 Found 502 hits with Last Name = 'maniara' and Initial = 'w'
Found 502 hits with Last Name = 'maniara' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403
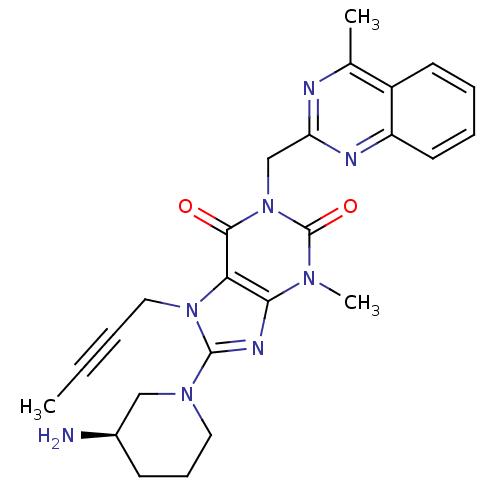
((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364171
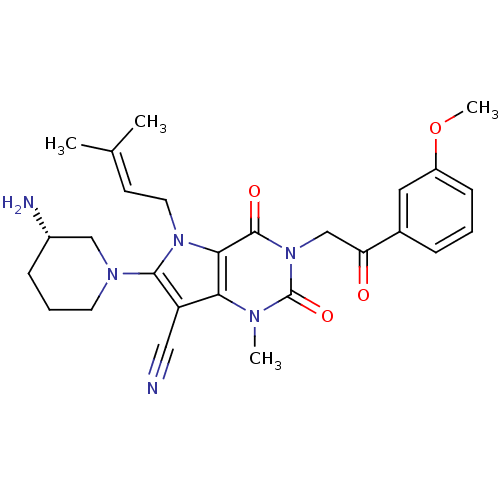
(CHEMBL1951598)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C27H32N6O4/c1-17(2)10-12-32-24-23(21(14-28)25(32)31-11-6-8-19(29)15-31)30(3)27(36)33(26(24)35)16-22(34)18-7-5-9-20(13-18)37-4/h5,7,9-10,13,19H,6,8,11-12,15-16,29H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364156

(CHEMBL1951432)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-7-20(29)15-32)31-17-33(27(25)35)16-23-21-8-4-3-6-19(21)9-11-30-23/h3-4,6,8-11,17,20H,5,7,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364158

(CHEMBL1951430)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364173
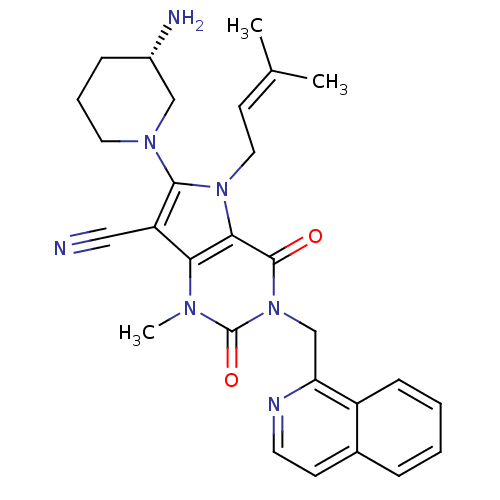
(CHEMBL1951599)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C28H31N7O2/c1-18(2)11-14-34-25-24(22(15-29)26(34)33-13-6-8-20(30)16-33)32(3)28(37)35(27(25)36)17-23-21-9-5-4-7-19(21)10-12-31-23/h4-5,7,9-12,20H,6,8,13-14,16-17,30H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364187

(CHEMBL1951416)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(22(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-23-21(15-29)20-9-6-5-8-19(20)17-32-23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364184
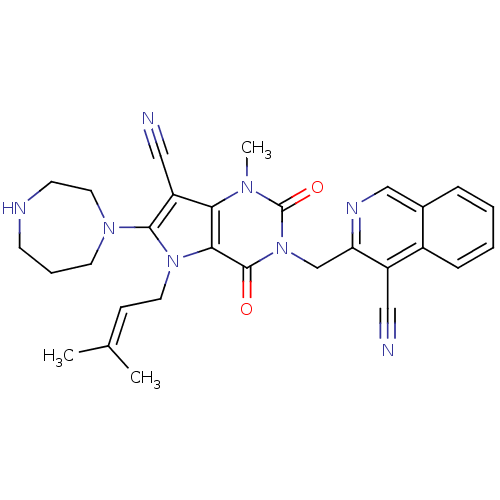
(CHEMBL1951611)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(23(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-24-22(15-30)21-8-5-4-7-20(21)17-33-24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Mus musculus) | BDBM50269112
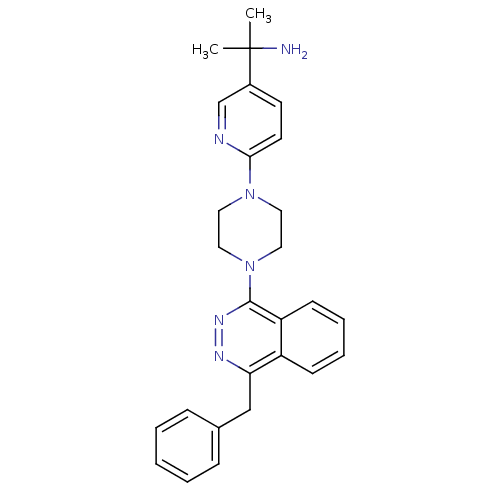
(1-{6-[4-(4-Benzylphthalazin-1-yl)piperazin-1-yl]py...)Show SMILES CC(C)(N)c1ccc(nc1)N1CCN(CC1)c1nnc(Cc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H30N6/c1-27(2,28)21-12-13-25(29-19-21)32-14-16-33(17-15-32)26-23-11-7-6-10-22(23)24(30-31-26)18-20-8-4-3-5-9-20/h3-13,19H,14-18,28H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Smoothened receptor in mouse TM3 cells transfected with pTA-8xGli-Luc reporter gene assessed as inhibition of 1 nM 3-chloro-4,7-difluor... |
J Med Chem 52: 3954-68 (2009)
Article DOI: 10.1021/jm900309j
BindingDB Entry DOI: 10.7270/Q2CN73T7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364147

(CHEMBL1951595)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C29H30N6O4/c1-32-25-23(15-30)27(33-13-7-11-21(31)17-33)34(16-19-8-4-3-5-9-19)26(25)28(37)35(29(32)38)18-24(36)20-10-6-12-22(14-20)39-2/h3-6,8-10,12,14,21H,7,11,13,16-18,31H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364167

(CHEMBL1951600)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C28H32N8O2/c1-17(2)11-13-35-25-24(21(14-29)26(35)34-12-7-8-19(30)15-34)33(4)28(38)36(27(25)37)16-23-31-18(3)20-9-5-6-10-22(20)32-23/h5-6,9-11,19H,7-8,12-13,15-16,30H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364177
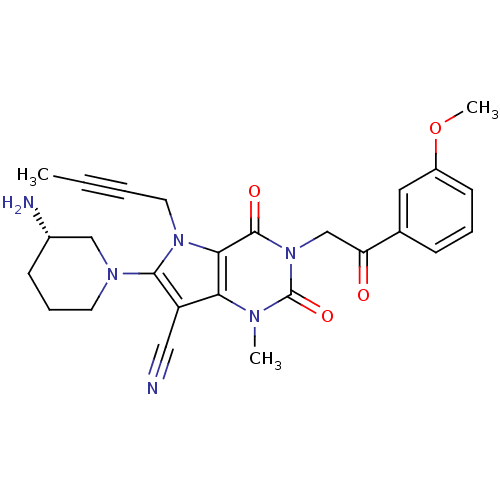
(CHEMBL1951603)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C26H28N6O4/c1-4-5-12-31-23-22(20(14-27)24(31)30-11-7-9-18(28)15-30)29(2)26(35)32(25(23)34)16-21(33)17-8-6-10-19(13-17)36-3/h6,8,10,13,18H,7,9,11-12,15-16,28H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364179

(CHEMBL1951607)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C27H32N6O4/c1-18(2)9-13-32-24-23(21(16-28)25(32)31-12-6-10-29-11-14-31)30(3)27(36)33(26(24)35)17-22(34)19-7-5-8-20(15-19)37-4/h5,7-9,15,29H,6,10-14,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364183
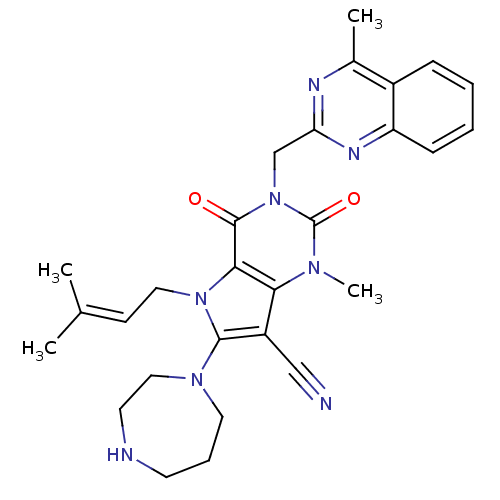
(CHEMBL1951609)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C28H32N8O2/c1-18(2)10-14-35-25-24(21(16-29)26(35)34-13-7-11-30-12-15-34)33(4)28(38)36(27(25)37)17-23-31-19(3)20-8-5-6-9-22(20)32-23/h5-6,8-10,30H,7,11-15,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364186
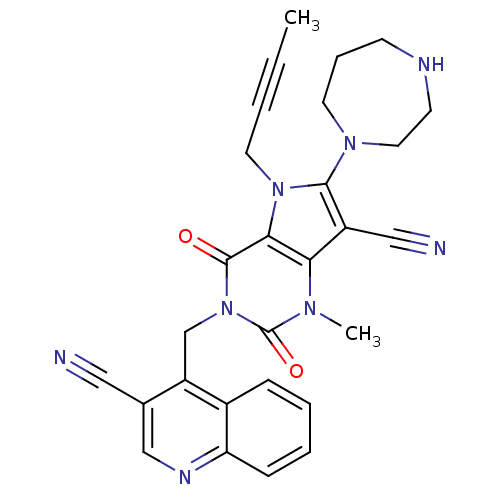
(CHEMBL1951614)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(21(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-22-19(15-29)17-32-23-9-6-5-8-20(22)23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Mus musculus) | BDBM50268313
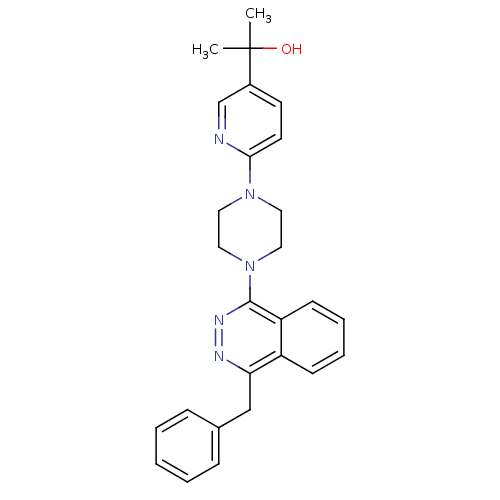
(2-{6-[4-(4-Benzylphthalazin-1-yl)piperazin-1-yl]py...)Show SMILES CC(C)(O)c1ccc(nc1)N1CCN(CC1)c1nnc(Cc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H29N5O/c1-27(2,33)21-12-13-25(28-19-21)31-14-16-32(17-15-31)26-23-11-7-6-10-22(23)24(29-30-26)18-20-8-4-3-5-9-20/h3-13,19,33H,14-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Smoothened receptor in mouse TM3 cells transfected with pTA-8xGli-Luc reporter gene assessed as inhibition of 1 nM 3-chloro-4,7-difluor... |
J Med Chem 52: 3954-68 (2009)
Article DOI: 10.1021/jm900309j
BindingDB Entry DOI: 10.7270/Q2CN73T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364155

(CHEMBL1951433)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O/c1-17(2)10-12-35-25-24(21(13-28)26(35)33-11-6-7-19(29)14-33)30-16-34(27(25)36)15-23-31-18(3)20-8-4-5-9-22(20)32-23/h4-5,8-10,16,19H,6-7,11-12,14-15,29H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364181
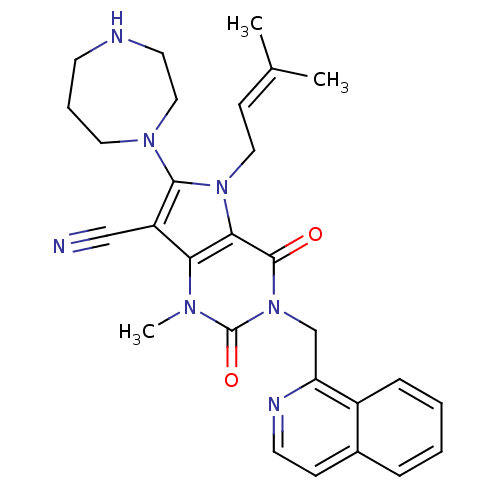
(CHEMBL1951608)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C28H31N7O2/c1-19(2)10-15-34-25-24(22(17-29)26(34)33-14-6-11-30-13-16-33)32(3)28(37)35(27(25)36)18-23-21-8-5-4-7-20(21)9-12-31-23/h4-5,7-10,12,30H,6,11,13-16,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial
(Rattus norvegicus) | BDBM50472804
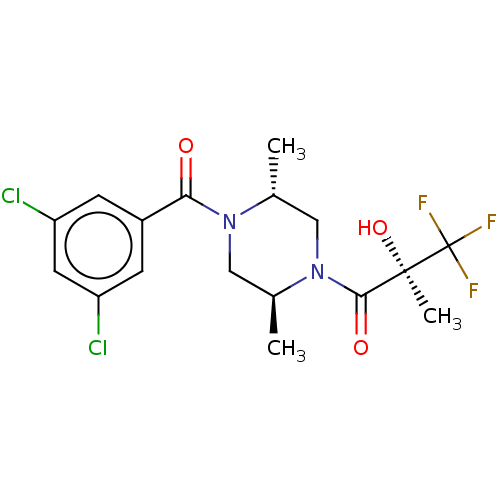
(CHEMBL83273)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)c1cc(Cl)cc(Cl)c1)C(=O)[C@@](C)(O)C(F)(F)F Show InChI InChI=1S/C17H19Cl2F3N2O3/c1-9-8-24(15(26)16(3,27)17(20,21)22)10(2)7-23(9)14(25)11-4-12(18)6-13(19)5-11/h4-6,9-10,27H,7-8H2,1-3H3/t9-,10+,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. |
J Med Chem 43: 236-49 (2000)
Article DOI: 10.1021/jm990358+
BindingDB Entry DOI: 10.7270/Q2B85BVQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364157

(CHEMBL1951431)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-6-20(29)16-32)31-17-33(27(25)35)15-19-9-11-30-23-8-4-3-7-21(19)23/h3-4,7-11,17,20H,5-6,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364159
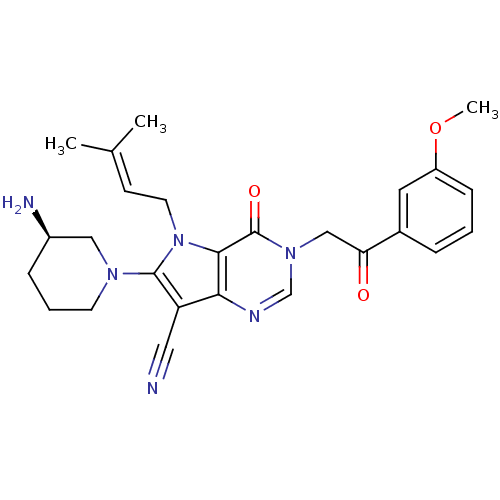
(CHEMBL1951429)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364165

(CHEMBL1951423)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C28H28N6O3/c1-37-22-11-5-9-20(13-22)24(35)17-33-18-31-25-23(14-29)27(32-12-6-10-21(30)16-32)34(26(25)28(33)36)15-19-7-3-2-4-8-19/h2-5,7-9,11,13,18,21H,6,10,12,15-17,30H2,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364170

(CHEMBL1951596)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(Cc2nccc3ccccc23)c1=O |r| Show InChI InChI=1S/C30H29N7O2/c1-34-26-24(16-31)28(35-15-7-11-22(32)18-35)36(17-20-8-3-2-4-9-20)27(26)29(38)37(30(34)39)19-25-23-12-6-5-10-21(23)13-14-33-25/h2-6,8-10,12-14,22H,7,11,15,17-19,32H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364176

(CHEMBL1951604)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H27N7O2/c1-3-4-14-33-24-23(21(15-28)25(33)32-13-7-9-19(29)16-32)31(2)27(36)34(26(24)35)17-22-20-10-6-5-8-18(20)11-12-30-22/h5-6,8,10-12,19H,7,9,13-14,16-17,29H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial
(Rattus norvegicus) | BDBM50472836
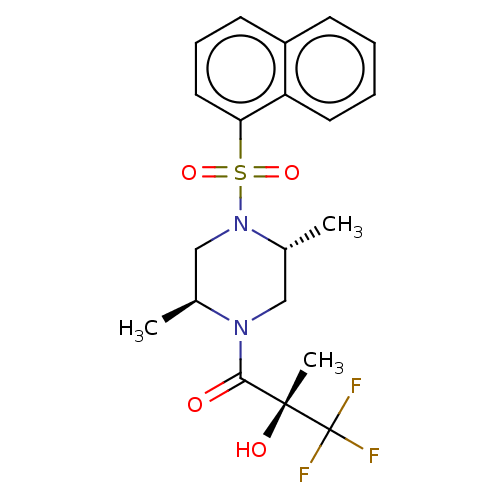
(CHEMBL311125)Show SMILES C[C@H]1CN([C@H](C)CN1C(=O)[C@@](C)(O)C(F)(F)F)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H23F3N2O4S/c1-13-12-25(14(2)11-24(13)18(26)19(3,27)20(21,22)23)30(28,29)17-10-6-8-15-7-4-5-9-16(15)17/h4-10,13-14,27H,11-12H2,1-3H3/t13-,14+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. |
J Med Chem 43: 236-49 (2000)
Article DOI: 10.1021/jm990358+
BindingDB Entry DOI: 10.7270/Q2B85BVQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364160

(CHEMBL1951428)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-8-19(28)15-31)30-17-32(26(24)34)16-22-20-9-5-4-7-18(20)10-11-29-22/h4-5,7,9-11,17,19H,6,8,12-13,15-16,28H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Mus musculus) | BDBM50268313

(2-{6-[4-(4-Benzylphthalazin-1-yl)piperazin-1-yl]py...)Show SMILES CC(C)(O)c1ccc(nc1)N1CCN(CC1)c1nnc(Cc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H29N5O/c1-27(2,33)21-12-13-25(28-19-21)31-14-16-32(17-15-31)26-23-11-7-6-10-22(23)24(29-30-26)18-20-8-4-3-5-9-20/h3-13,19,33H,14-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]3-chloro-4,7-difluoro-N-(4-methoxy-3-(pyridin-4-yl)benzyl)-N-(4-(methylamino)cyclohexyl)benzo[b]thiophene-2-carboxamide from mous... |
J Med Chem 52: 3954-68 (2009)
Article DOI: 10.1021/jm900309j
BindingDB Entry DOI: 10.7270/Q2CN73T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364169

(CHEMBL1951612)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H27N7O2/c1-3-4-15-33-24-23(21(17-28)25(33)32-14-7-11-29-13-16-32)31(2)27(36)34(26(24)35)18-22-20-9-6-5-8-19(20)10-12-30-22/h5-6,8-10,12,29H,7,11,13-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364185

(CHEMBL1949693)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(22(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-23-20(15-30)17-33-24-8-5-4-7-21(23)24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Mus musculus) | BDBM50269112

(1-{6-[4-(4-Benzylphthalazin-1-yl)piperazin-1-yl]py...)Show SMILES CC(C)(N)c1ccc(nc1)N1CCN(CC1)c1nnc(Cc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H30N6/c1-27(2,28)21-12-13-25(29-19-21)32-14-16-33(17-15-32)26-23-11-7-6-10-22(23)24(30-31-26)18-20-8-4-3-5-9-20/h3-13,19H,14-18,28H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]3-chloro-4,7-difluoro-N-(4-methoxy-3-(pyridin-4-yl)benzyl)-N-(4-(methylamino)cyclohexyl)benzo[b]thiophene-2-carboxamide from mous... |
J Med Chem 52: 3954-68 (2009)
Article DOI: 10.1021/jm900309j
BindingDB Entry DOI: 10.7270/Q2CN73T7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364150

(CHEMBL1951438)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C26H25N7O/c1-2-3-14-33-24-23(21(16-27)25(33)31-13-6-10-28-12-15-31)30-18-32(26(24)34)17-22-20-8-5-4-7-19(20)9-11-29-22/h4-5,7-9,11,18,28H,6,10,12-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364152

(CHEMBL1951437)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H29N7O/c1-19(2)9-14-34-25-24(22(16-28)26(34)32-13-5-10-29-12-15-32)31-18-33(27(25)35)17-23-21-7-4-3-6-20(21)8-11-30-23/h3-4,6-9,11,18,29H,5,10,12-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial
(Homo sapiens (Human)) | BDBM50472713
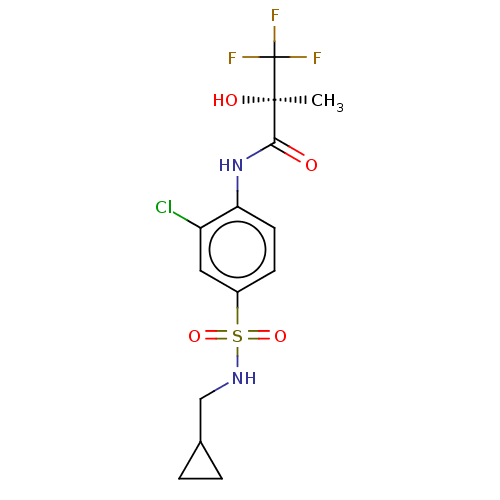
(CHEMBL73068)Show SMILES C[C@@](O)(C(=O)Nc1ccc(cc1Cl)S(=O)(=O)NCC1CC1)C(F)(F)F Show InChI InChI=1S/C14H16ClF3N2O4S/c1-13(22,14(16,17)18)12(21)20-11-5-4-9(6-10(11)15)25(23,24)19-7-8-2-3-8/h4-6,8,19,22H,2-3,7H2,1H3,(H,20,21)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of porcine pyruvate dehydrogenase kinase (PDHK)in a primary enzymatic assay |
J Med Chem 43: 2248-57 (2000)
Article DOI: 10.1021/jm0000923
BindingDB Entry DOI: 10.7270/Q2C82D1Z |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364168
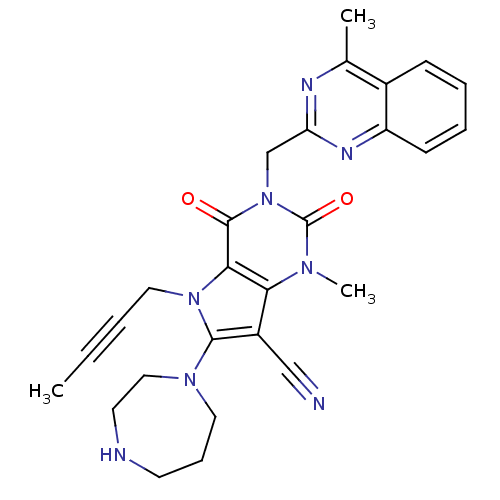
(CHEMBL1951613)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C27H28N8O2/c1-4-5-14-34-24-23(20(16-28)25(34)33-13-8-11-29-12-15-33)32(3)27(37)35(26(24)36)17-22-30-18(2)19-9-6-7-10-21(19)31-22/h6-7,9-10,29H,8,11-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364154
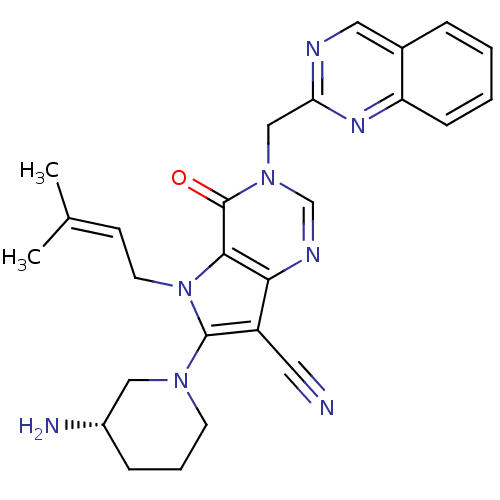
(CHEMBL1951434)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ncc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C26H28N8O/c1-17(2)9-11-34-24-23(20(12-27)25(34)32-10-5-7-19(28)14-32)30-16-33(26(24)35)15-22-29-13-18-6-3-4-8-21(18)31-22/h3-4,6,8-9,13,16,19H,5,7,10-11,14-15,28H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50268313

(2-{6-[4-(4-Benzylphthalazin-1-yl)piperazin-1-yl]py...)Show SMILES CC(C)(O)c1ccc(nc1)N1CCN(CC1)c1nnc(Cc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H29N5O/c1-27(2,33)21-12-13-25(28-19-21)31-14-16-32(17-15-31)26-23-11-7-6-10-22(23)24(29-30-26)18-20-8-4-3-5-9-20/h3-13,19,33H,14-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]3-chloro-4,7-difluoro-N-(4-methoxy-3-(pyridin-4-yl)benzyl)-N-(4-(methylamino)cyclohexyl)benzo[b]thiophene-2-carboxamide from huma... |
J Med Chem 52: 3954-68 (2009)
Article DOI: 10.1021/jm900309j
BindingDB Entry DOI: 10.7270/Q2CN73T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial
(Homo sapiens (Human)) | BDBM50472668

(CHEMBL74582)Show SMILES C[C@@](O)(C(=O)Nc1ccc(cc1Cl)S(=O)(=O)NCC=C)C(F)(F)F Show InChI InChI=1S/C13H14ClF3N2O4S/c1-3-6-18-24(22,23)8-4-5-10(9(14)7-8)19-11(20)12(2,21)13(15,16)17/h3-5,7,18,21H,1,6H2,2H3,(H,19,20)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of porcine pyruvate dehydrogenase kinase (PDHK)in a primary enzymatic assay |
J Med Chem 43: 2248-57 (2000)
Article DOI: 10.1021/jm0000923
BindingDB Entry DOI: 10.7270/Q2C82D1Z |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial
(Homo sapiens (Human)) | BDBM50472648
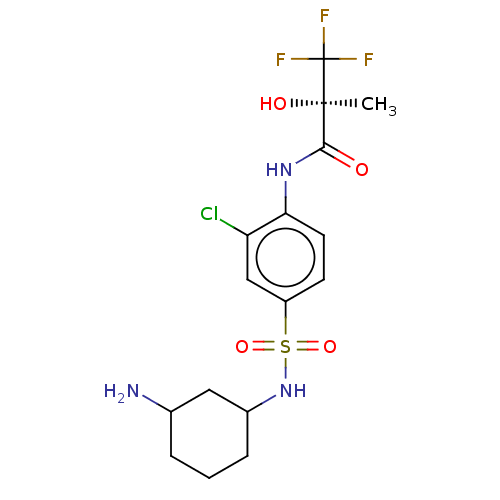
(CHEMBL73204)Show SMILES C[C@@](O)(C(=O)Nc1ccc(cc1Cl)S(=O)(=O)NC1CCCC(N)C1)C(F)(F)F Show InChI InChI=1S/C16H21ClF3N3O4S/c1-15(25,16(18,19)20)14(24)22-13-6-5-11(8-12(13)17)28(26,27)23-10-4-2-3-9(21)7-10/h5-6,8-10,23,25H,2-4,7,21H2,1H3,(H,22,24)/t9?,10?,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of porcine pyruvate dehydrogenase kinase (PDHK)in a primary enzymatic assay |
J Med Chem 43: 2248-57 (2000)
Article DOI: 10.1021/jm0000923
BindingDB Entry DOI: 10.7270/Q2C82D1Z |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364153
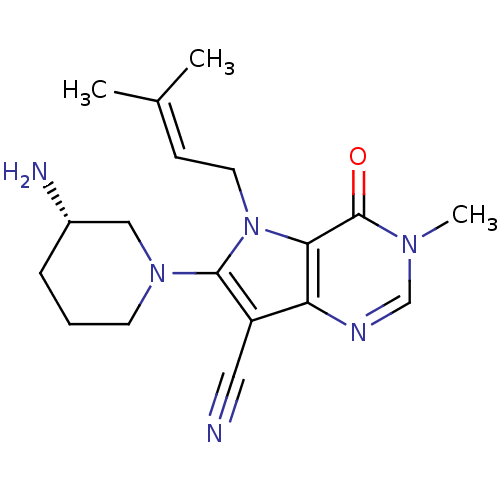
(CHEMBL1951435)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6])c(=O)c12 |r| Show InChI InChI=1S/C18H24N6O/c1-12(2)6-8-24-16-15(21-11-22(3)18(16)25)14(9-19)17(24)23-7-4-5-13(20)10-23/h6,11,13H,4-5,7-8,10,20H2,1-3H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364174

(CHEMBL1951601)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cnc4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O2/c1-17(2)10-12-34-24-23(20(13-28)25(34)33-11-6-7-18(29)15-33)32(3)27(37)35(26(24)36)16-19-14-30-21-8-4-5-9-22(21)31-19/h4-5,8-10,14,18H,6-7,11-12,15-16,29H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
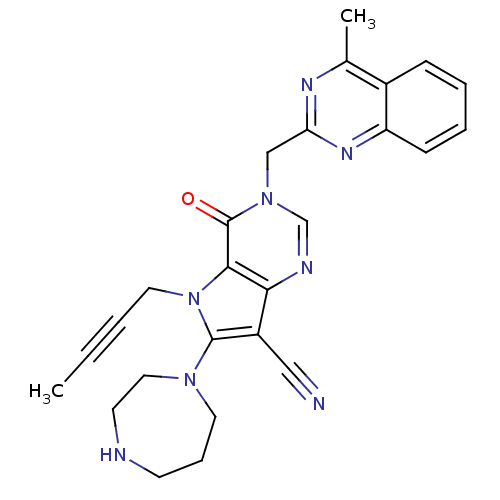
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364148
(CHEMBL1951439)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H26N8O/c1-3-4-13-34-24-23(20(15-27)25(34)32-12-7-10-28-11-14-32)29-17-33(26(24)35)16-22-30-18(2)19-8-5-6-9-21(19)31-22/h5-6,8-9,17,28H,7,10-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial
(Rattus norvegicus) | BDBM50472839

(CHEMBL3349325)Show SMILES C[C@@H]1CN([C@@H](C)CN1\C(Oc1ccccc1)=N\C#N)C(=O)[C@@](C)(O)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3/c1-12-10-25(16(23-11-22)28-14-7-5-4-6-8-14)13(2)9-24(12)15(26)17(3,27)18(19,20)21/h4-8,12-13,27H,9-10H2,1-3H3/b23-16-/t12-,13+,17+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. |
J Med Chem 43: 236-49 (2000)
Article DOI: 10.1021/jm990358+
BindingDB Entry DOI: 10.7270/Q2B85BVQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364172

(CHEMBL1951597)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H24N6O2/c1-24-17-16(11-22)19(26-10-6-9-15(23)13-26)27(12-14-7-4-3-5-8-14)18(17)20(28)25(2)21(24)29/h3-5,7-8,15H,6,9-10,12-13,23H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364182

(CHEMBL1951610)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3cn4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C26H30N8O2/c1-18(2)8-13-33-23-22(20(15-27)24(33)31-12-6-9-28-10-14-31)30(3)26(36)34(25(23)35)17-19-16-32-11-5-4-7-21(32)29-19/h4-5,7-8,11,16,28H,6,9-10,12-14,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364161
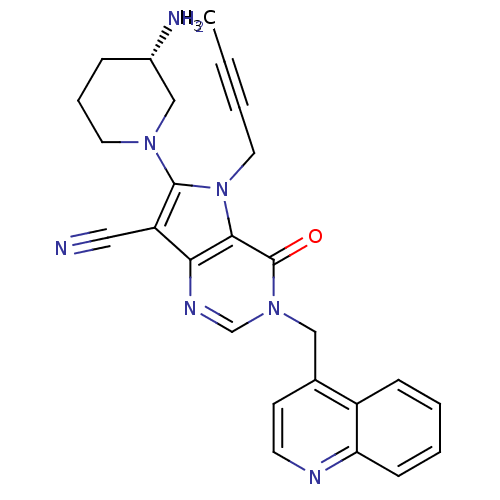
(CHEMBL1951427)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-7-19(28)16-31)30-17-32(26(24)34)15-18-10-11-29-22-9-5-4-8-20(18)22/h4-5,8-11,17,19H,6-7,12-13,15-16,28H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Mus musculus) | BDBM50268314
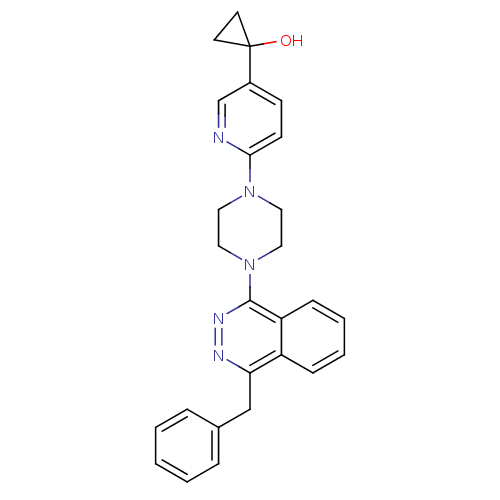
(1-{6-[4-(4-Benzylphthalazin-1-yl)piperazin-1-yl]py...)Show SMILES OC1(CC1)c1ccc(nc1)N1CCN(CC1)c1nnc(Cc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H27N5O/c33-27(12-13-27)21-10-11-25(28-19-21)31-14-16-32(17-15-31)26-23-9-5-4-8-22(23)24(29-30-26)18-20-6-2-1-3-7-20/h1-11,19,33H,12-18H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]3-chloro-4,7-difluoro-N-(4-methoxy-3-(pyridin-4-yl)benzyl)-N-(4-(methylamino)cyclohexyl)benzo[b]thiophene-2-carboxamide from mous... |
J Med Chem 52: 3954-68 (2009)
Article DOI: 10.1021/jm900309j
BindingDB Entry DOI: 10.7270/Q2CN73T7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364145

(CHEMBL1951420)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C25H28N6O3/c1-17(2)7-10-31-23-22(20(14-26)24(31)29-11-8-27-9-12-29)28-16-30(25(23)33)15-21(32)18-5-4-6-19(13-18)34-3/h4-7,13,16,27H,8-12,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial
(Homo sapiens (Human)) | BDBM50472670
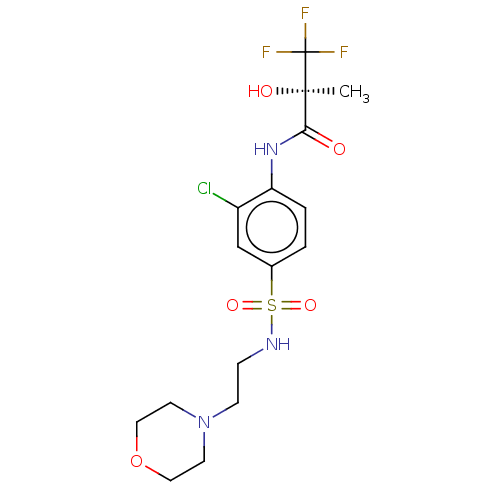
(CHEMBL75821)Show SMILES C[C@@](O)(C(=O)Nc1ccc(cc1Cl)S(=O)(=O)NCCN1CCOCC1)C(F)(F)F Show InChI InChI=1S/C16H21ClF3N3O5S/c1-15(25,16(18,19)20)14(24)22-13-3-2-11(10-12(13)17)29(26,27)21-4-5-23-6-8-28-9-7-23/h2-3,10,21,25H,4-9H2,1H3,(H,22,24)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of porcine pyruvate dehydrogenase kinase (PDHK)in a primary enzymatic assay |
J Med Chem 43: 2248-57 (2000)
Article DOI: 10.1021/jm0000923
BindingDB Entry DOI: 10.7270/Q2C82D1Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1,/2,/3,/4, mitochondrial
(Homo sapiens (Human)) | BDBM50472647

(CHEMBL73099)Show SMILES CC(C)CNS(=O)(=O)c1ccc(NC(=O)[C@@](C)(O)C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C14H18ClF3N2O4S/c1-8(2)7-19-25(23,24)9-4-5-11(10(15)6-9)20-12(21)13(3,22)14(16,17)18/h4-6,8,19,22H,7H2,1-3H3,(H,20,21)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of porcine pyruvate dehydrogenase kinase (PDHK)in a primary enzymatic assay |
J Med Chem 43: 2248-57 (2000)
Article DOI: 10.1021/jm0000923
BindingDB Entry DOI: 10.7270/Q2C82D1Z |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial
(Rattus norvegicus) | BDBM50472841

(CHEMBL443012)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)C1CCCCC1)C(=O)[C@@](C)(O)C(F)(F)F Show InChI InChI=1S/C17H27F3N2O3/c1-11-10-22(15(24)16(3,25)17(18,19)20)12(2)9-21(11)14(23)13-7-5-4-6-8-13/h11-13,25H,4-10H2,1-3H3/t11-,12+,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity tested against Pyruvate Dehydrogenase Kinase (PDHK) receptor from rats. |
J Med Chem 43: 236-49 (2000)
Article DOI: 10.1021/jm990358+
BindingDB Entry DOI: 10.7270/Q2B85BVQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data