

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50010044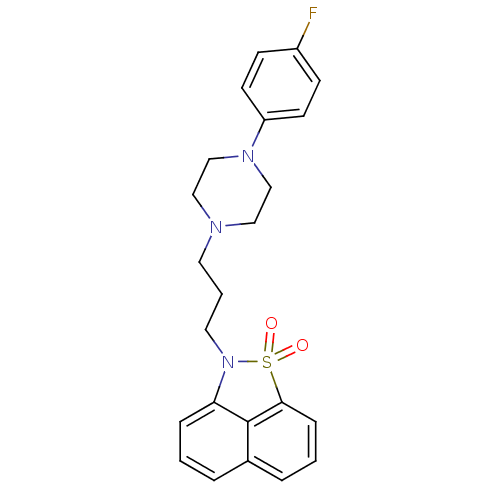 (2-{3-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-propyl}-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry Alfortville Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Binding constant against 5-hydroxytryptamine 2 receptor (in vivo) | J Med Chem 34: 2477-83 (1991) BindingDB Entry DOI: 10.7270/Q2R78FT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584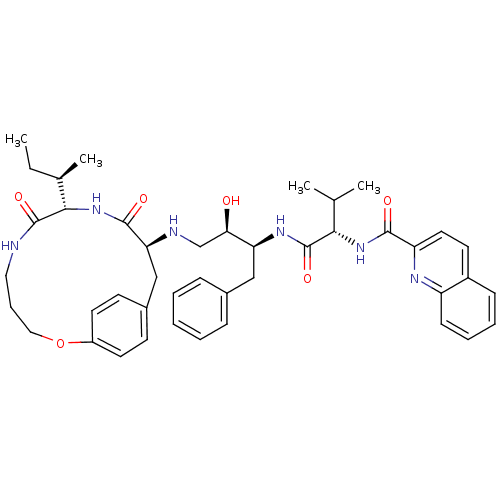 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142990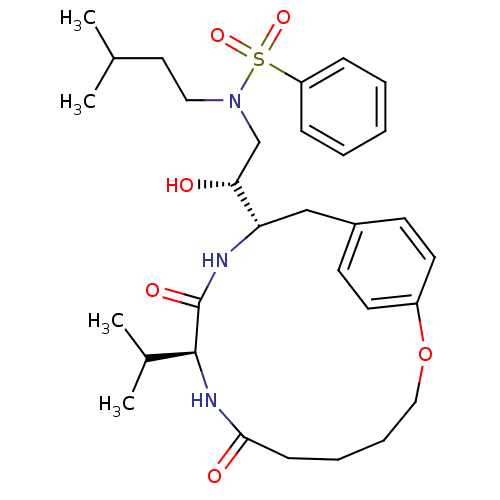 (CHEMBL288836 | N-[(R)-2-Hydroxy-2-((9S,12S)-9-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476730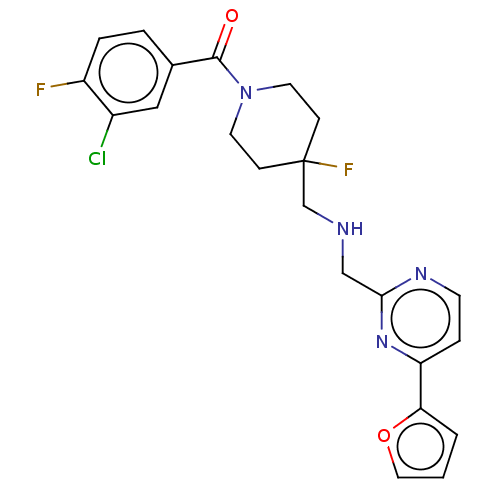 (CHEMBL231471) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370377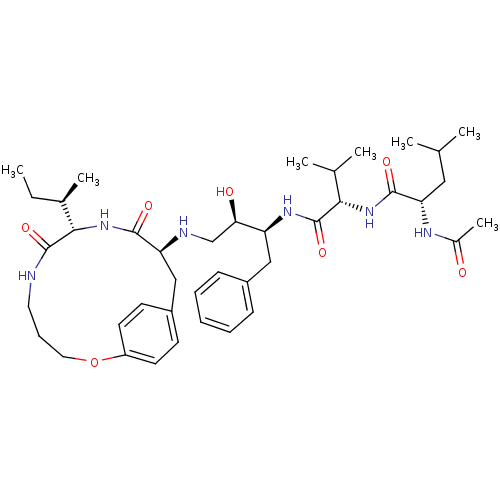 (CHEMBL1790231) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13928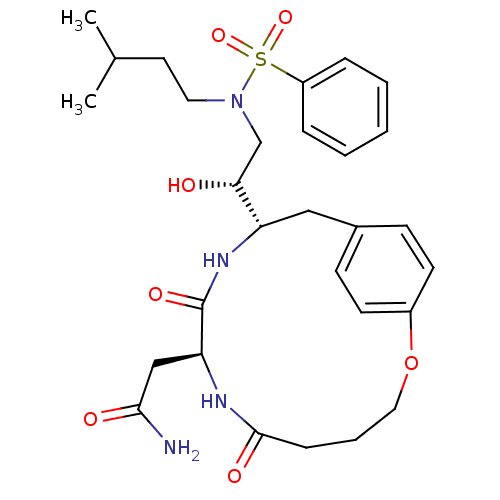 (2-[(8S,11S)-11-[(1R)-2-[benzene(3-methylbutyl)sulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152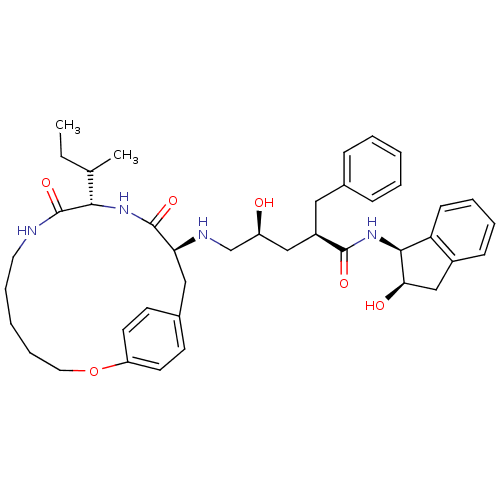 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry Alfortville Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Binding constant against 5-hydroxytryptamine 2 receptor (in vitro) | J Med Chem 34: 2477-83 (1991) BindingDB Entry DOI: 10.7270/Q2R78FT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476729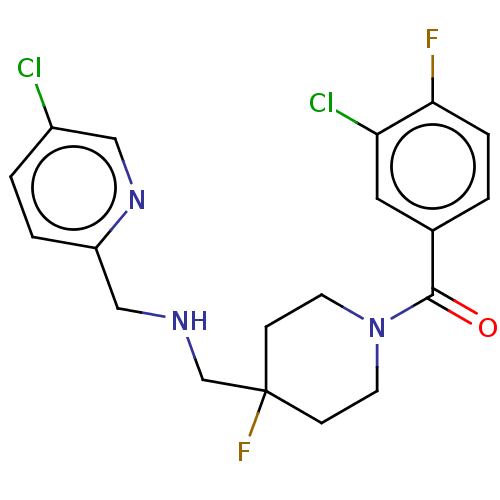 (CHEMBL425833) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568920 (CHEMBL4846823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476740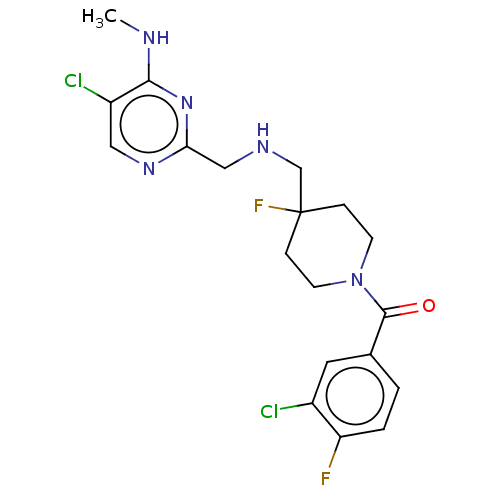 (CHEMBL396062) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10439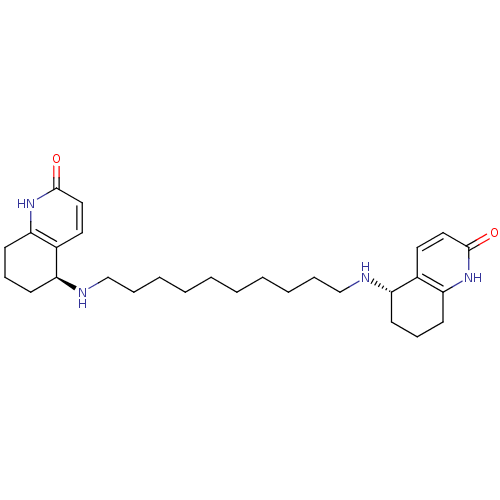 ((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | -51.4 | 2.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50328639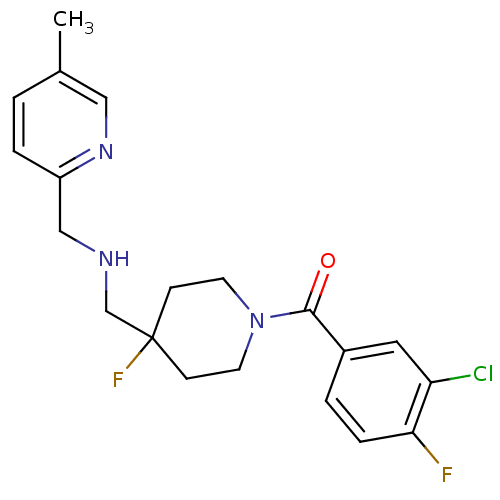 ((3-Chloro-4-fluoro-phenyl)-(4-fluoro-4-{[(5-methyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142965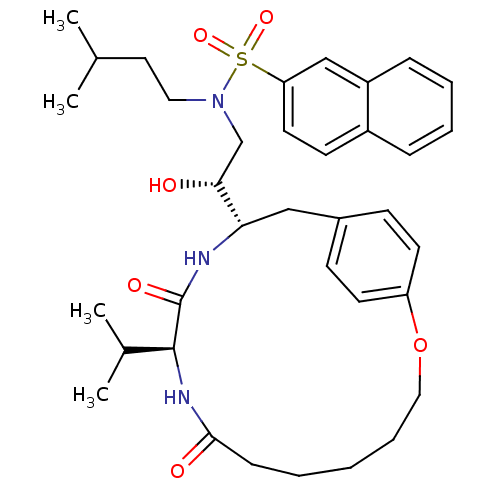 (CHEMBL296937 | Naphthalene-2-sulfonic acid [(S)-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142978 ((S)-11-{(S)-2-[Benzenesulfonyl-(3-methyl-butyl)-am...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370379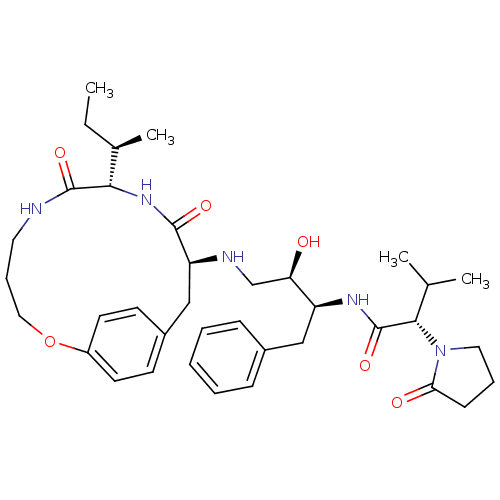 (CHEMBL1790229) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50403100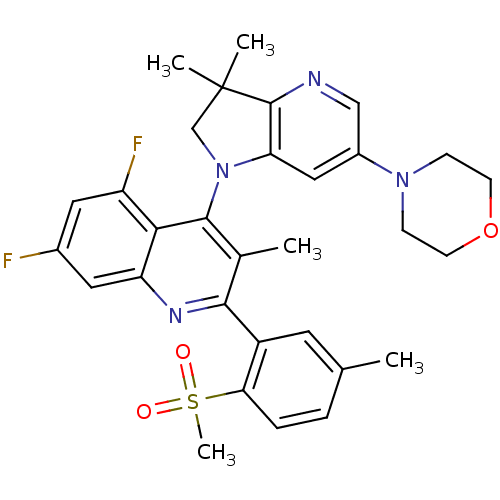 (CHEMBL2216891 | US8765940, 4-(3,3-dimethyl-6-(4-mo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50362863 (CHEMBL1940418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476727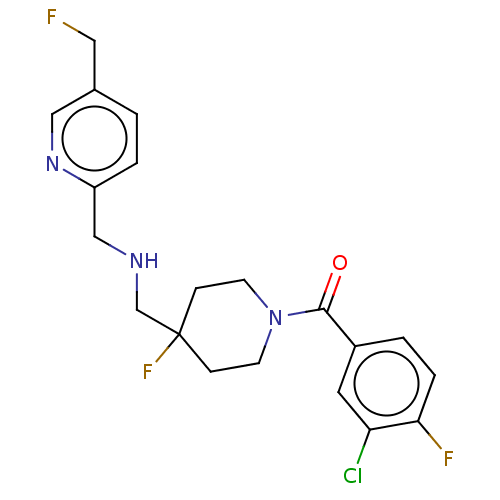 (CHEMBL394530) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124797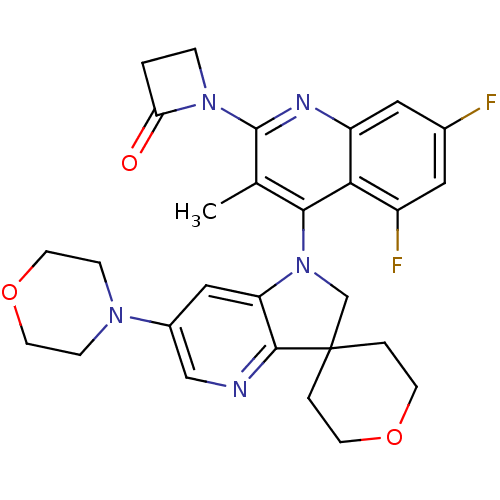 (US8765940, 1-(5,7-difluoro-3-methyl-4-(6'-(4-morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.27 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124781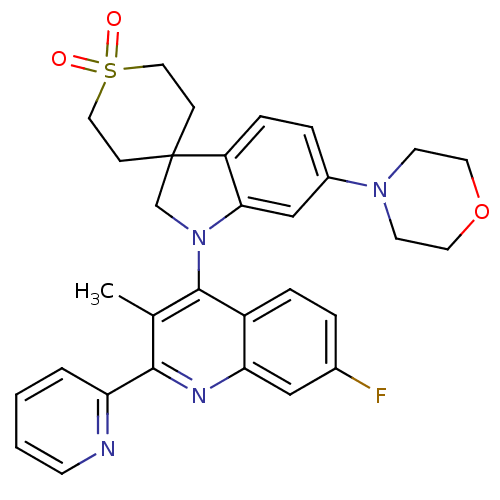 (US8765940, 1-(7-fluoro-3-methyl-2-(2-pyridinyl)-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124778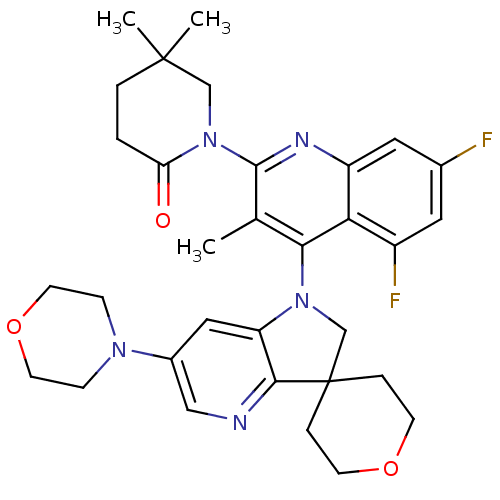 (US8765940, 1-(5,7-difluoro-3-methyl-4-(6'-(4-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124765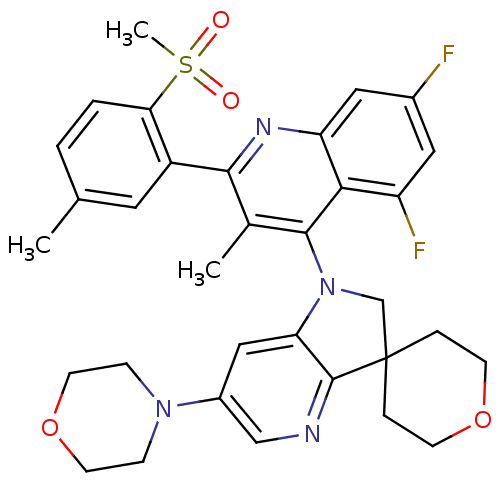 (US8765940, 1'-(5,7-difluoro-3-methyl-2-(5-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124764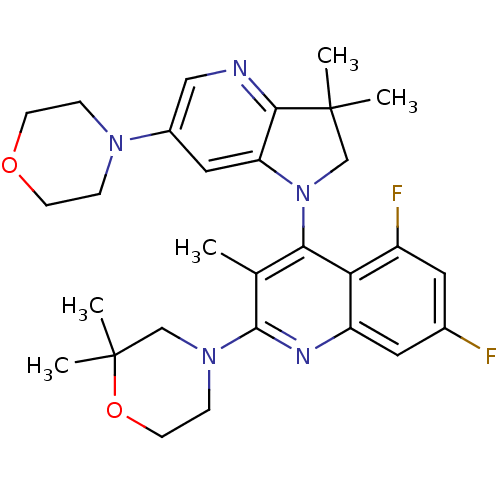 (US8765940, 2-(2,2-dimethyl-4-morpholinyl)-4-(3,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124749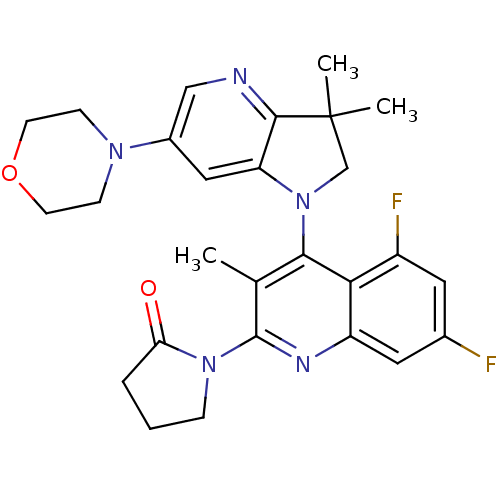 (US8765940, 1-(4-(3,3-dimethyl-6-(4-morpholinyl)-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM13014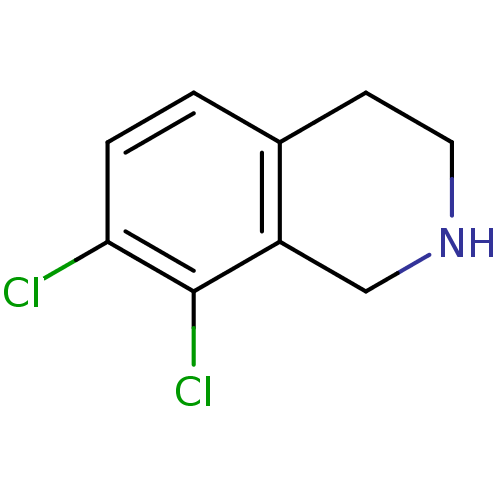 (7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to human wild type his-tagged PNMT | J Med Chem 48: 7243-52 (2005) Article DOI: 10.1021/jm050568o BindingDB Entry DOI: 10.7270/Q2QC049M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142986 (CHEMBL295464 | N-[(S)-2-(R)-Hydroxy-2-((S)-10-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124759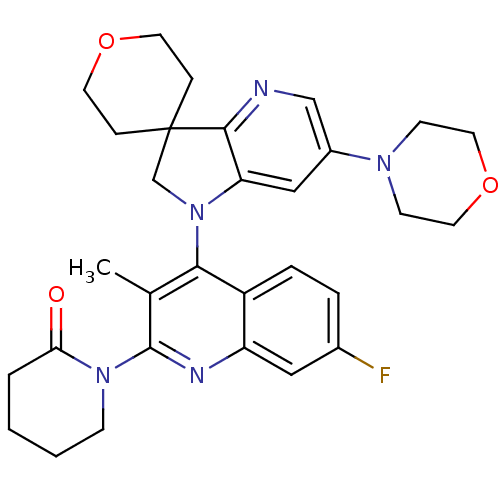 (US8765940, 1-(7-fluoro-3-methyl-4-(6'-(4-morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151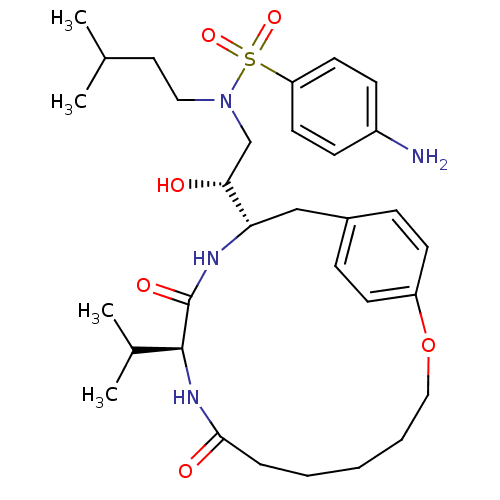 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476732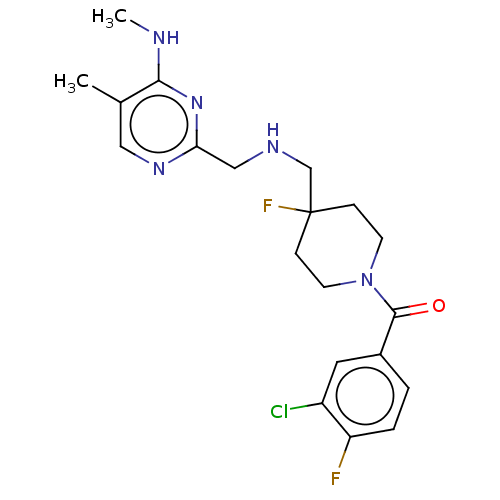 (CHEMBL394606) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13931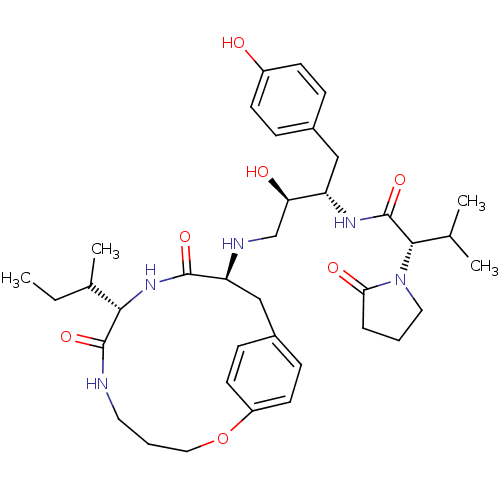 ((2S)-N-[(2S,3R)-4-{[(8S,11S)-8-[(2R)-butan-2-yl]-7...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | -51.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124767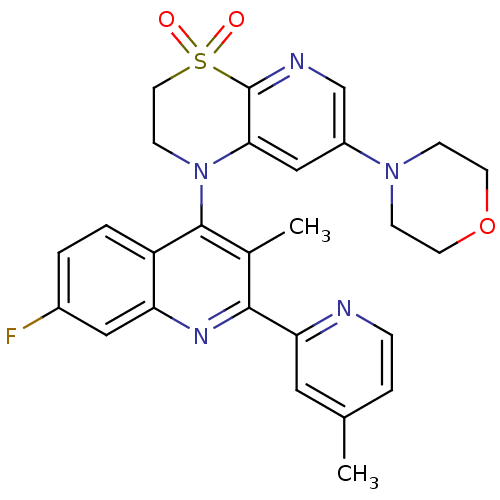 (US8765940, 1-(7-fluoro-3-methyl-2-(4-methyl-2-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50142974 (4-Amino-N-sec-butyl-N-[(S)-2-(R)-hydroxy-2-((S)-10...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay | J Med Chem 47: 1641-51 (2004) Article DOI: 10.1021/jm030337m BindingDB Entry DOI: 10.7270/Q2JD4XJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476734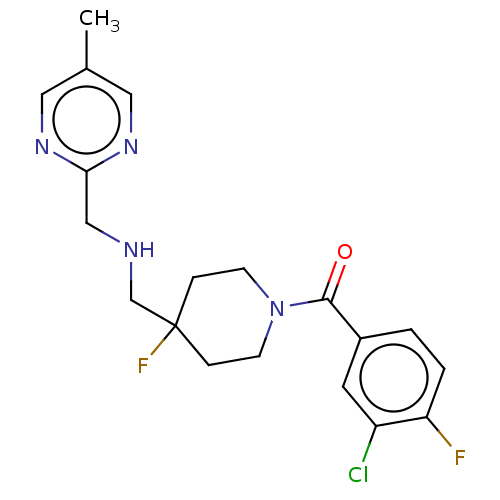 (CHEMBL230963) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124766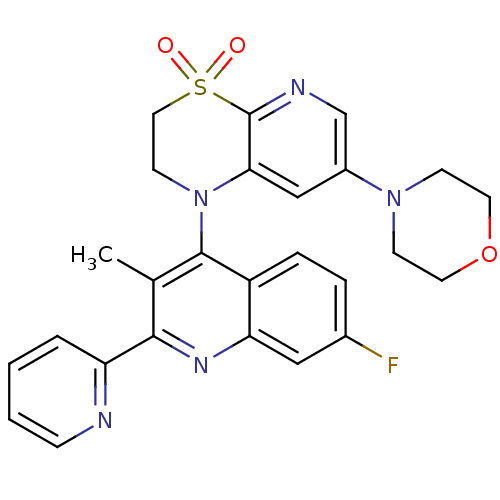 (US8765940, 1-(7-fluoro-3-methyl-2-(2-pyridinyl)-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124799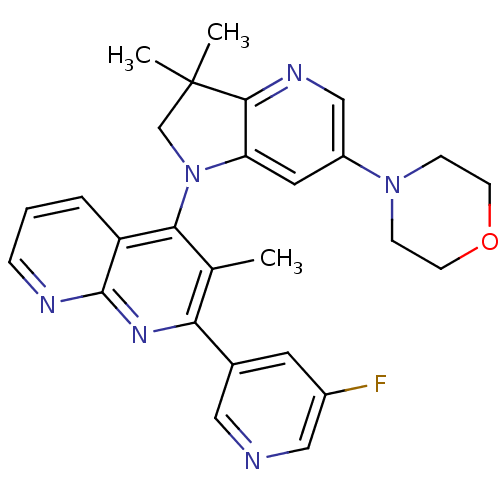 (US8765940, 4-(3,3-dimethyl-6-(4-morpholinyl)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.24 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124798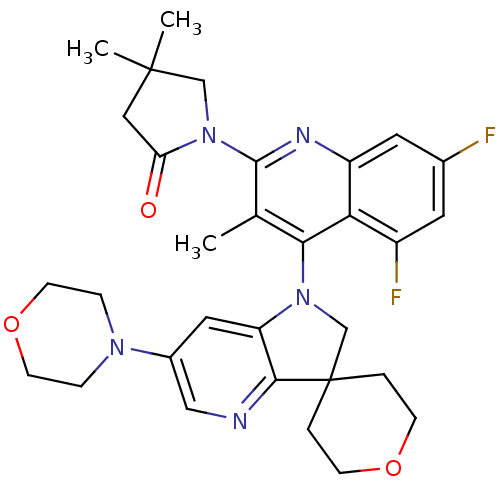 (US8765940, 1-(5,7-difluoro-3-methyl-4-(6'-(4-morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124783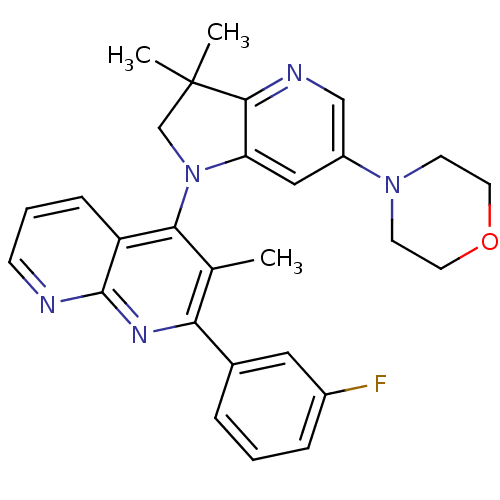 (US8765940, 4-(3,3-dimethyl-6-(4-morpholinyl)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568924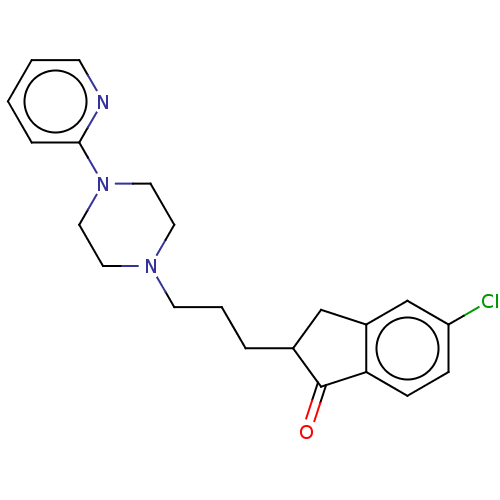 (CHEMBL4862180) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50476736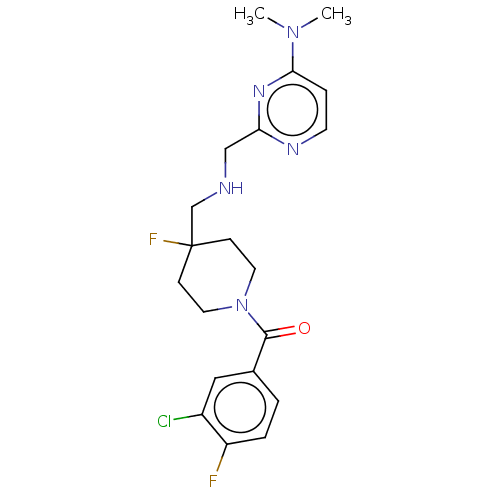 (CHEMBL394778) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Research Center Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor in rat cortex membrane | J Med Chem 50: 5024-33 (2007) Article DOI: 10.1021/jm070714l BindingDB Entry DOI: 10.7270/Q2HH6NTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568924 (CHEMBL4862180) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124743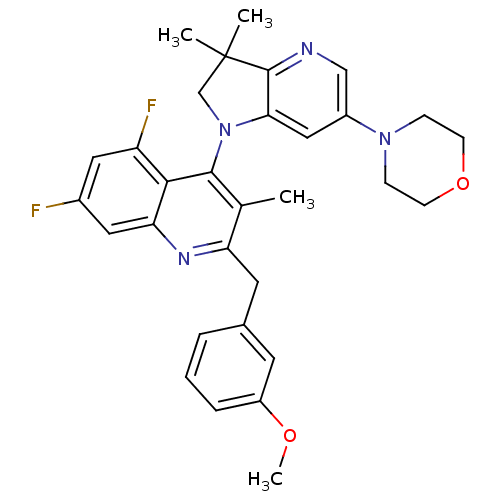 (US8765940, 4-(3,3-dimethyl-6-(4-morpholinyl)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124758 (US8765940, 1-(5,7-difluoro-3-methyl-4-(6'-(4-morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM124775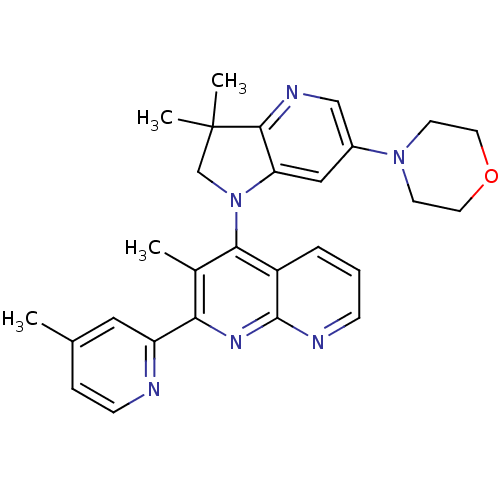 (US8765940, 4-(3,3-dimethyl-6-(4-morpholinyl)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc. US Patent | Assay Description A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... | US Patent US8765940 (2014) BindingDB Entry DOI: 10.7270/Q2WW7G94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568921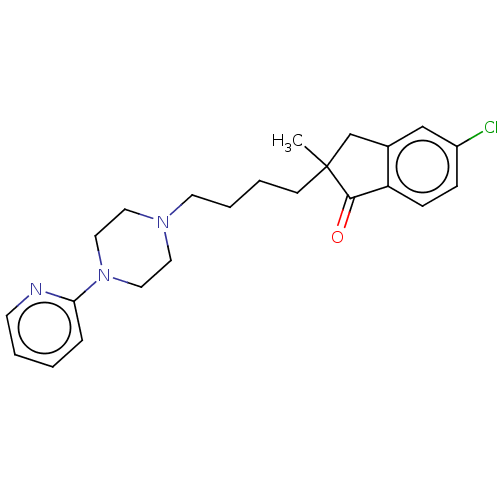 (CHEMBL4871868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor incubated for 1 hr by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113243 BindingDB Entry DOI: 10.7270/Q29C726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4428 total ) | Next | Last >> |