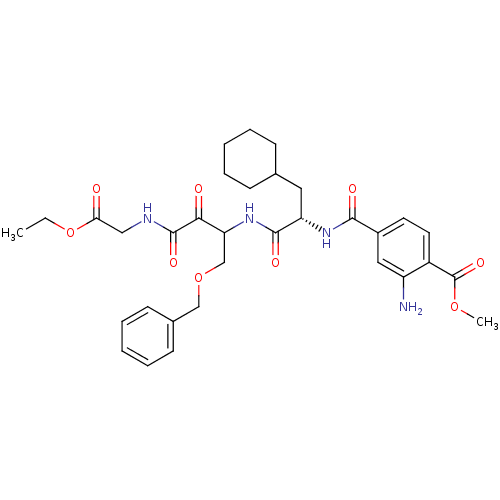 (CHEMBL1085969 | Methyl 2-amino-4-([(1S)-2-(1-[(ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320053 (CHEMBL1085976 | ethyl 2-(4-(benzyloxy)-3-((S)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121549 (CHEMBL347111 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human B cells | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320058 (CHEMBL1085980 | ethyl 2-(4-(benzyloxy)-3-((S)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320052 (CHEMBL1085975 | ethyl 2-(4-(benzyloxy)-3-((S)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320056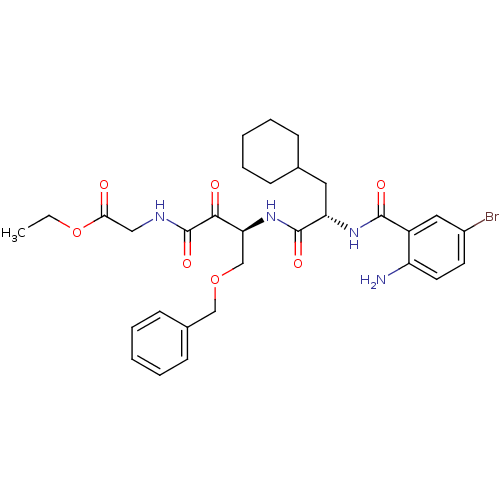 (CHEMBL1085979 | ethyl 2-((S)-3-((S)-2-(2-amino-5-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320043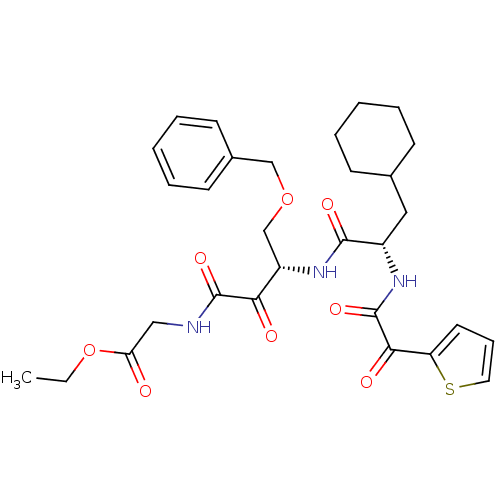 (CHEMBL1085730 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320061 (CHEMBL1085983 | ethyl 2-(4-(benzyloxy)-3-((S)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320044 (CHEMBL1083923 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206328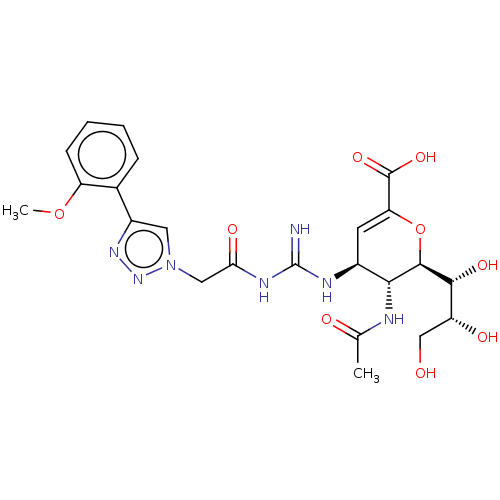 (CHEMBL3932696) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320037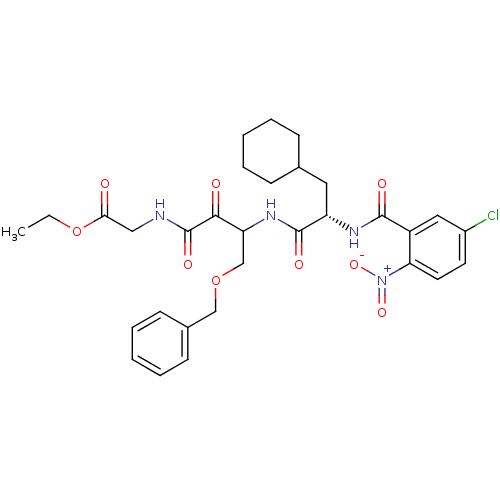 (CHEMBL1085727 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320048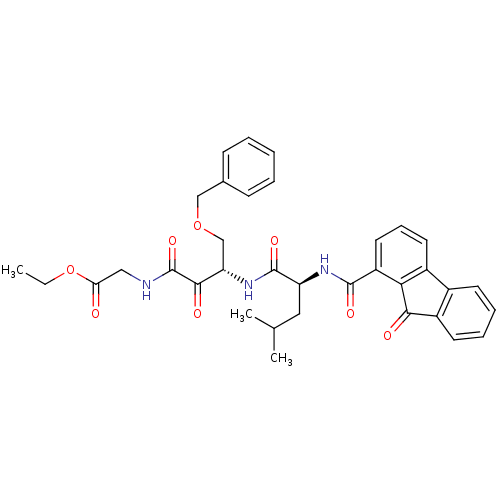 (CHEMBL1085971 | Glycine, N-[(3S)-4-benzyloxy-(2S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM248160 (Cathepsin S inhibitor alpha-ketoamide, 6r) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Tsing Hua University | Assay Description A microplate-based screening procedure was used to study the effect of inhibition of re-Cat S, re-Cat L and re-Cat K on several compounds. The assays... | J Enzyme Inhib Med Chem 29: 538-46 (2014) Article DOI: 10.3109/14756366.2013.823957 BindingDB Entry DOI: 10.7270/Q2RN36RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206329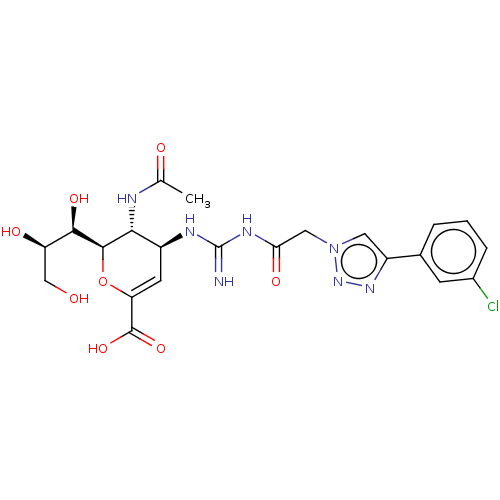 (CHEMBL3982739) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206323 (CHEMBL3924843) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Competitive inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed b... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206322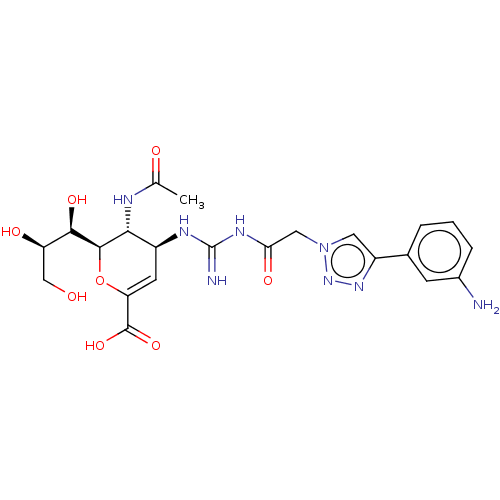 (CHEMBL3974750) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320045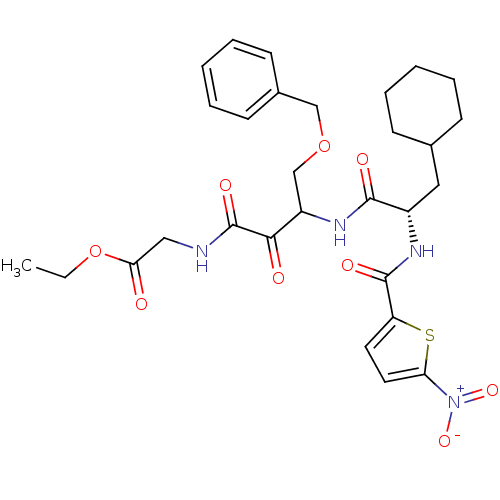 (CHEMBL1085731 | Glycine, N-[(3S)-4-benzyloxy-3-[[[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50320054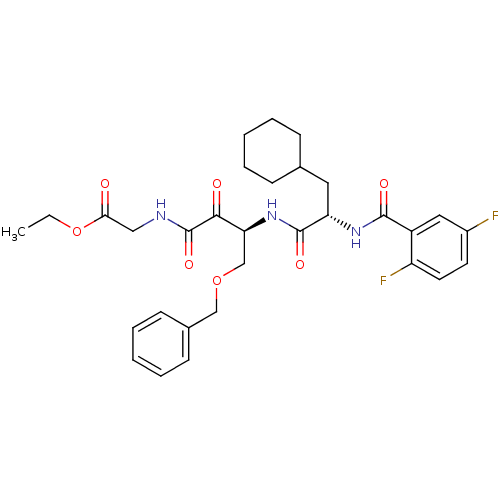 (CHEMBL1085977 | ethyl 2-((S)-4-(benzyloxy)-3-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in Escherichia coli BL21 (DE3) after 10 mins by fluorescence assay | J Med Chem 53: 4545-9 (2010) Article DOI: 10.1021/jm100089e BindingDB Entry DOI: 10.7270/Q2TX3FJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50063416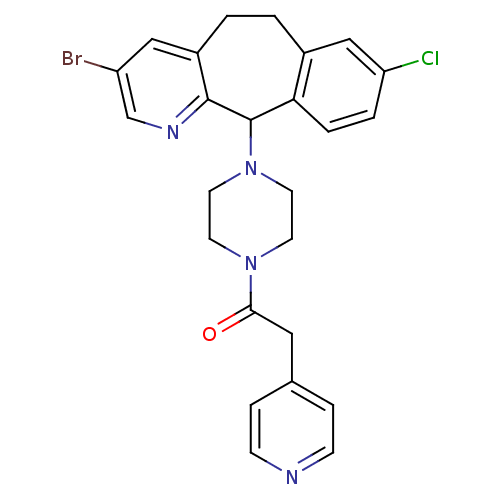 (1-[4-(3-Bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of farnesyltransferase farnesylation of the H-ras oncogene | J Med Chem 41: 877-93 (1998) Article DOI: 10.1021/jm970462w BindingDB Entry DOI: 10.7270/Q2M61JDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206355 (CHEMBL3936449) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206330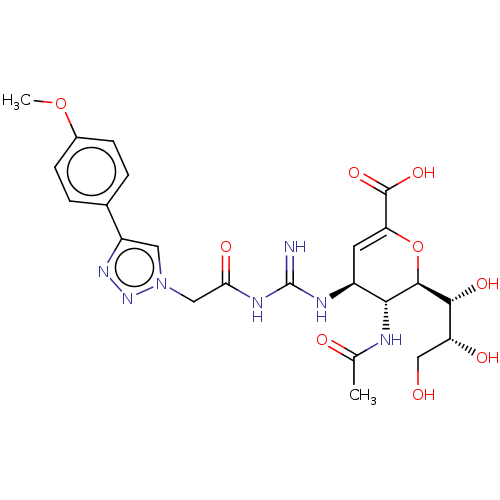 (CHEMBL3966255) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206321 (CHEMBL3926666) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206359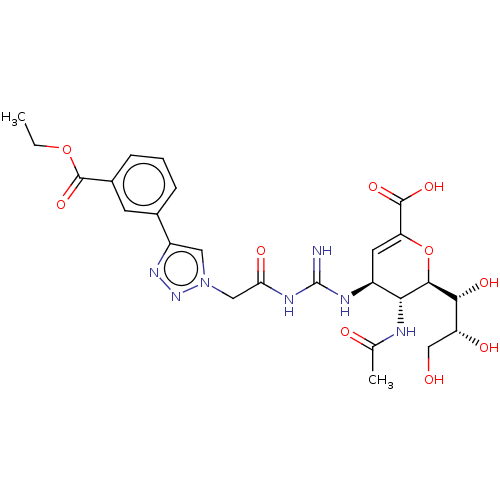 (CHEMBL3945341) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206356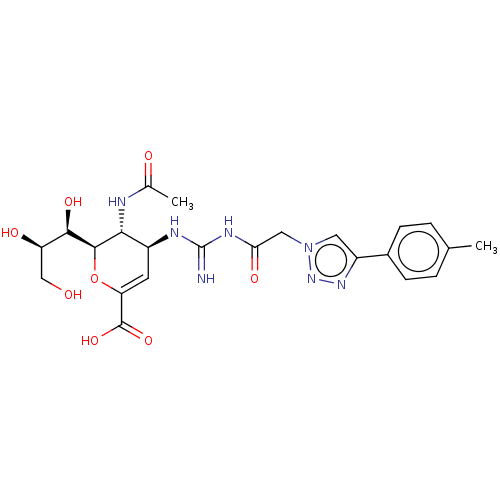 (CHEMBL3955100) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206324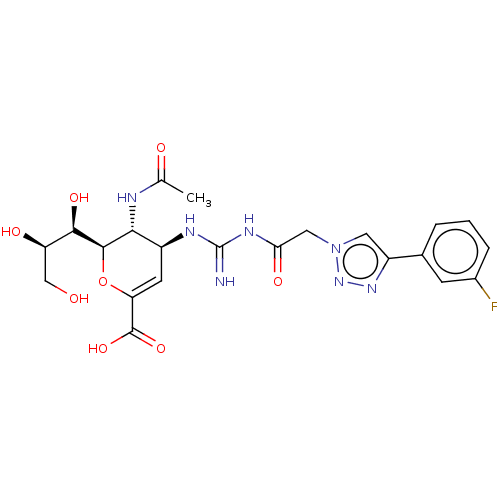 (CHEMBL3954074) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206327 (CHEMBL3951461) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50206331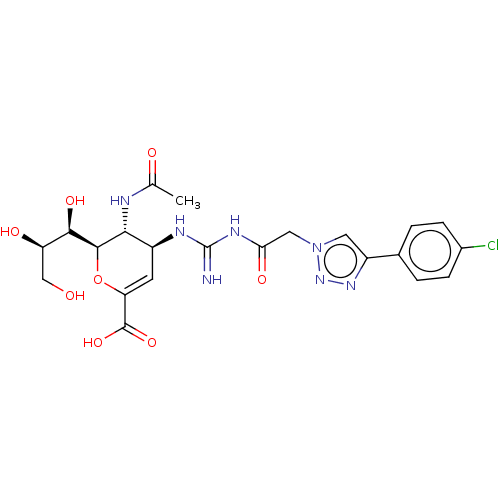 (CHEMBL3927490) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Tsing Hua University Curated by ChEMBL | Assay Description Inhibition of MDCK infected Influenza A virus A/WSN/33(H1N1) neuraminidase using MU-NANA as substrate preincubated for 30 mins followed by substrate ... | Eur J Med Chem 123: 397-406 (2016) Article DOI: 10.1016/j.ejmech.2016.07.064 BindingDB Entry DOI: 10.7270/Q2KH0QB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 484 total ) | Next | Last >> |