 Found 73 hits with Last Name = 'little' and Initial = 's'
Found 73 hits with Last Name = 'little' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Human rhinovirus B) | BDBM50065621

(CHEMBL94688 | [(S)-1-((S)-1-{(S)-3-Carbamoyl-1-[2-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)\C=C1/CCCOC1=O Show InChI InChI=1S/C33H42N4O7/c1-22(2)18-27(37-33(42)44-21-24-12-7-4-8-13-24)31(40)36-28(19-23-10-5-3-6-11-23)30(39)35-26(15-16-29(34)38)20-25-14-9-17-43-32(25)41/h3-8,10-13,20,22,26-28H,9,14-19,21H2,1-2H3,(H2,34,38)(H,35,39)(H,36,40)(H,37,42)/b25-20+/t26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Catalytic rate constant (Kobs/[I]) of the compound was evaluated against human rhinovirus (HRV) serotype 14 3C Protease (3CP) |
J Med Chem 41: 2806-18 (1998)
Article DOI: 10.1021/jm980068d
BindingDB Entry DOI: 10.7270/Q29G5KZQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288762
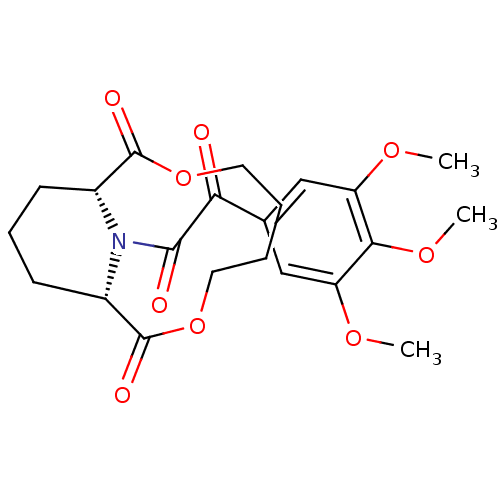
((1S,10R)-14-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ace...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCCOC2=O Show InChI InChI=1S/C22H27NO9/c1-28-16-11-13(12-17(29-2)19(16)30-3)18(24)20(25)23-14-7-6-8-15(23)22(27)32-10-5-4-9-31-21(14)26/h11-12,14-15H,4-10H2,1-3H3/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288763

((1S,9R)-5-Benzyloxymethyl-13-[2-oxo-2-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(COCc1ccccc1)COC2=O Show InChI InChI=1S/C29H33NO10/c1-35-23-12-20(13-24(36-2)26(23)37-3)25(31)27(32)30-21-10-7-11-22(30)29(34)40-17-19(16-39-28(21)33)15-38-14-18-8-5-4-6-9-18/h4-6,8-9,12-13,19,21-22H,7,10-11,14-17H2,1-3H3/t19?,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288765

((1S,9R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO[Si](C)(C)C(C)(C)C)COC2=O Show InChI InChI=1S/C28H41NO10Si/c1-28(2,3)40(7,8)39-16-17-14-37-26(32)19-10-9-11-20(27(33)38-15-17)29(19)25(31)23(30)18-12-21(34-4)24(36-6)22(13-18)35-5/h12-13,17,19-20H,9-11,14-16H2,1-8H3/t17?,19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288764
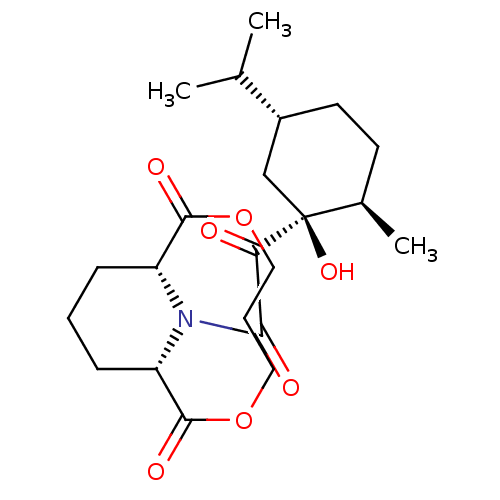
((1S,9R)-13-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C22H33NO7/c1-13(2)15-9-8-14(3)22(28,12-15)18(24)19(25)23-16-6-4-7-17(23)21(27)30-11-5-10-29-20(16)26/h13-17,28H,4-12H2,1-3H3/t14-,15-,16-,17+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288768
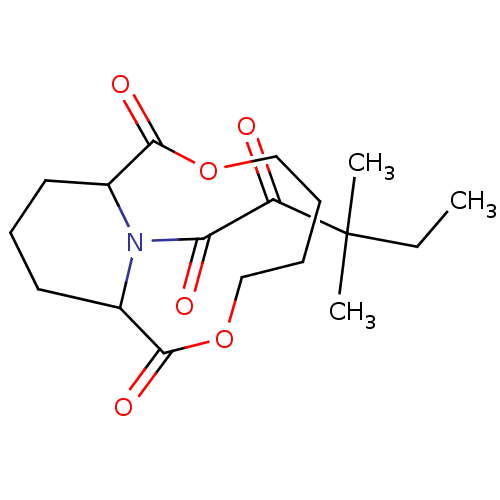
((1R,10S)-14-(3,3-Dimethyl-2-oxo-pentanoyl)-3,8-dio...)Show SMILES CCC(C)(C)C(=O)C(=O)N1C2CCCC1C(=O)OCCCCOC2=O Show InChI InChI=1S/C18H27NO6/c1-4-18(2,3)14(20)15(21)19-12-8-7-9-13(19)17(23)25-11-6-5-10-24-16(12)22/h12-13H,4-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288767
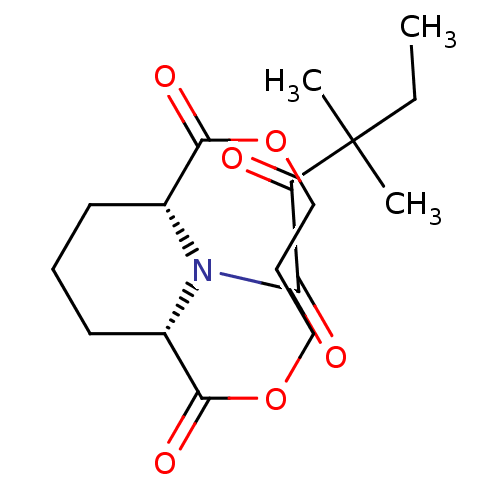
((1R,9S)-13-(3,3-Dimethyl-2-oxo-pentanoyl)-3,7-diox...)Show SMILES CCC(C)(C)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C17H25NO6/c1-4-17(2,3)13(19)14(20)18-11-7-5-8-12(18)16(22)24-10-6-9-23-15(11)21/h11-12H,4-10H2,1-3H3/t11-,12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288766
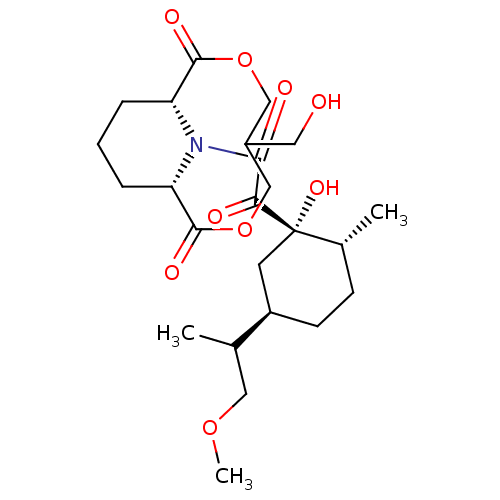
((1S,9R)-13-{2-[(1S,2R,5R)-1-Hydroxy-5-(2-methoxy-1...)Show SMILES COCC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO)COC2=O Show InChI InChI=1S/C24H37NO9/c1-14(11-32-3)17-8-7-15(2)24(31,9-17)20(27)21(28)25-18-5-4-6-19(25)23(30)34-13-16(10-26)12-33-22(18)29/h14-19,26,31H,4-13H2,1-3H3/t14?,15-,16?,17-,18-,19+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288769
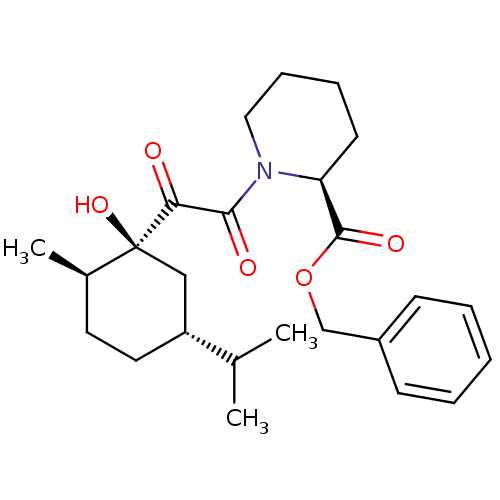
((S)-1-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-methy...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C25H35NO5/c1-17(2)20-13-12-18(3)25(30,15-20)22(27)23(28)26-14-8-7-11-21(26)24(29)31-16-19-9-5-4-6-10-19/h4-6,9-10,17-18,20-21,30H,7-8,11-16H2,1-3H3/t18-,20-,21+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195627
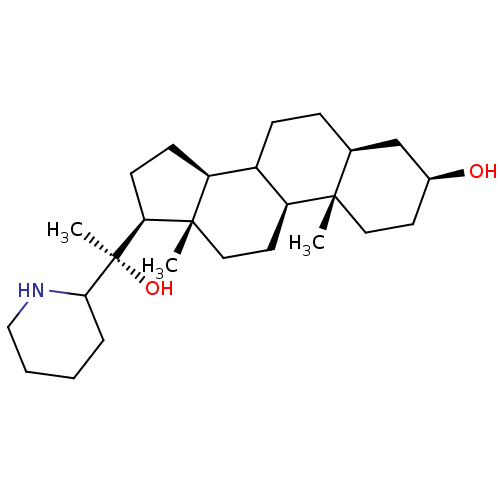
(22,26-azasterol | CHEMBL425634)Show SMILES C[C@@](O)([C@H]1CC[C@H]2C3CC[C@H]4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C1CCCCN1 Show InChI InChI=1S/C26H45NO2/c1-24-13-11-18(28)16-17(24)7-8-19-20-9-10-22(25(20,2)14-12-21(19)24)26(3,29)23-6-4-5-15-27-23/h17-23,27-29H,4-16H2,1-3H3/t17-,18-,19?,20-,21-,22-,23?,24-,25-,26+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50084636
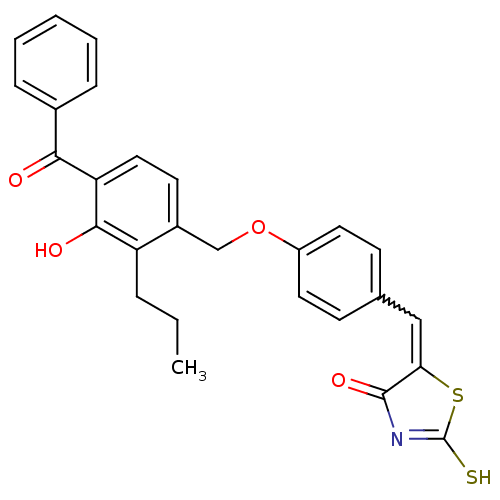
(5-[1-[4-(4-Benzoyl-3-hydroxy-2-propyl-benzyloxy)-p...)Show SMILES CCCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1 |w:16.16,c:21| Show InChI InChI=1S/C27H23NO4S2/c1-2-6-21-19(11-14-22(25(21)30)24(29)18-7-4-3-5-8-18)16-32-20-12-9-17(10-13-20)15-23-26(31)28-27(33)34-23/h3-5,7-15,30H,2,6,16H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080991
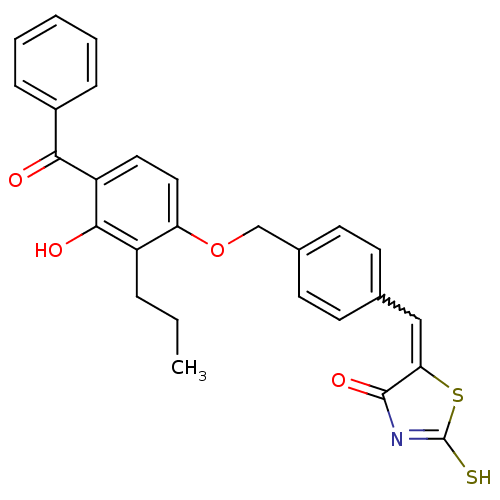
(5-[1-[4-(4-Benzoyl-3-hydroxy-2-propyl-phenoxymethy...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1 |w:16.16,c:21| Show InChI InChI=1S/C27H23NO4S2/c1-2-6-20-22(14-13-21(25(20)30)24(29)19-7-4-3-5-8-19)32-16-18-11-9-17(10-12-18)15-23-26(31)28-27(33)34-23/h3-5,7-15,30H,2,6,16H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287763
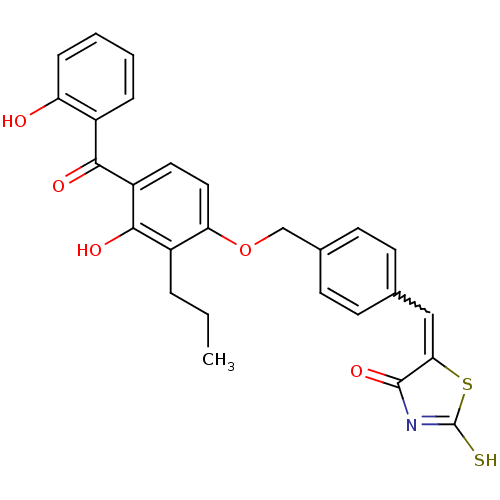
(5-[1-{4-[3-Hydroxy-4-(2-hydroxy-benzoyl)-2-propyl-...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1O |w:16.16,c:21| Show InChI InChI=1S/C27H23NO5S2/c1-2-5-19-22(13-12-20(25(19)31)24(30)18-6-3-4-7-21(18)29)33-15-17-10-8-16(9-11-17)14-23-26(32)28-27(34)35-23/h3-4,6-14,29,31H,2,5,15H2,1H3,(H,28,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287749
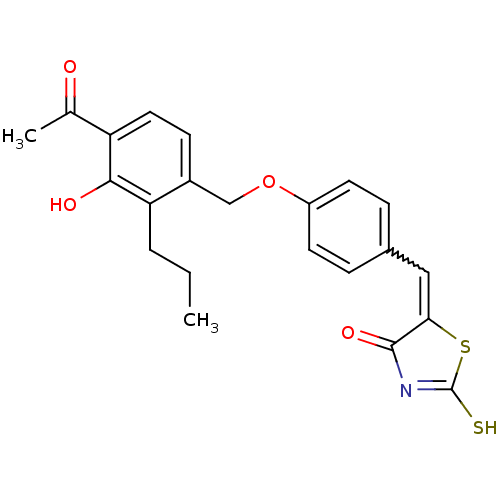
(5-[1-[4-(4-Acetyl-3-hydroxy-2-propyl-benzyloxy)-ph...)Show SMILES CCCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(C)=O |w:16.16,c:21| Show InChI InChI=1S/C22H21NO4S2/c1-3-4-18-15(7-10-17(13(2)24)20(18)25)12-27-16-8-5-14(6-9-16)11-19-21(26)23-22(28)29-19/h5-11,25H,3-4,12H2,1-2H3,(H,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287758
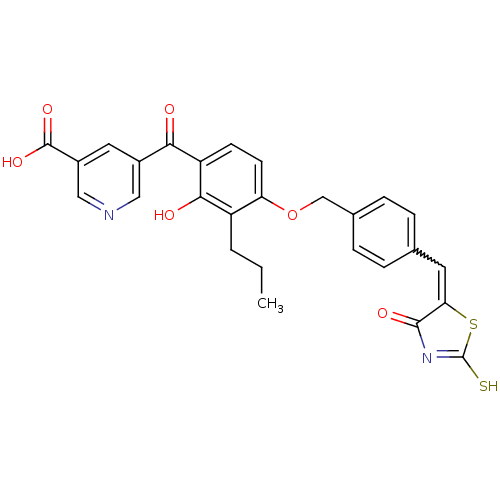
(5-(2-Hydroxy-4-{4-[4-oxo-2-thioxo-thiazolidin-(5Z)...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1cncc(c1)C(O)=O |w:16.16,c:21| Show InChI InChI=1S/C27H22N2O6S2/c1-2-3-19-21(9-8-20(24(19)31)23(30)17-11-18(26(33)34)13-28-12-17)35-14-16-6-4-15(5-7-16)10-22-25(32)29-27(36)37-22/h4-13,31H,2-3,14H2,1H3,(H,33,34)(H,29,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287743
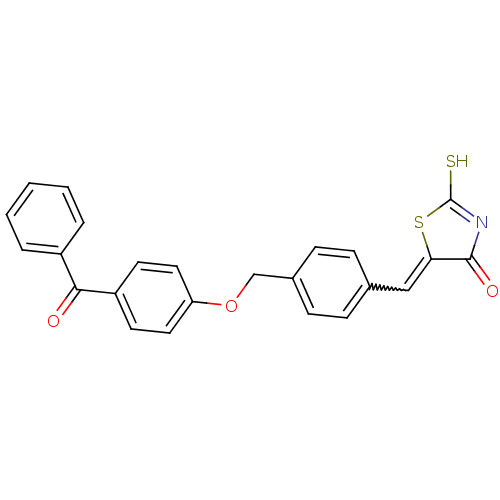
(5-[1-[4-(4-Benzoyl-phenoxymethyl)-phenyl]-meth-(Z)...)Show SMILES SC1=NC(=O)C(S1)=Cc1ccc(COc2ccc(cc2)C(=O)c2ccccc2)cc1 |w:7.8,t:1| Show InChI InChI=1S/C24H17NO3S2/c26-22(18-4-2-1-3-5-18)19-10-12-20(13-11-19)28-15-17-8-6-16(7-9-17)14-21-23(27)25-24(29)30-21/h1-14H,15H2,(H,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287747
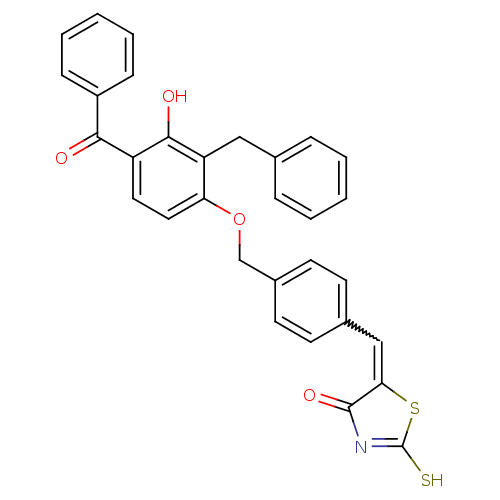
(5-[1-[4-(4-Benzoyl-2-benzyl-3-hydroxy-phenoxymethy...)Show SMILES Oc1c(Cc2ccccc2)c(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc1C(=O)c1ccccc1 |w:17.17,c:22| Show InChI InChI=1S/C31H23NO4S2/c33-28(23-9-5-2-6-10-23)24-15-16-26(25(29(24)34)17-20-7-3-1-4-8-20)36-19-22-13-11-21(12-14-22)18-27-30(35)32-31(37)38-27/h1-16,18,34H,17,19H2,(H,32,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080991

(5-[1-[4-(4-Benzoyl-3-hydroxy-2-propyl-phenoxymethy...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccccc1 |w:16.16,c:21| Show InChI InChI=1S/C27H23NO4S2/c1-2-6-20-22(14-13-21(25(20)30)24(29)19-7-4-3-5-8-19)32-16-18-11-9-17(10-12-18)15-23-26(31)28-27(33)34-23/h3-5,7-15,30H,2,6,16H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of cathepsin D at an enzyme level of 50 ng/mL |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287761
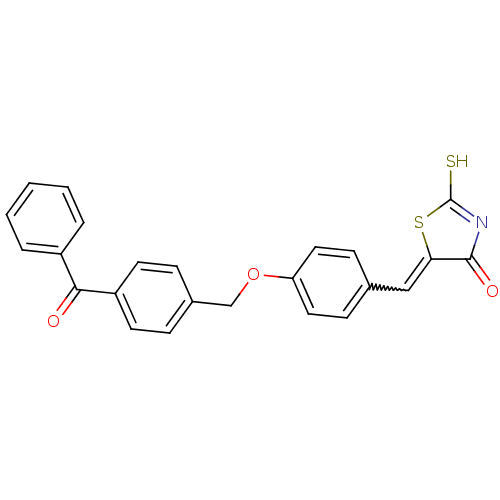
(5-[1-[4-(4-Benzoyl-benzyloxy)-phenyl]-meth-(Z)-yli...)Show SMILES SC1=NC(=O)C(S1)=Cc1ccc(OCc2ccc(cc2)C(=O)c2ccccc2)cc1 |w:7.8,t:1| Show InChI InChI=1S/C24H17NO3S2/c26-22(18-4-2-1-3-5-18)19-10-6-17(7-11-19)15-28-20-12-8-16(9-13-20)14-21-23(27)25-24(29)30-21/h1-14H,15H2,(H,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287759
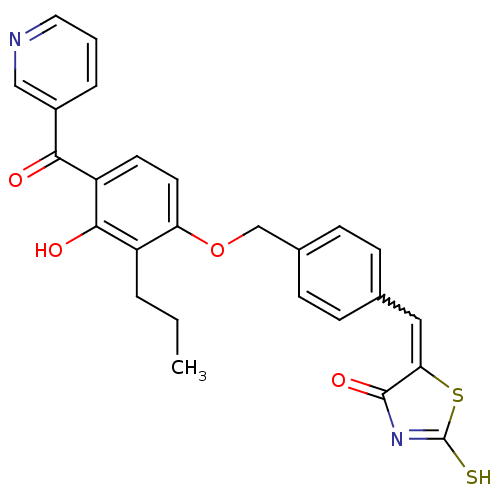
(5-[1-{4-[3-Hydroxy-2-propyl-4-(pyridine-3-carbonyl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1cccnc1 |w:16.16,c:21| Show InChI InChI=1S/C26H22N2O4S2/c1-2-4-19-21(11-10-20(24(19)30)23(29)18-5-3-12-27-14-18)32-15-17-8-6-16(7-9-17)13-22-25(31)28-26(33)34-22/h3,5-14,30H,2,4,15H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195627

(22,26-azasterol | CHEMBL425634)Show SMILES C[C@@](O)([C@H]1CC[C@H]2C3CC[C@H]4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C1CCCCN1 Show InChI InChI=1S/C26H45NO2/c1-24-13-11-18(28)16-17(24)7-8-19-20-9-10-22(25(20,2)14-12-21(19)24)26(3,29)23-6-4-5-15-27-23/h17-23,27-29H,4-16H2,1-3H3/t17-,18-,19?,20-,21-,22-,23?,24-,25-,26+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei brucei recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287742
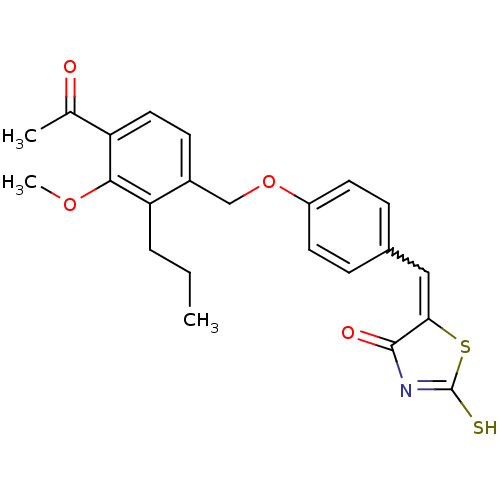
(5-[1-[4-(4-Acetyl-3-methoxy-2-propyl-benzyloxy)-ph...)Show SMILES CCCc1c(COc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC |w:11.10,c:15| Show InChI InChI=1S/C23H23NO4S2/c1-4-5-19-16(8-11-18(14(2)25)21(19)27-3)13-28-17-9-6-15(7-10-17)12-20-22(26)24-23(29)30-20/h6-12H,4-5,13H2,1-3H3,(H,24,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287750

(5-[1-[4-(4-Acetyl-3-hydroxy-2-propyl-phenoxymethyl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(C)=O |w:16.16,c:21| Show InChI InChI=1S/C22H21NO4S2/c1-3-4-17-18(10-9-16(13(2)24)20(17)25)27-12-15-7-5-14(6-8-15)11-19-21(26)23-22(28)29-19/h5-11,25H,3-4,12H2,1-2H3,(H,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287757
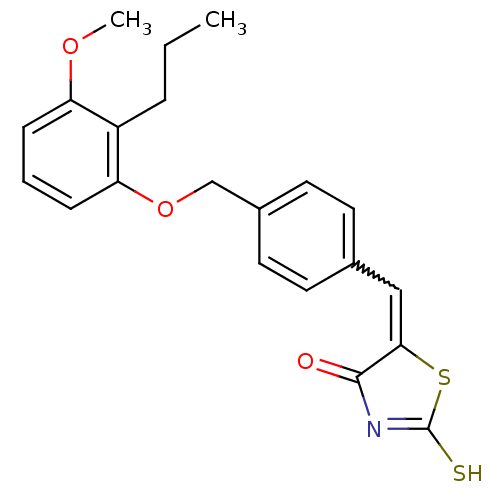
(5-[1-[4-(3-Methoxy-2-propyl-phenoxymethyl)-phenyl]...)Show SMILES CCCc1c(OC)cccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1 |w:17.17,c:22| Show InChI InChI=1S/C21H21NO3S2/c1-3-5-16-17(24-2)6-4-7-18(16)25-13-15-10-8-14(9-11-15)12-19-20(23)22-21(26)27-19/h4,6-12H,3,5,13H2,1-2H3,(H,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287762
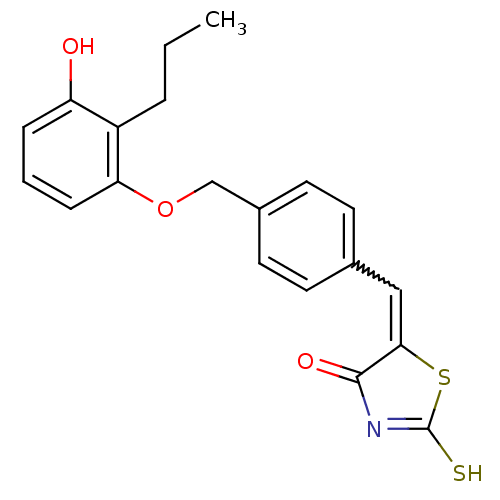
(5-[1-[4-(3-Hydroxy-2-propyl-phenoxymethyl)-phenyl]...)Show SMILES CCCc1c(O)cccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1 |w:16.16,c:21| Show InChI InChI=1S/C20H19NO3S2/c1-2-4-15-16(22)5-3-6-17(15)24-12-14-9-7-13(8-10-14)11-18-19(23)21-20(25)26-18/h3,5-11,22H,2,4,12H2,1H3,(H,21,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287752
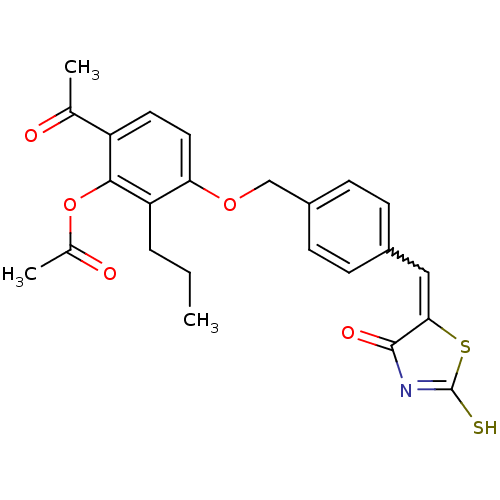
(Acetic acid 6-acetyl-3-{4-[4-oxo-2-thioxo-thiazoli...)Show SMILES CCCc1c(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC(C)=O |w:11.10,c:15| Show InChI InChI=1S/C24H23NO5S2/c1-4-5-19-20(11-10-18(14(2)26)22(19)30-15(3)27)29-13-17-8-6-16(7-9-17)12-21-23(28)25-24(31)32-21/h6-12H,4-5,13H2,1-3H3,(H,25,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287745
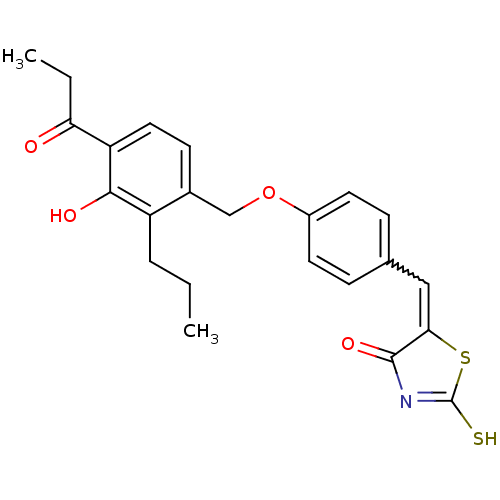
(5-[1-[4-(3-Hydroxy-4-propionyl-2-propyl-benzyloxy)...)Show SMILES CCCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)CC |w:16.16,c:21| Show InChI InChI=1S/C23H23NO4S2/c1-3-5-17-15(8-11-18(21(17)26)19(25)4-2)13-28-16-9-6-14(7-10-16)12-20-22(27)24-23(29)30-20/h6-12,26H,3-5,13H2,1-2H3,(H,24,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195636

(3beta-ol-23,24-bisnor-5-en-22-(butanoic acid) amin...)Show SMILES C[C@H](CNCCCC(O)=O)[C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |t:16| Show InChI InChI=1S/C26H43NO3/c1-17(16-27-14-4-5-24(29)30)21-8-9-22-20-7-6-18-15-19(28)10-12-25(18,2)23(20)11-13-26(21,22)3/h6,17,19-23,27-28H,4-5,7-16H2,1-3H3,(H,29,30)/t17-,19+,20?,21-,22+,23+,25+,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287760

(2-Hydroxy-4-{4-[4-oxo-2-thioxo-thiazolidin-(5Z)-yl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(O)=O |w:16.16,c:21| Show InChI InChI=1S/C21H19NO5S2/c1-2-3-14-16(9-8-15(18(14)23)20(25)26)27-11-13-6-4-12(5-7-13)10-17-19(24)22-21(28)29-17/h4-10,23H,2-3,11H2,1H3,(H,25,26)(H,22,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287751
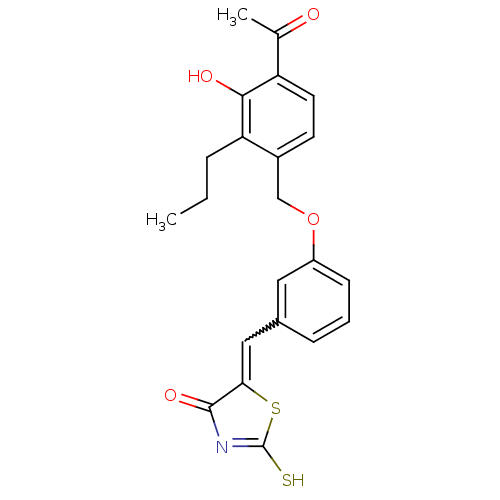
(5-[1-[3-(4-Acetyl-3-hydroxy-2-propyl-benzyloxy)-ph...)Show SMILES CCCc1c(O)c(ccc1COc1cccc(C=C2SC(S)=NC2=O)c1)C(C)=O |w:17.17,c:22| Show InChI InChI=1S/C22H21NO4S2/c1-3-5-18-15(8-9-17(13(2)24)20(18)25)12-27-16-7-4-6-14(10-16)11-19-21(26)23-22(28)29-19/h4,6-11,25H,3,5,12H2,1-2H3,(H,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287753
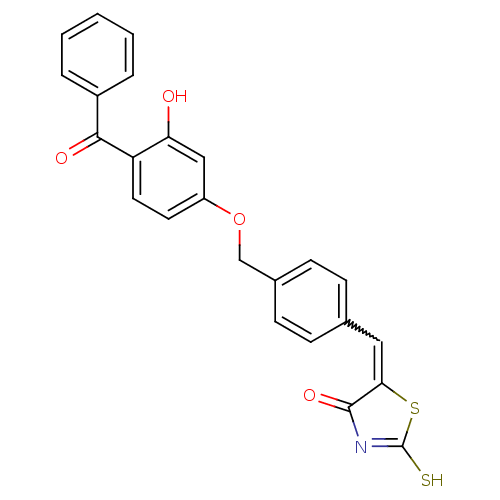
(5-[1-[4-(4-Benzoyl-3-hydroxy-phenoxymethyl)-phenyl...)Show SMILES Oc1cc(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc1C(=O)c1ccccc1 |w:10.9,c:14| Show InChI InChI=1S/C24H17NO4S2/c26-20-13-18(10-11-19(20)22(27)17-4-2-1-3-5-17)29-14-16-8-6-15(7-9-16)12-21-23(28)25-24(30)31-21/h1-13,26H,14H2,(H,25,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287748
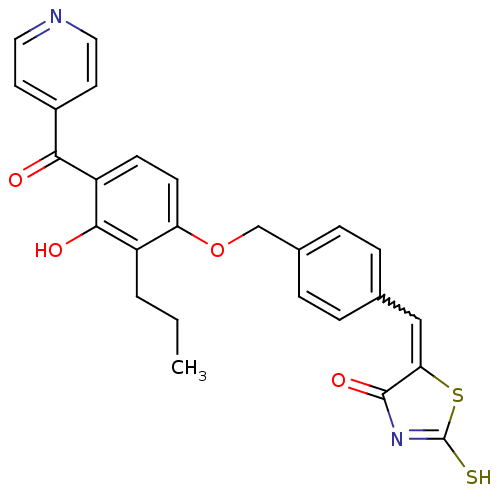
(5-[1-{4-[3-Hydroxy-2-propyl-4-(pyridine-4-carbonyl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)c1ccncc1 |w:16.16,c:21| Show InChI InChI=1S/C26H22N2O4S2/c1-2-3-19-21(9-8-20(24(19)30)23(29)18-10-12-27-13-11-18)32-15-17-6-4-16(5-7-17)14-22-25(31)28-26(33)34-22/h4-14,30H,2-3,15H2,1H3,(H,28,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287746
(2-Hydroxy-4-{4-[4-oxo-2-thioxo-thiazolidin-(5Z)-yl...)Show SMILES CCCc1c(O)c(ccc1OCc1ccc(C=C2SC(S)=NC2=O)cc1)C(=O)OC |w:16.16,c:21| Show InChI InChI=1S/C22H21NO5S2/c1-3-4-15-17(10-9-16(19(15)24)21(26)27-2)28-12-14-7-5-13(6-8-14)11-18-20(25)23-22(29)30-18/h5-11,24H,3-4,12H2,1-2H3,(H,23,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287755
(5-[1-[4-(4-Acetyl-3-hydroxy-benzyloxy)-phenyl]-met...)Show SMILES CC(=O)c1ccc(COc2ccc(C=C3SC(S)=NC3=O)cc2)cc1O |w:13.12,c:17| Show InChI InChI=1S/C19H15NO4S2/c1-11(21)15-7-4-13(8-16(15)22)10-24-14-5-2-12(3-6-14)9-17-18(23)20-19(25)26-17/h2-9,22H,10H2,1H3,(H,20,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287754
(5-[1-[4-(4-Acetyl-2-ethyl-3-hydroxy-benzyloxy)-phe...)Show SMILES CCc1c(O)c(ccc1COc1ccc(C=C2SC(S)=NC2=O)cc1)C(C)=O |w:15.15,c:20| Show InChI InChI=1S/C21H19NO4S2/c1-3-16-14(6-9-17(12(2)23)19(16)24)11-26-15-7-4-13(5-8-15)10-18-20(25)22-21(27)28-18/h4-10,24H,3,11H2,1-2H3,(H,22,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287744
(Acetic acid 6-acetyl-3-{4-[4-oxo-2-thioxo-thiazoli...)Show SMILES CCCc1c(COc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC(C)=O |w:11.10,c:15| Show InChI InChI=1S/C24H23NO5S2/c1-4-5-20-17(8-11-19(14(2)26)22(20)30-15(3)27)13-29-18-9-6-16(7-10-18)12-21-23(28)25-24(31)32-21/h6-12H,4-5,13H2,1-3H3,(H,25,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of cathepsin D at concentration of 4.15 microg/mL |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50287756
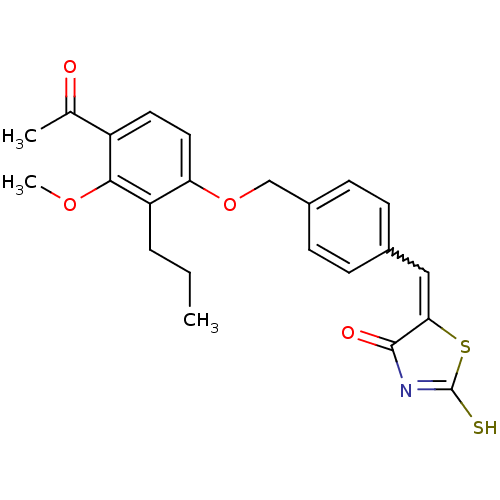
(5-[1-[4-(4-Acetyl-3-methoxy-2-propyl-phenoxymethyl...)Show SMILES CCCc1c(OCc2ccc(C=C3SC(S)=NC3=O)cc2)ccc(C(C)=O)c1OC |w:11.10,c:15| Show InChI InChI=1S/C23H23NO4S2/c1-4-5-18-19(11-10-17(14(2)25)21(18)27-3)28-13-16-8-6-15(7-9-16)12-20-22(26)24-23(29)30-20/h6-12H,4-5,13H2,1-3H3,(H,24,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of cathepsin D. |
Bioorg Med Chem Lett 6: 2157-2162 (1996)
Article DOI: 10.1016/0960-894X(96)00393-9
BindingDB Entry DOI: 10.7270/Q28G8KPZ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195640
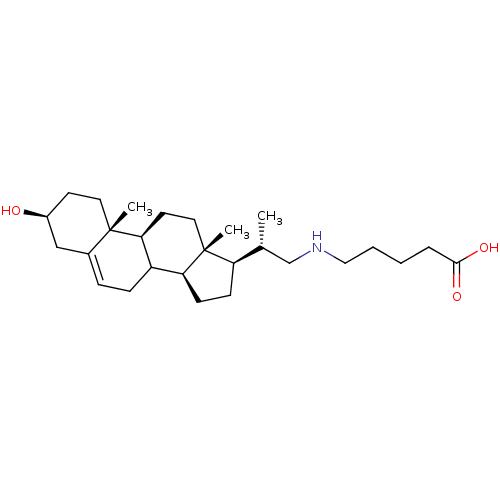
(3beta-ol-23,24-bisnor-5-en-22-(pentanoic acid) ami...)Show SMILES C[C@H](CNCCCCC(O)=O)[C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |t:17| Show InChI InChI=1S/C27H45NO3/c1-18(17-28-15-5-4-6-25(30)31)22-9-10-23-21-8-7-19-16-20(29)11-13-26(19,2)24(21)12-14-27(22,23)3/h7,18,20-24,28-29H,4-6,8-17H2,1-3H3,(H,30,31)/t18-,20+,21?,22-,23+,24+,26+,27-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195646
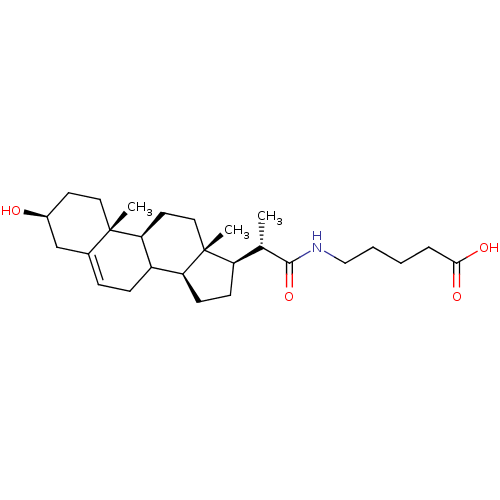
(3beta-ol-23,24-bisnor-5-en-22-(pentanoic acid) ami...)Show SMILES C[C@@H]([C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(=O)NCCCCC(O)=O |t:8| Show InChI InChI=1S/C27H43NO4/c1-17(25(32)28-15-5-4-6-24(30)31)21-9-10-22-20-8-7-18-16-19(29)11-13-26(18,2)23(20)12-14-27(21,22)3/h7,17,19-23,29H,4-6,8-16H2,1-3H3,(H,28,32)(H,30,31)/t17-,19-,20?,21+,22-,23-,26-,27+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195642
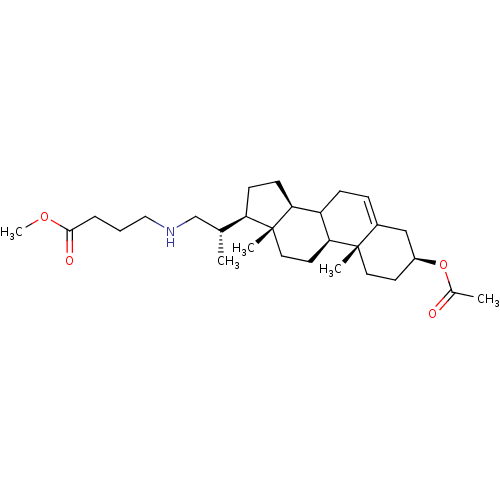
(3beta-acetoxy-23,24-bisnor-chol-5-en-22-(methyl bu...)Show SMILES COC(=O)CCCNC[C@@H](C)[C@H]1CC[C@H]2C3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OC(C)=O |t:17| Show InChI InChI=1S/C29H47NO4/c1-19(18-30-16-6-7-27(32)33-5)24-10-11-25-23-9-8-21-17-22(34-20(2)31)12-14-28(21,3)26(23)13-15-29(24,25)4/h8,19,22-26,30H,6-7,9-18H2,1-5H3/t19-,22+,23?,24-,25+,26+,28+,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei brucei recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195649

(3beta-ol-23,24-bisnor-5-en-22-(hexanoic acid) amin...)Show SMILES C[C@H](CNCCCCCC(O)=O)[C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |t:18| Show InChI InChI=1S/C28H47NO3/c1-19(18-29-16-6-4-5-7-26(31)32)23-10-11-24-22-9-8-20-17-21(30)12-14-27(20,2)25(22)13-15-28(23,24)3/h8,19,21-25,29-30H,4-7,9-18H2,1-3H3,(H,31,32)/t19-,21+,22?,23-,24+,25+,27+,28-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195651
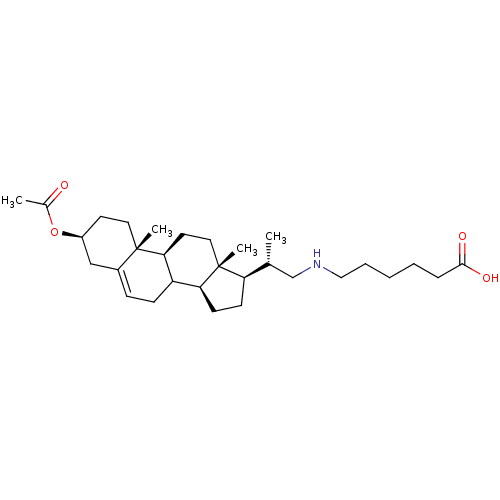
(3beta-acetoxy-23,24-bisnor-chol-5-en-22-(hexanoic ...)Show SMILES C[C@H](CNCCCCCC(O)=O)[C@H]1CC[C@H]2C3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OC(C)=O |t:18| Show InChI InChI=1S/C30H49NO4/c1-20(19-31-17-7-5-6-8-28(33)34)25-11-12-26-24-10-9-22-18-23(35-21(2)32)13-15-29(22,3)27(24)14-16-30(25,26)4/h9,20,23-27,31H,5-8,10-19H2,1-4H3,(H,33,34)/t20-,23+,24?,25-,26+,27+,29+,30-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195653
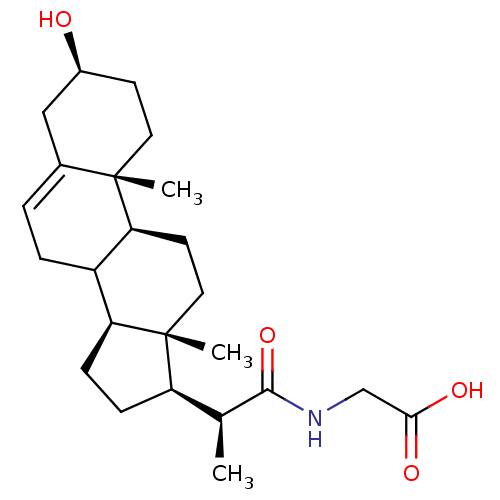
(3beta-ol-23,24-bisnor-5-en-22-(ethanoic acid) amid...)Show SMILES C[C@@H]([C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(=O)NCC(O)=O |t:8| Show InChI InChI=1S/C24H37NO4/c1-14(22(29)25-13-21(27)28)18-6-7-19-17-5-4-15-12-16(26)8-10-23(15,2)20(17)9-11-24(18,19)3/h4,14,16-20,26H,5-13H2,1-3H3,(H,25,29)(H,27,28)/t14-,16-,17?,18+,19-,20-,23-,24+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195652

(3beta-ol-23,24-bisnor-5-en-22-(5-(2-Z-amino)pentan...)Show SMILES C[C@@H]([C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(=O)NCCC[C@H](NC(=O)OCc1ccccc1)C(O)=O |t:8| Show InChI InChI=1S/C35H50N2O6/c1-22(31(39)36-19-7-10-30(32(40)41)37-33(42)43-21-23-8-5-4-6-9-23)27-13-14-28-26-12-11-24-20-25(38)15-17-34(24,2)29(26)16-18-35(27,28)3/h4-6,8-9,11,22,25-30,38H,7,10,12-21H2,1-3H3,(H,36,39)(H,37,42)(H,40,41)/t22-,25-,26?,27+,28-,29-,30-,34-,35+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195654

(3beta-acetoxy-23,24-bisnor-5-en-22-(4-(2-Boc-amino...)Show SMILES C[C@H](CNCC[C@H](NC(=O)OC(C)(C)C)C(O)=O)[C@H]1CC[C@H]2C3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OC(C)=O |t:24| Show InChI InChI=1S/C33H54N2O6/c1-20(19-34-17-14-28(29(37)38)35-30(39)41-31(3,4)5)25-10-11-26-24-9-8-22-18-23(40-21(2)36)12-15-32(22,6)27(24)13-16-33(25,26)7/h8,20,23-28,34H,9-19H2,1-7H3,(H,35,39)(H,37,38)/t20-,23+,24?,25-,26+,27+,28+,32+,33-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195621
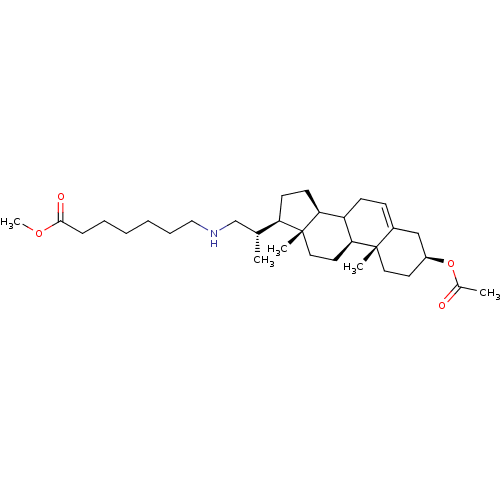
(3beta-acetoxy-23,24-bisnor-chol-5-en-22-(methyl he...)Show SMILES COC(=O)CCCCCCNC[C@@H](C)[C@H]1CC[C@H]2C3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OC(C)=O |t:20| Show InChI InChI=1S/C32H53NO4/c1-22(21-33-19-9-7-6-8-10-30(35)36-5)27-13-14-28-26-12-11-24-20-25(37-23(2)34)15-17-31(24,3)29(26)16-18-32(27,28)4/h11,22,25-29,33H,6-10,12-21H2,1-5H3/t22-,25+,26?,27-,28+,29+,31+,32-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195645
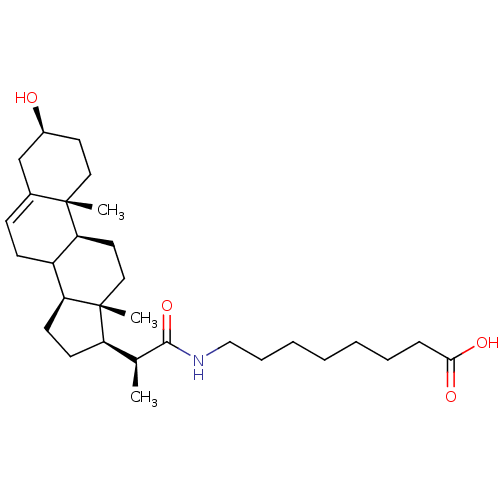
(3beta-ol-23,24-bisnor-5-en-22-(octanoic acid) amid...)Show SMILES C[C@@H]([C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(=O)NCCCCCCCC(O)=O |t:8| Show InChI InChI=1S/C30H49NO4/c1-20(28(35)31-18-8-6-4-5-7-9-27(33)34)24-12-13-25-23-11-10-21-19-22(32)14-16-29(21,2)26(23)15-17-30(24,25)3/h10,20,22-26,32H,4-9,11-19H2,1-3H3,(H,31,35)(H,33,34)/t20-,22-,23?,24+,25-,26-,29-,30+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195644
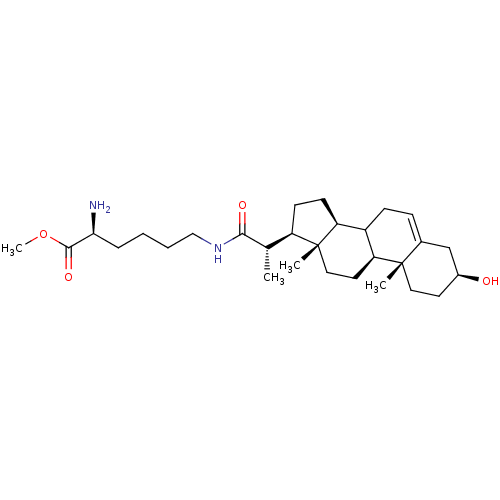
(3beta-ol-23,24-bisnor-5-en-22-(6-(2-amino) hexanoa...)Show SMILES COC(=O)[C@@H](N)CCCCNC(=O)[C@@H](C)[C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |t:21| Show InChI InChI=1S/C29H48N2O4/c1-18(26(33)31-16-6-5-7-25(30)27(34)35-4)22-10-11-23-21-9-8-19-17-20(32)12-14-28(19,2)24(21)13-15-29(22,23)3/h8,18,20-25,32H,5-7,9-17,30H2,1-4H3,(H,31,33)/t18-,20-,21?,22+,23-,24-,25-,28-,29+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195647
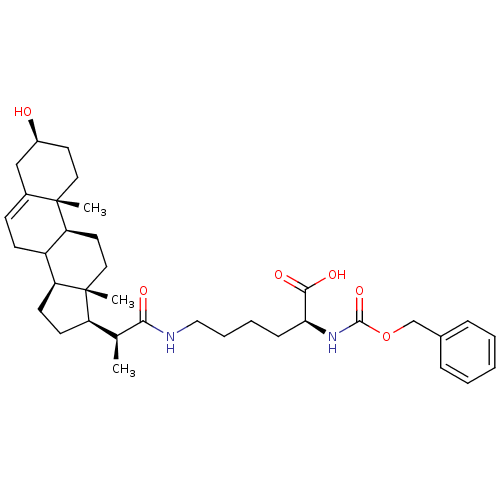
(3beat-ol-23,24-bisnor-5-en-22-(6-(2-Z-amino) hexan...)Show SMILES C[C@@H]([C@H]1CC[C@H]2C3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(=O)NCCCC[C@H](NC(=O)OCc1ccccc1)C(O)=O |t:8| Show InChI InChI=1S/C36H52N2O6/c1-23(32(40)37-20-8-7-11-31(33(41)42)38-34(43)44-22-24-9-5-4-6-10-24)28-14-15-29-27-13-12-25-21-26(39)16-18-35(25,2)30(27)17-19-36(28,29)3/h4-6,9-10,12,23,26-31,39H,7-8,11,13-22H2,1-3H3,(H,37,40)(H,38,43)(H,41,42)/t23-,26-,27?,28+,29-,30-,31-,35-,36+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |
Cycloartenol-C-24-methyltransferase
(Arabidopsis thaliana) | BDBM50195650
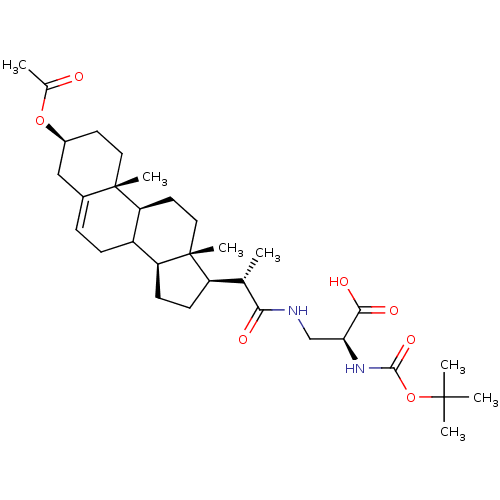
(3beta-acetoxy-23,24-bisnor-5-en-22-(3-(2-Boc-amino...)Show SMILES C[C@@H]([C@H]1CC[C@H]2C3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OC(C)=O)C(=O)NC[C@H](NC(=O)OC(C)(C)C)C(O)=O |t:8| Show InChI InChI=1S/C32H50N2O7/c1-18(27(36)33-17-26(28(37)38)34-29(39)41-30(3,4)5)23-10-11-24-22-9-8-20-16-21(40-19(2)35)12-14-31(20,6)25(22)13-15-32(23,24)7/h8,18,21-26H,9-17H2,1-7H3,(H,33,36)(H,34,39)(H,37,38)/t18-,21-,22?,23+,24-,25-,26-,31-,32+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major recombinant 24-SMT |
J Med Chem 49: 6094-103 (2006)
Article DOI: 10.1021/jm060290f
BindingDB Entry DOI: 10.7270/Q2TB16JJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data