

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50010904 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012938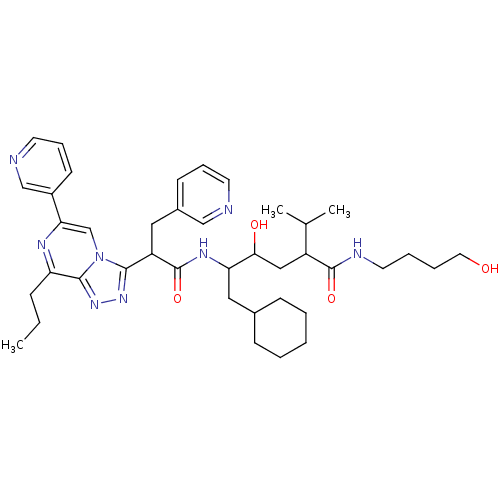 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012937 (CHEMBL76697 | Less polar Epimer-6-Cyclohexyl-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012937 (CHEMBL76697 | Less polar Epimer-6-Cyclohexyl-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012946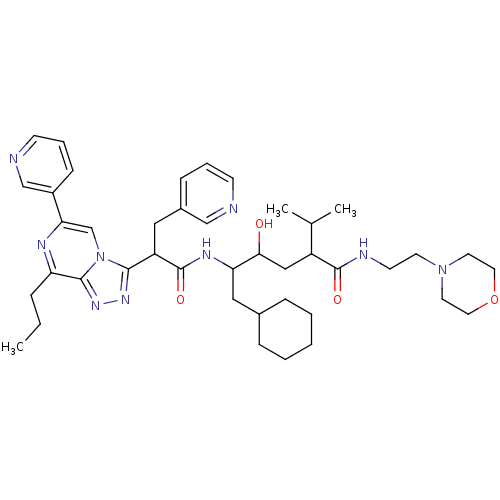 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012939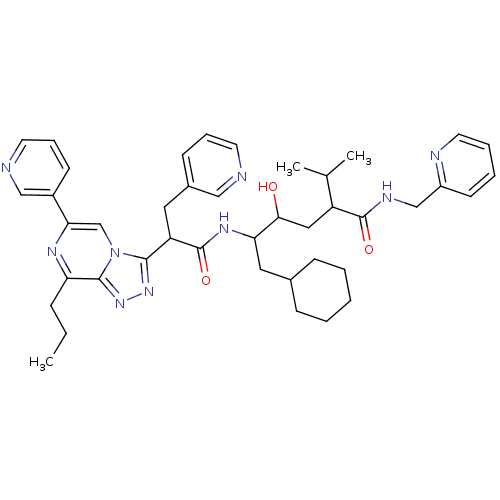 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012944 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012922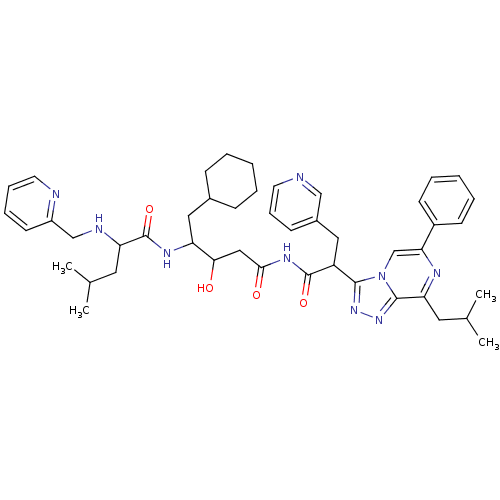 (4-Methyl-2-[(pyridin-2-ylmethyl)-amino]-pentanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012922 (4-Methyl-2-[(pyridin-2-ylmethyl)-amino]-pentanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012928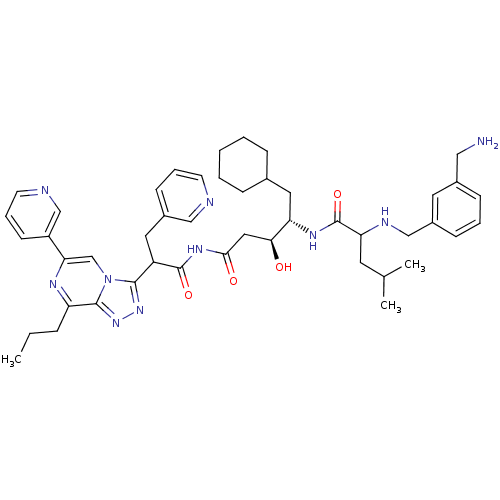 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012923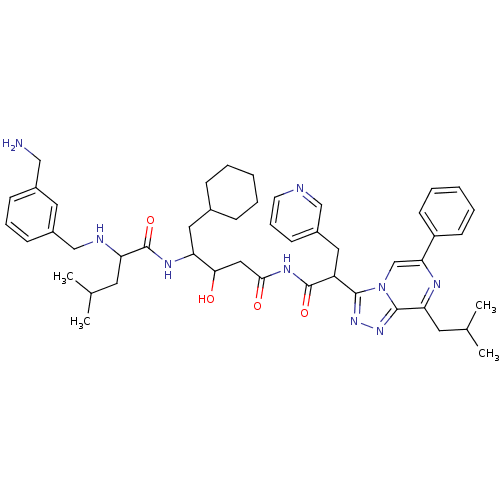 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012923 (2-(3-Aminomethyl-benzylamino)-4-methyl-pentanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012932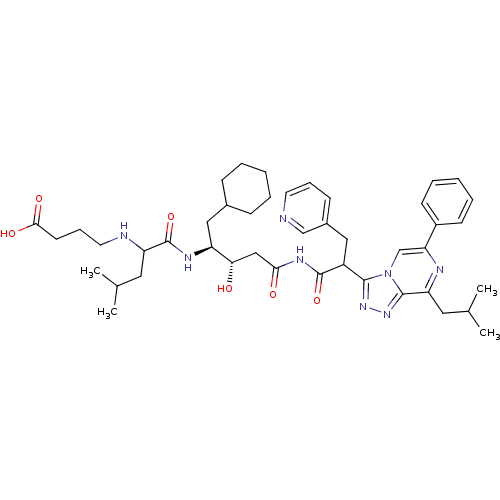 (4-(1-{1-Cyclohexylmethyl-2-hydroxy-4-[2-(8-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012943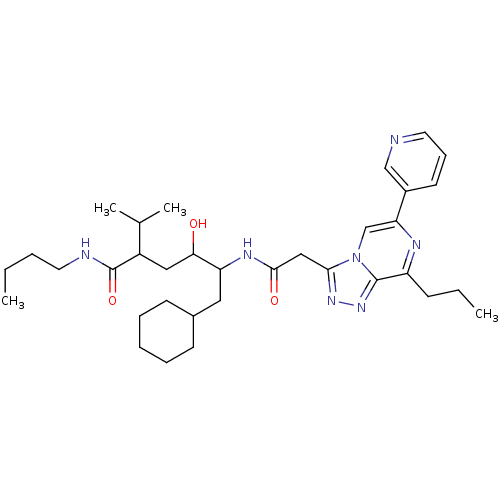 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012925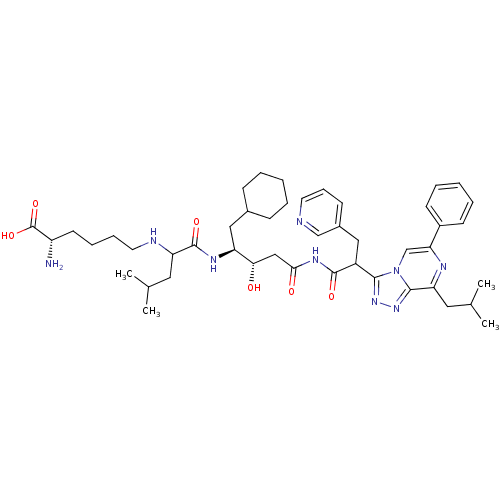 (2-Amino-6-(1-{1-cyclohexylmethyl-2-hydroxy-4-[2-(8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047133 (CHEMBL434768 | {6-Ethyl-2-methyl-4-[2'-(1H-tetrazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047111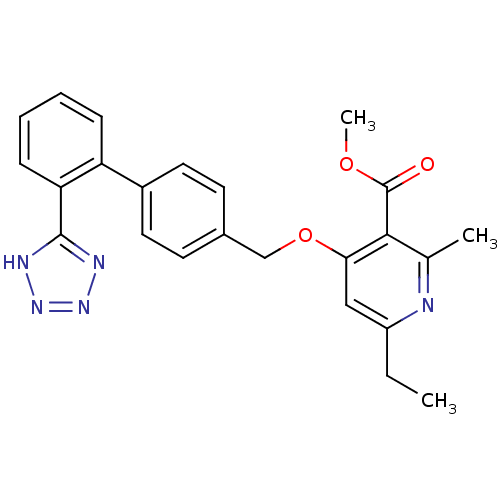 (6-Ethyl-2-methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047117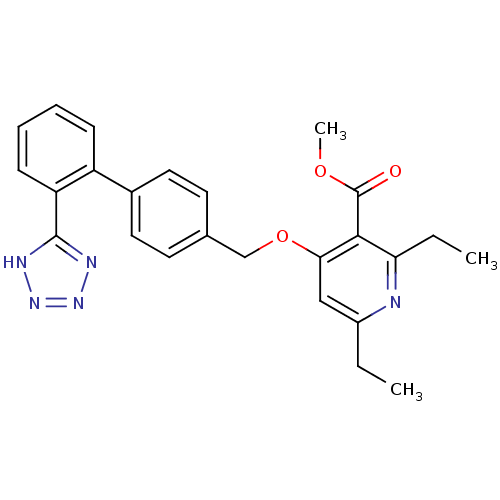 (2,6-Diethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047113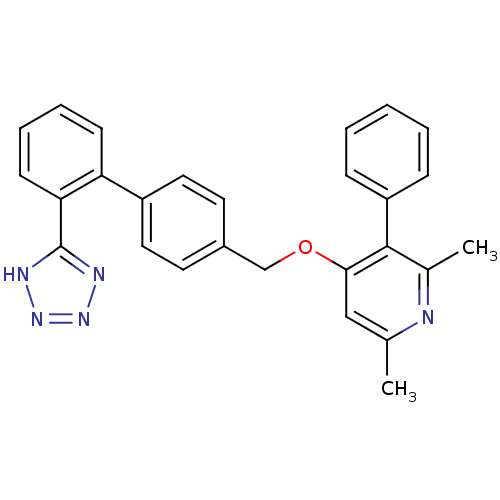 (2,6-Dimethyl-3-phenyl-4-[2'-(1H-tetrazol-5-yl)-bip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047130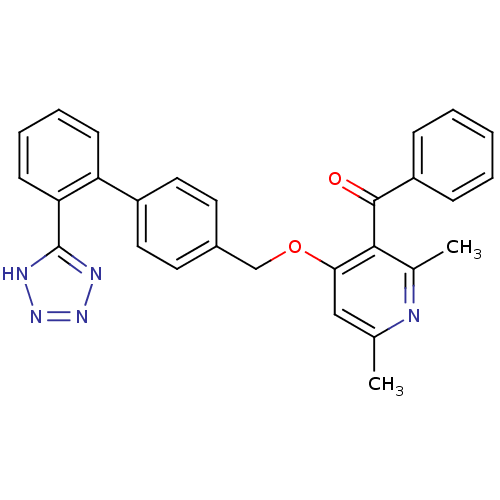 (CHEMBL280189 | {2,6-Dimethyl-4-[2'-(1H-tetrazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047129 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047122 (1-{6-Ethyl-2-methyl-4-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047112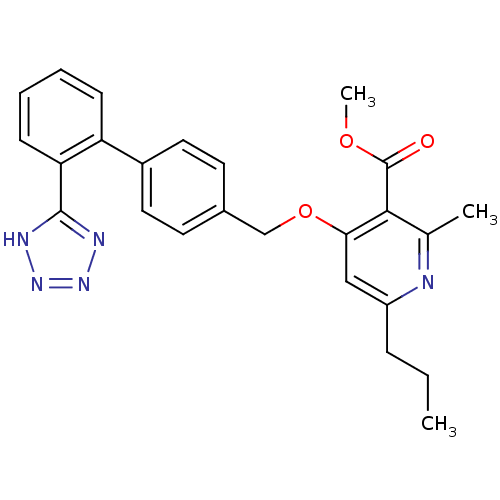 (2-Methyl-6-propyl-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003386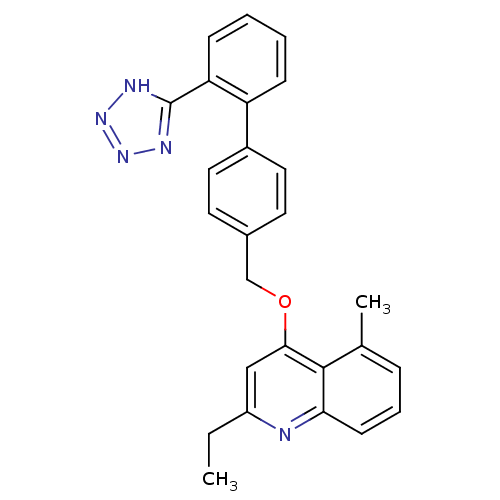 (2-Ethyl-5-methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047132 (2,6-Diethyl-3-iodo-4-[2'-(2H-tetrazol-5-yl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047119 (3-Benzyl-2,6-dimethyl-4-[2'-(1H-tetrazol-5-yl)-bip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003392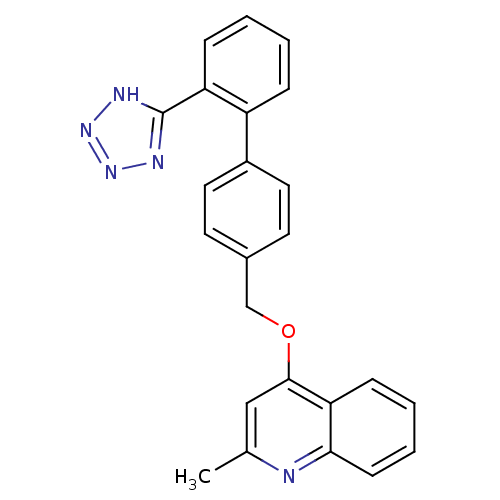 (2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003392 (2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50009714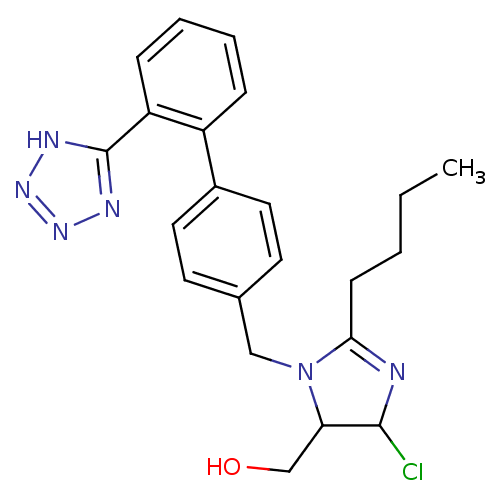 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50406795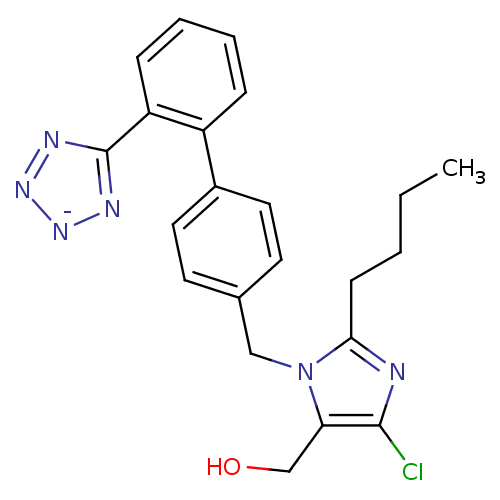 (Cozaar | LOSARTAN POTASSIUM) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003400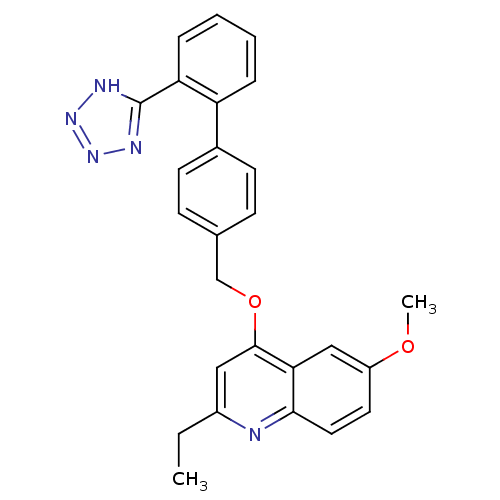 (2-Ethyl-6-methoxy-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047125 (2-Ethyl-6-methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047115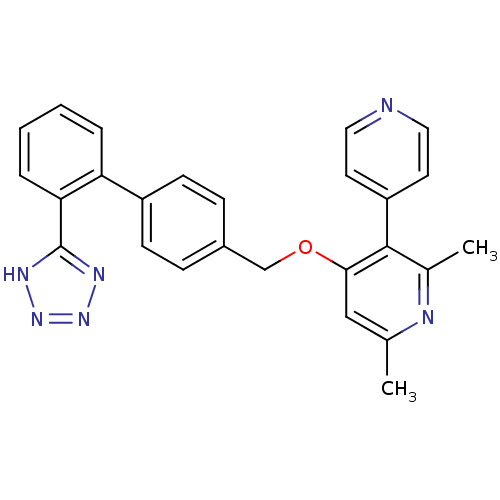 (2,6-Dimethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047127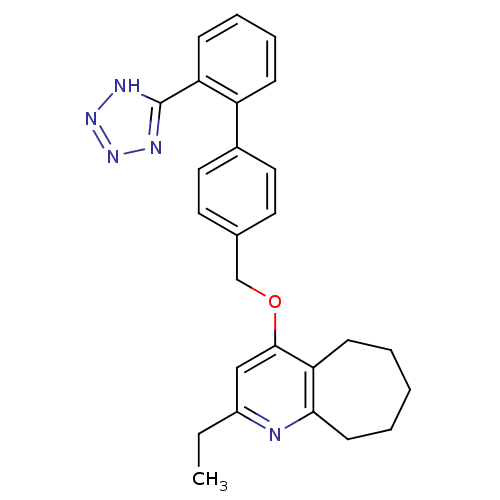 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50014134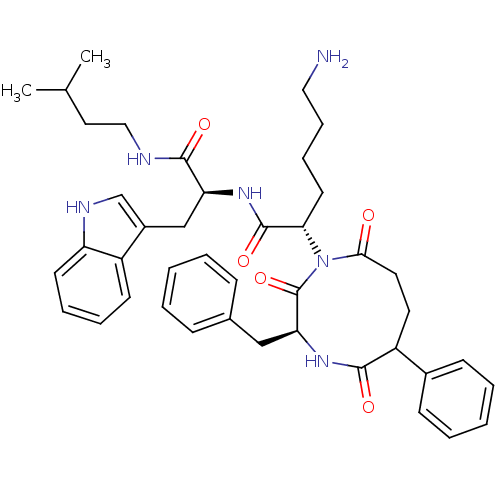 (6-Amino-2-(3-benzyl-2,5,9-trioxo-6-phenyl-[1,4]dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2560-8 (1990) BindingDB Entry DOI: 10.7270/Q20V8BR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012941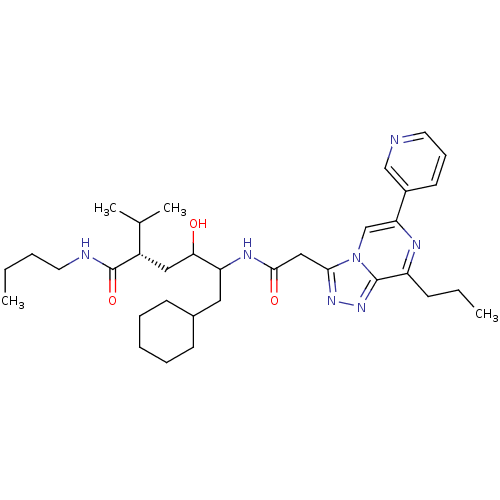 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003377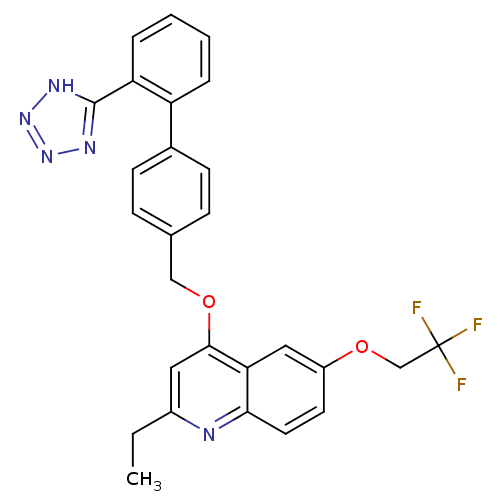 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003403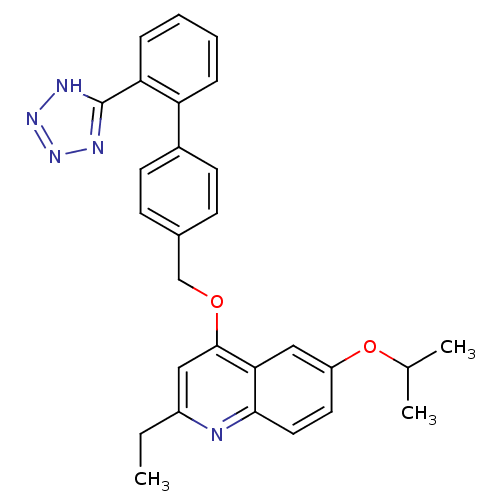 (2-Ethyl-6-isopropoxy-4-[2'-(1H-tetrazol-5-yl)-biph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047131 (1-{2,6-Dimethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012940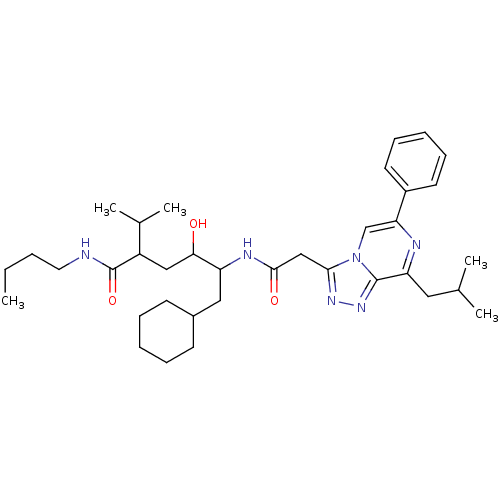 (6-Cyclohexyl-4-hydroxy-5-[2-(8-isobutyl-6-phenyl-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003387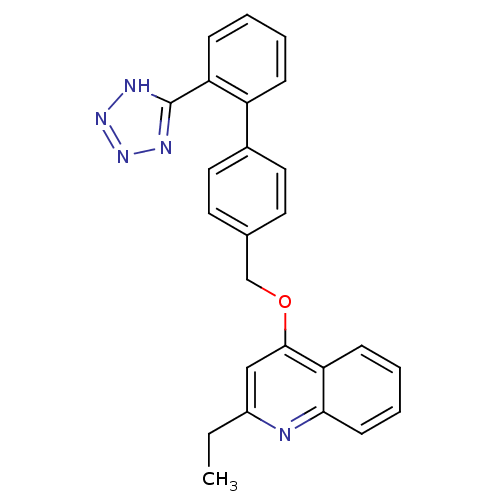 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003387 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047118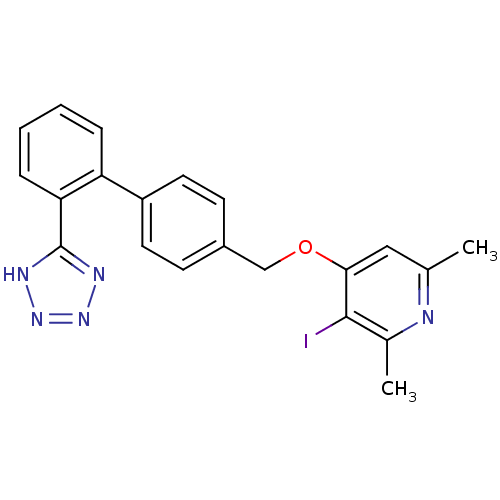 (3-Iodo-2,6-dimethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003394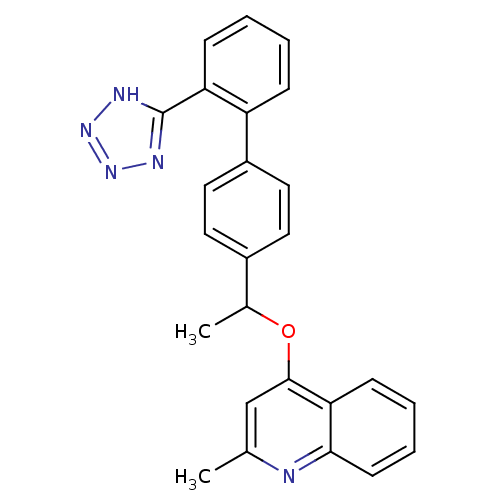 (2-Methyl-4-{1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012942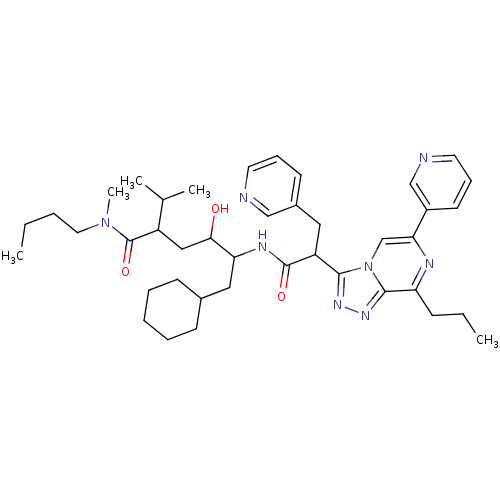 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012935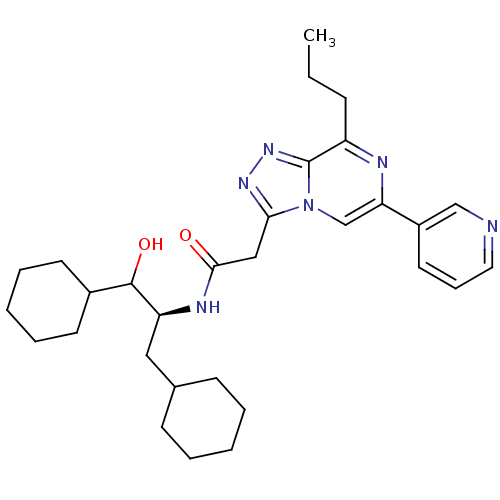 (CHEMBL431401 | N-(2-Cyclohexyl-1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Tested in vitro for their ability to inhibit human renin incubated with human angiotensinogen | J Med Chem 33: 2326-34 (1990) BindingDB Entry DOI: 10.7270/Q2H13105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047126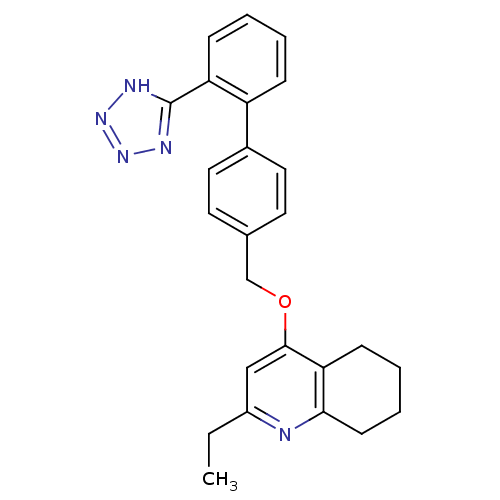 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047124 (2,6-Dimethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047121 (6-Methyl-2-propyl-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [125I]-Angiotensin binding to a guinea pig adrenal membrane preparation which corresponds to Angiotensin II receptor, type 1 | J Med Chem 36: 1245-54 (1993) BindingDB Entry DOI: 10.7270/Q2GM86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50003376 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation | J Med Chem 35: 4027-38 (1992) BindingDB Entry DOI: 10.7270/Q2G44P8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 163 total ) | Next | Last >> |