

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114862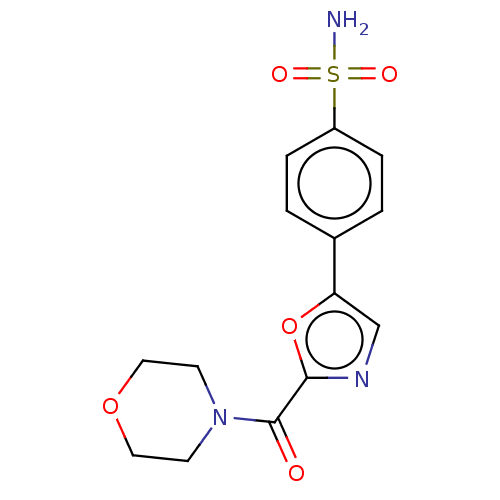 (CHEMBL3608874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114861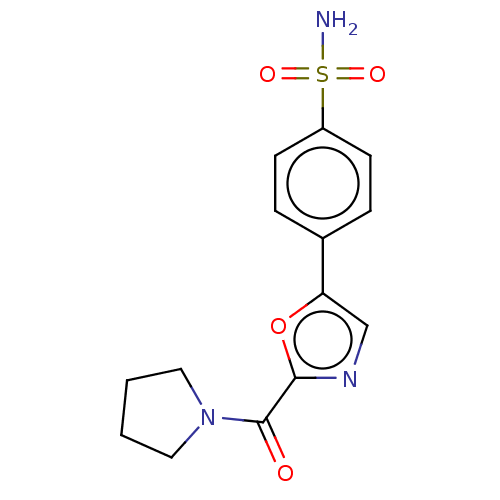 (CHEMBL3608873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50114861 (CHEMBL3608873) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50114862 (CHEMBL3608874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114855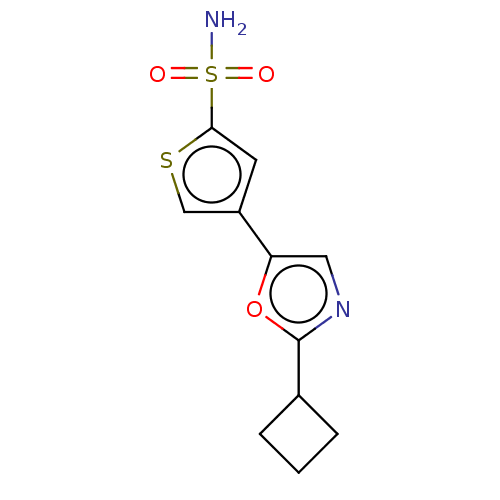 (CHEMBL3608892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114853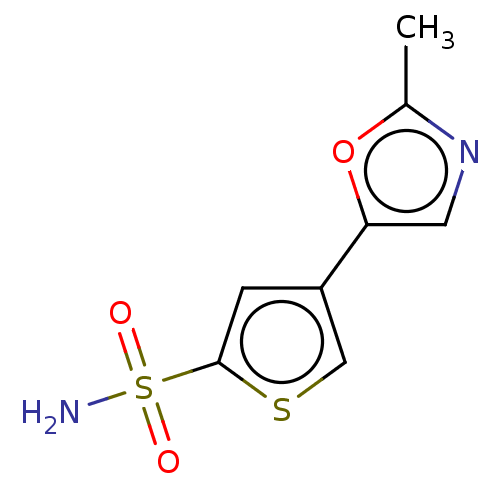 (CHEMBL3608890) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114851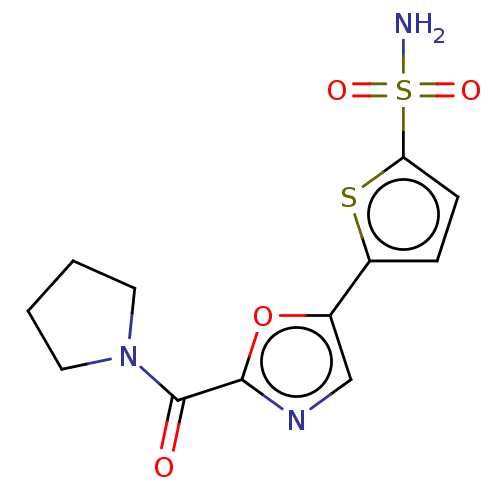 (CHEMBL3608888) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114850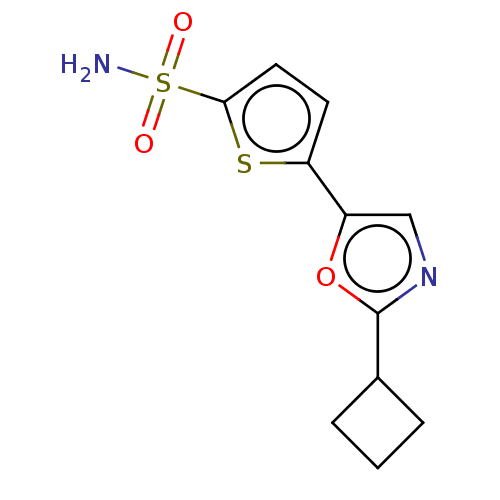 (CHEMBL3608887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114846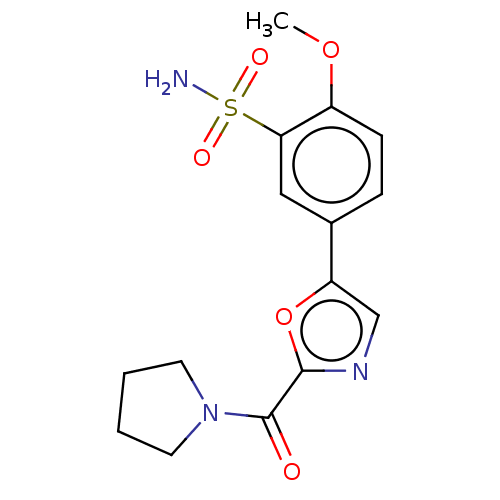 (CHEMBL3608883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114869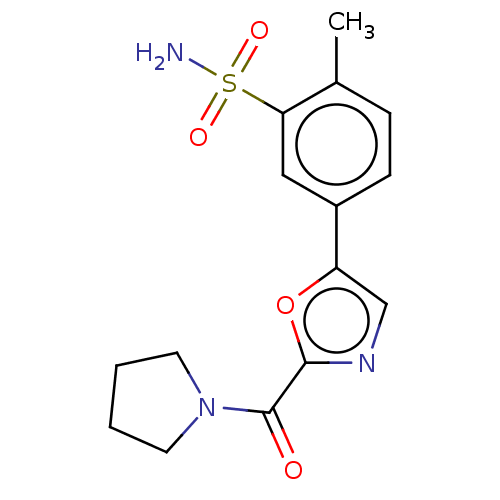 (CHEMBL3608881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114859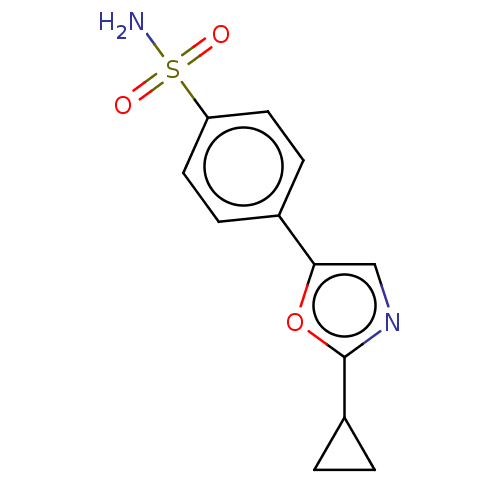 (CHEMBL3608871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114856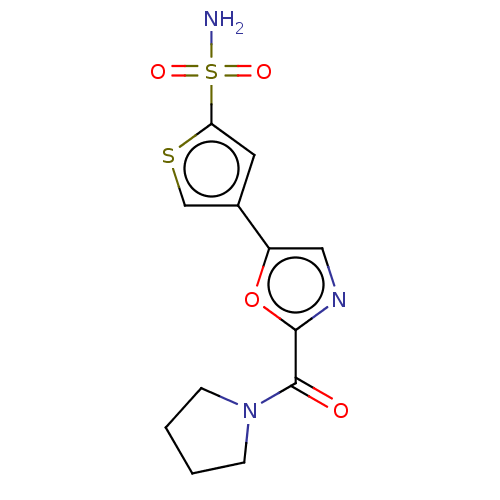 (CHEMBL3608893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114852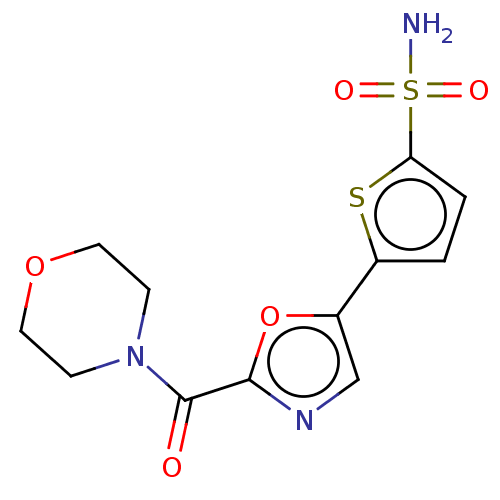 (CHEMBL3608889) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114860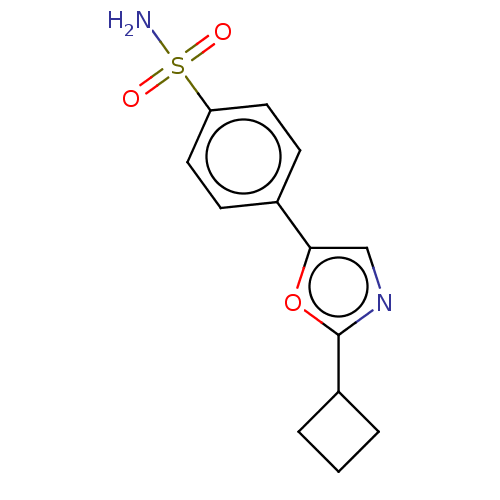 (CHEMBL3608872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114857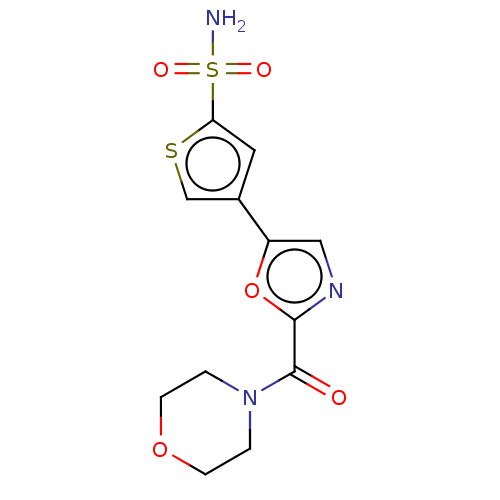 (CHEMBL3608894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114847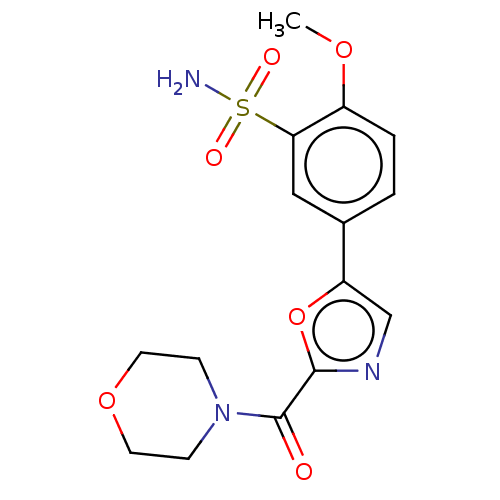 (CHEMBL3608884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114858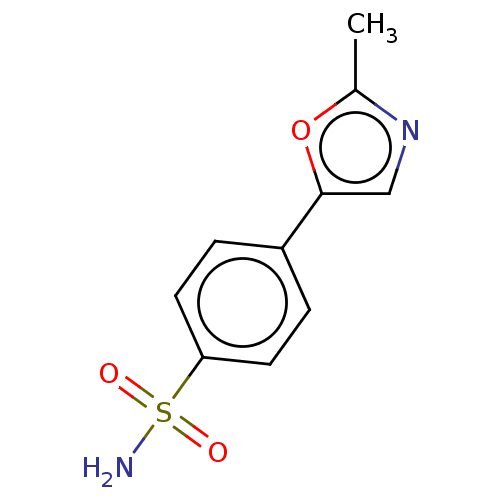 (CHEMBL3608870) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50114853 (CHEMBL3608890) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50114857 (CHEMBL3608894) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283288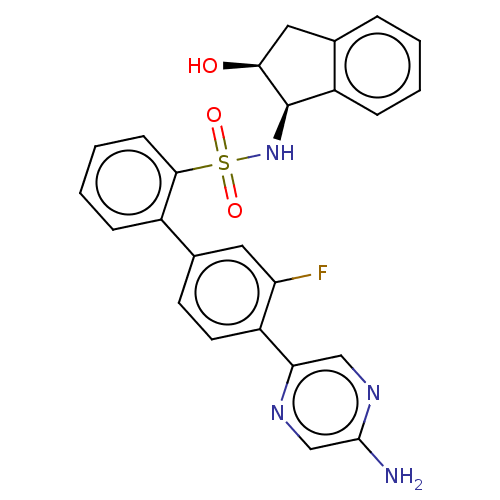 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-((1R,2S)- 2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283229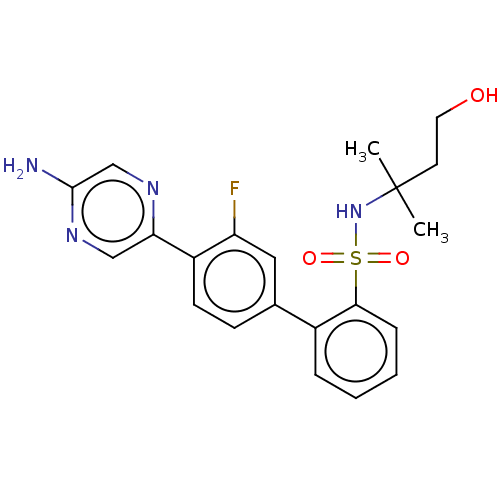 (4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-(3- hydroxy-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283230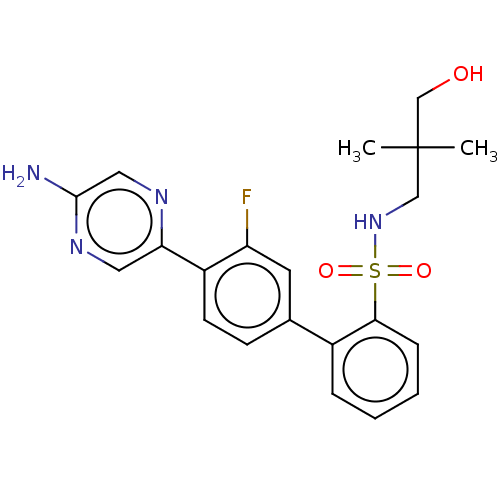 (4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-(3- hydroxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283262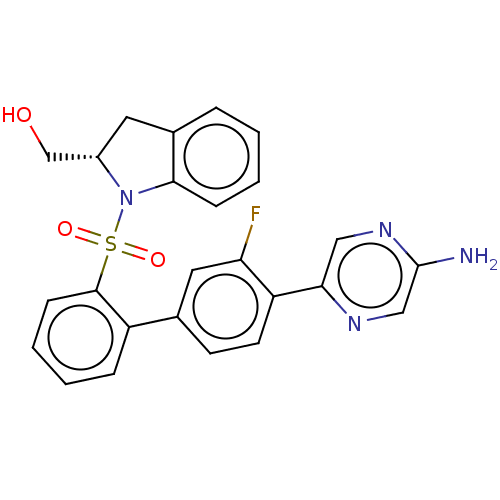 ((S)-(1-{[4'-(5-Aminopyrazin-2-yl)-3'- fluorobiphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283287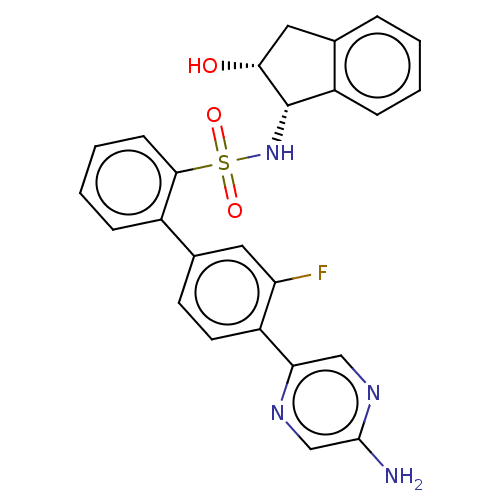 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-((1S,2R)- 2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283303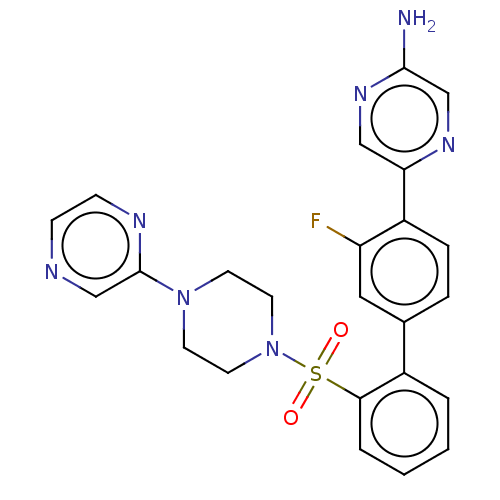 (5-{3-Fluoro-2'-[(4-pyrazin-2-ylpiperazin-1- yl)sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283304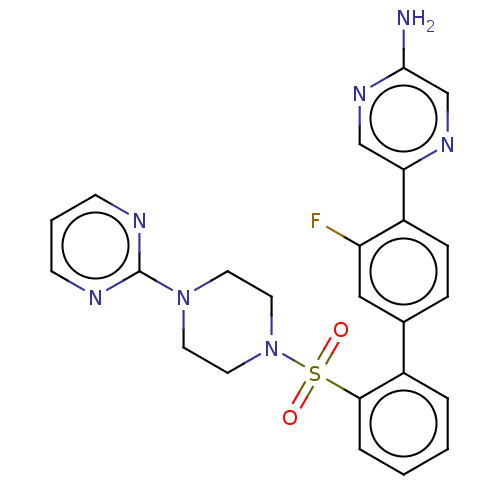 (5-{3-Fluoro-2'-[(4-pyrimidin-2-ylpiperazin-1- yl)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283306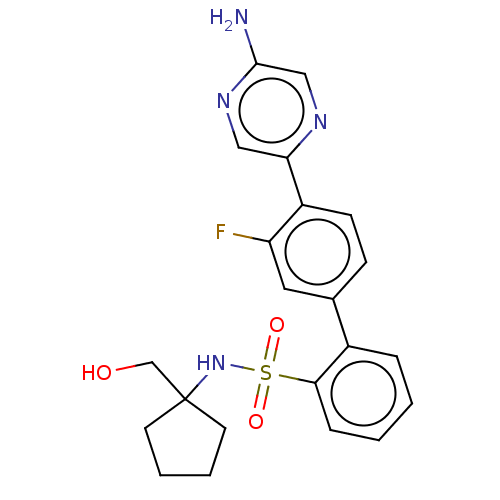 (4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-[1- (hydroxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283335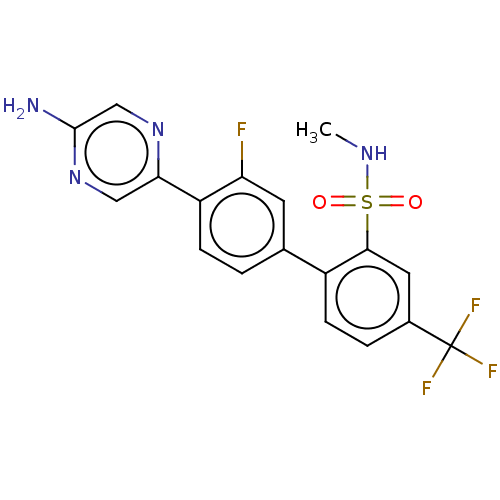 (4'-(5-Aminopyrazxin-2-yl)-3'-fluoro-N-methyl-4- (t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283337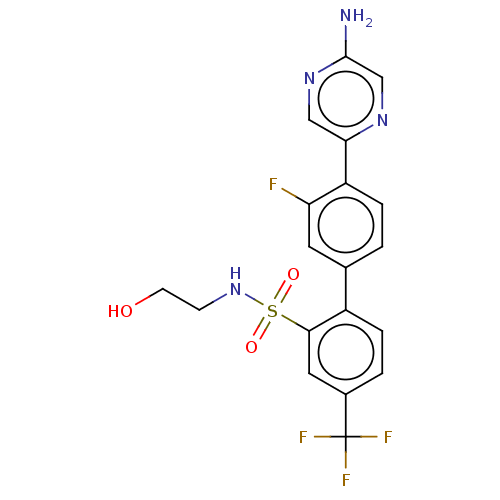 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-(2- hydroxyet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283508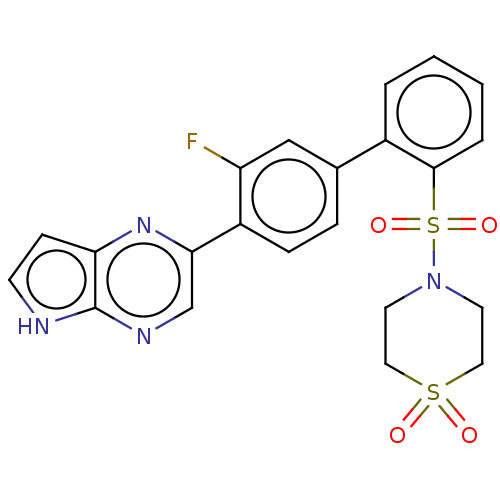 (2-{2'-[(1,1-Dioxidothiomorpholin-4-yl)sulfonyl]- 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283516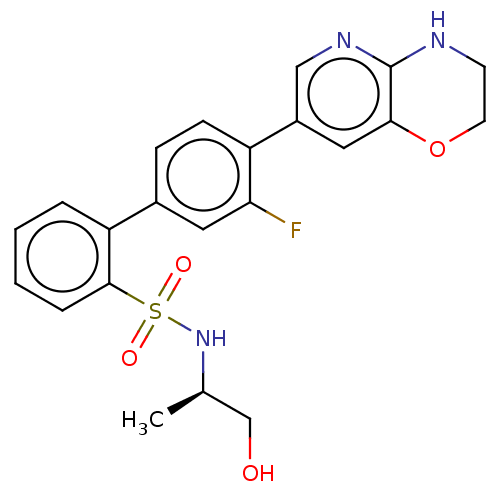 (4'-(3,4-Dihydro-2H-pyrido[3,2-b][1,4]oxazin-7- yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283502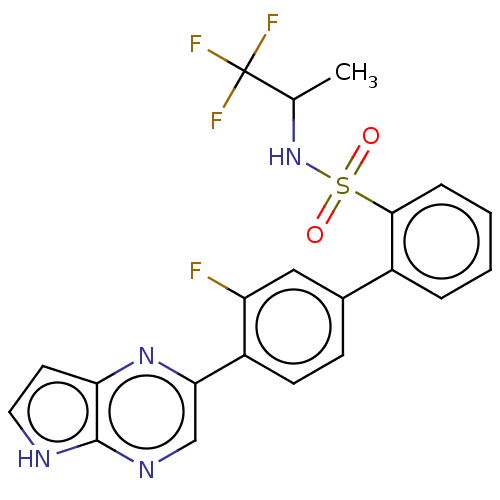 (3'-Fluoro-4'-(5H-pyrrolo[2,3-b]pyrazin-2-yl)N-- N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283248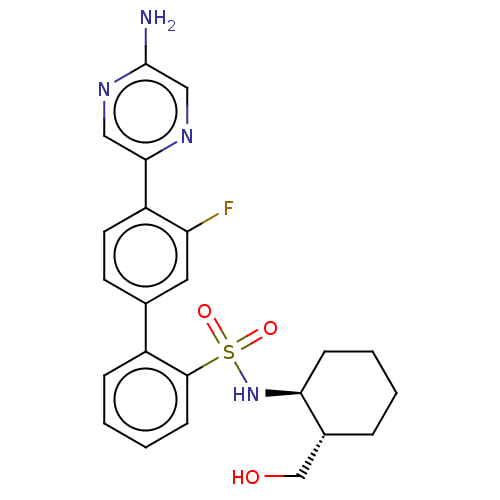 ((trans)-4'-(5-Aminopyrazin-2-yl)-3'-fluoro-N-[2- (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283677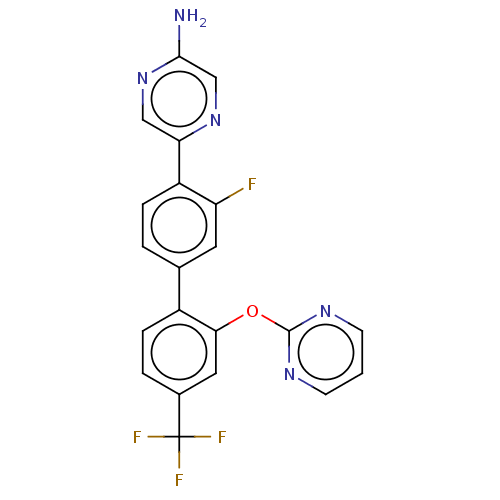 (5-[3-Fluoro-2'-(pyrimidin-2-yloxy)-4'- (trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM283245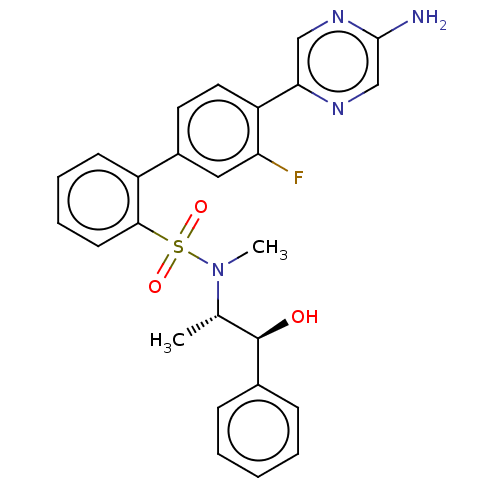 (4'-(5-aminopyrazin-2-yl)-3'-fluoro-N-((1S,2S)- 1-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50343731 ((R)-N-alpha-(2,2-Diphenylacetyl)-N-(4-ureidomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells | Bioorg Med Chem 19: 2859-78 (2011) Article DOI: 10.1016/j.bmc.2011.03.045 BindingDB Entry DOI: 10.7270/Q2F47PG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50114870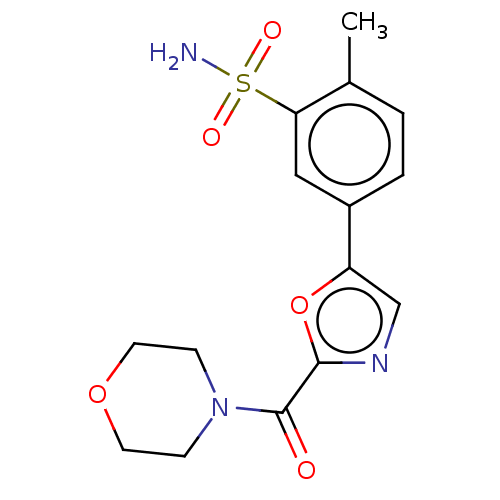 (CHEMBL3608882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Eur J Med Chem 101: 334-47 (2015) Article DOI: 10.1016/j.ejmech.2015.06.022 BindingDB Entry DOI: 10.7270/Q2W95BZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM337621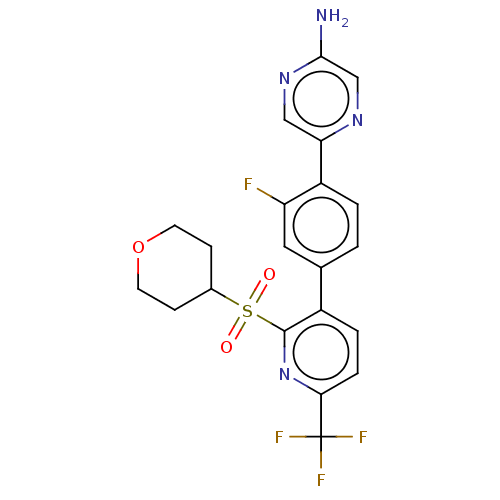 (5-{2-Fluoro-4-[2-tetrahydro-2H- pyran-4-ylsulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description The FLAP binding assay is performed in HTRF format (homogeneous time resolved fluorescence). FLAP-containing membranes (1 μg/well final for huma... | US Patent US9745328 (2017) BindingDB Entry DOI: 10.7270/Q2H13446 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM169403 (US9079866, 808 | US9745328, Compound 808 | US98848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9079866 (2015) BindingDB Entry DOI: 10.7270/Q2FF3R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167060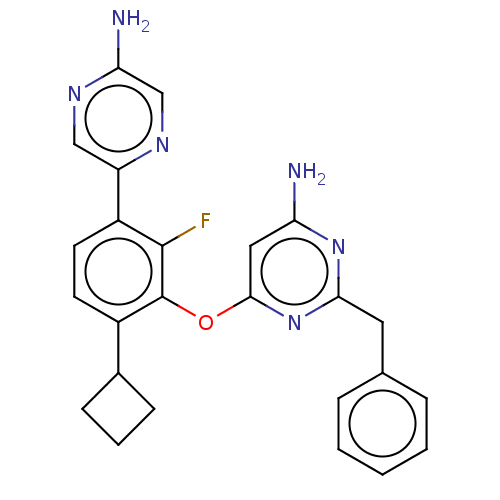 (US9073876, 146 | US9732093, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167060 (US9073876, 146 | US9732093, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM169403 (US9079866, 808 | US9745328, Compound 808 | US98848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description The FLAP binding assay is performed in HTRF format (homogeneous time resolved fluorescence). FLAP-containing membranes (1 μg/well final for huma... | US Patent US9745328 (2017) BindingDB Entry DOI: 10.7270/Q2H13446 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM169403 (US9079866, 808 | US9745328, Compound 808 | US98848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167060 (US9073876, 146 | US9732093, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167191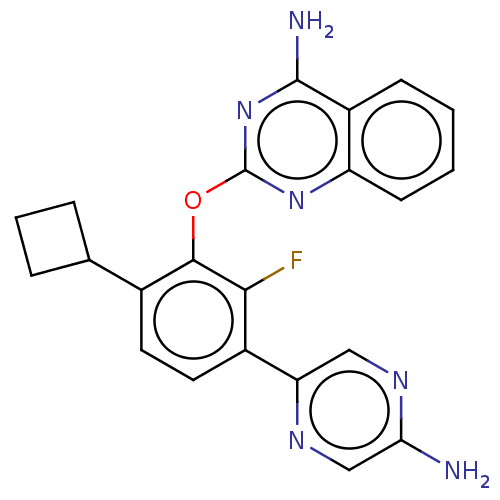 (US9073876, 272 | US9732093, Compound 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM337627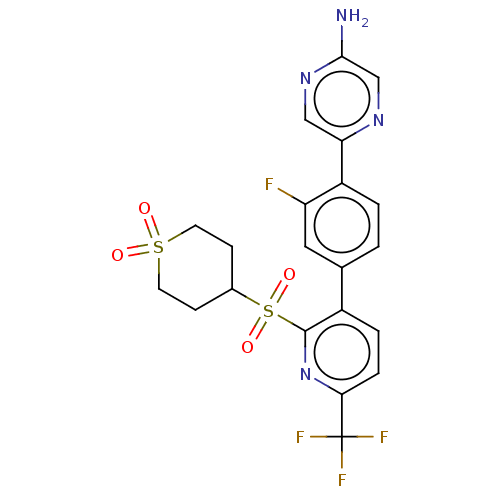 (5-(4-{2-[(1,1-Dioxidotetrahydra-2H- thiopyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description The FLAP binding assay is performed in HTRF format (homogeneous time resolved fluorescence). FLAP-containing membranes (1 μg/well final for huma... | US Patent US9745328 (2017) BindingDB Entry DOI: 10.7270/Q2H13446 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM167191 (US9073876, 272 | US9732093, Compound 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9073876 (2015) BindingDB Entry DOI: 10.7270/Q28S4NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Flap endonuclease 1 (Homo sapiens (Human)) | BDBM167191 (US9073876, 272 | US9732093, Compound 272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9732093 (2017) BindingDB Entry DOI: 10.7270/Q2NV9MCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM169201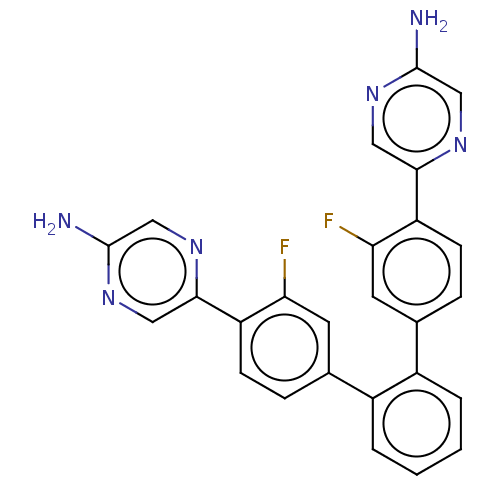 (US9079866, 602 | US9745328, Compound 602 | US98848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM169405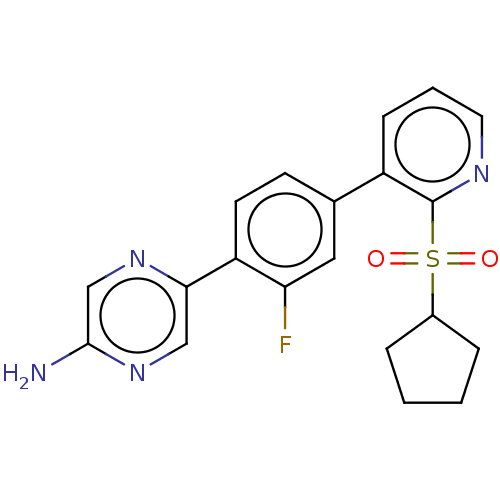 (US9079866, 810 | US9745328, Compound 810 | US98848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9884878 (2018) BindingDB Entry DOI: 10.7270/Q2R78H8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8368 total ) | Next | Last >> |