

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435812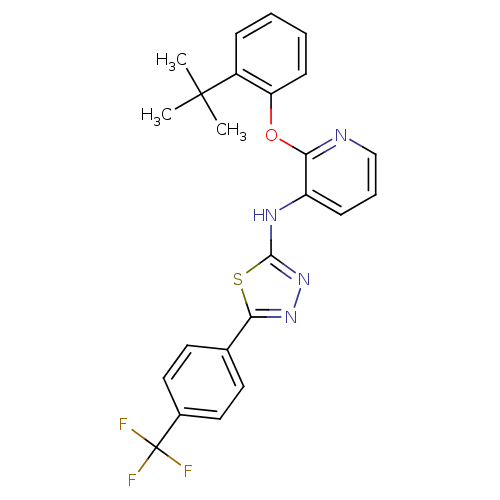 (CHEMBL2393201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14142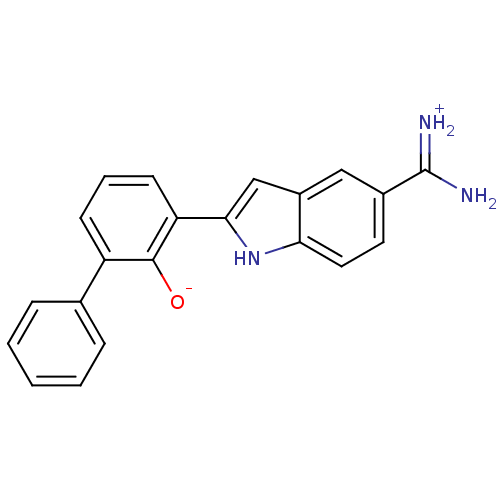 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115874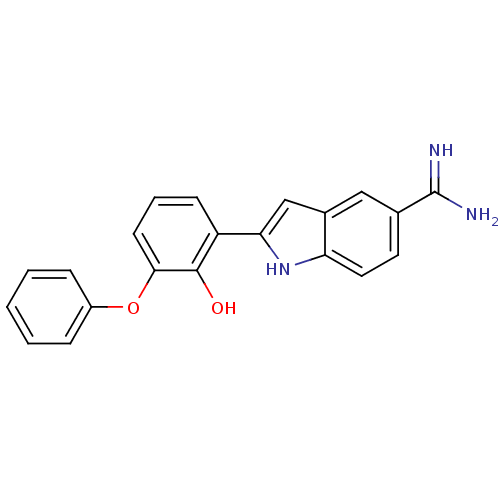 (2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14152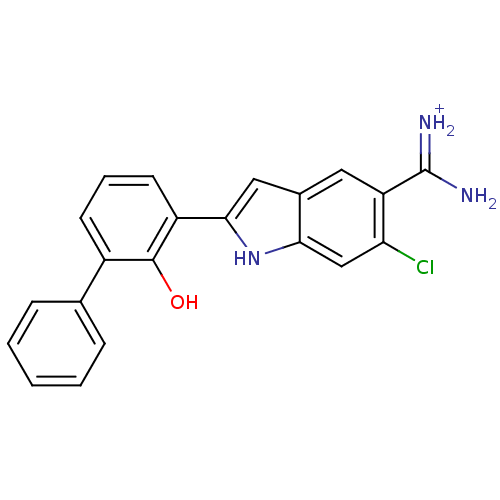 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435813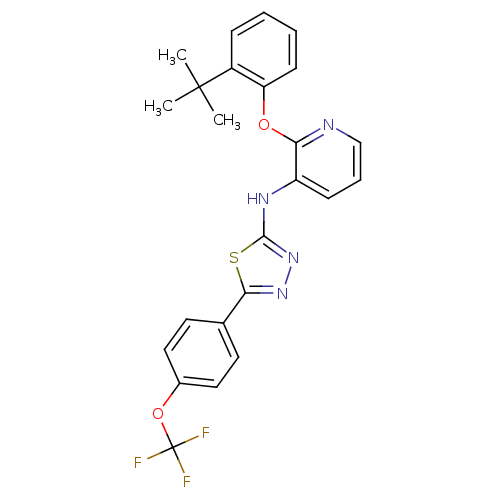 (CHEMBL2393200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444488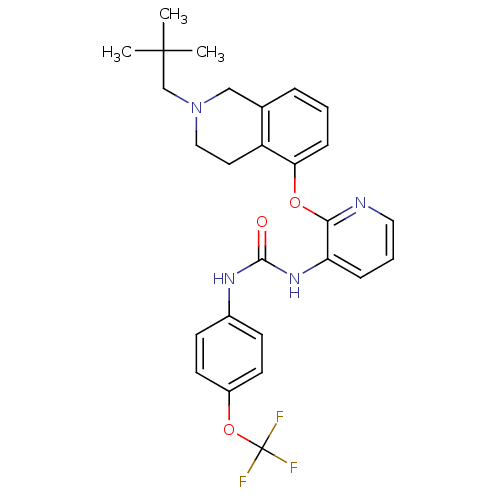 (CHEMBL3092613) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115868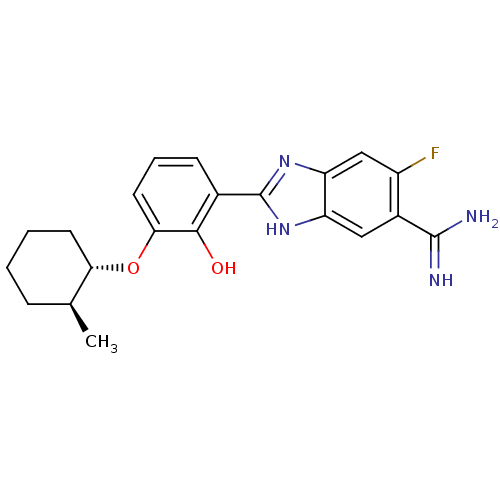 (2-{5-[AMINO(IMINIO)METHYL]-6-FLUORO-1H-BENZIMIDAZO...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14149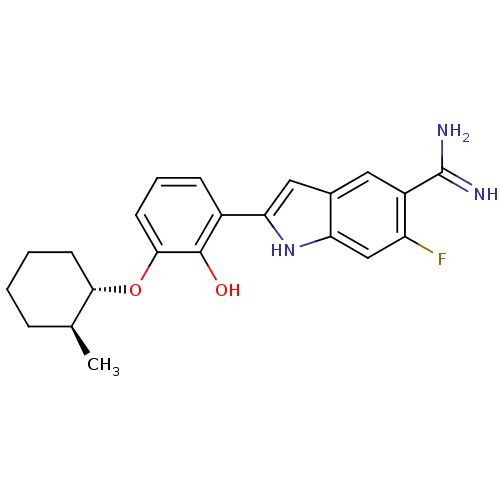 (6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435825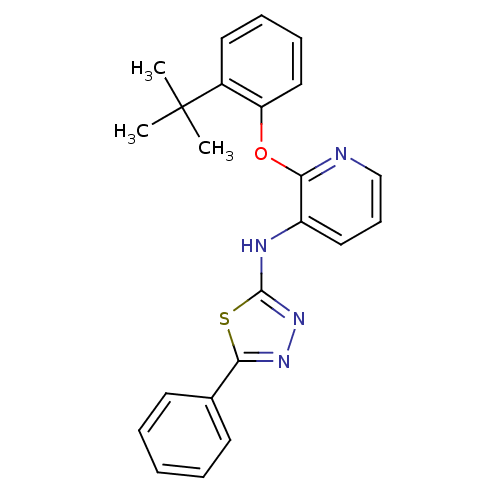 (CHEMBL2393213) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435828 (CHEMBL2390979) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435829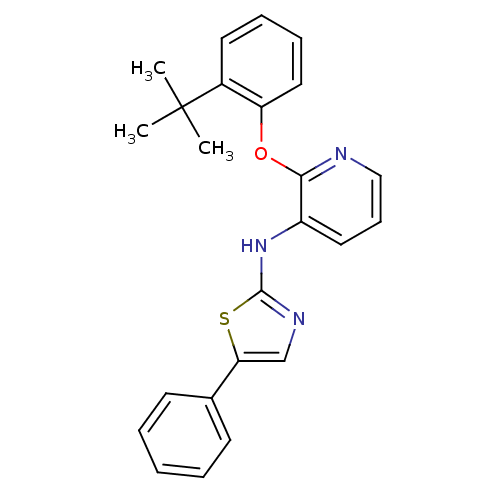 (CHEMBL2393211) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444487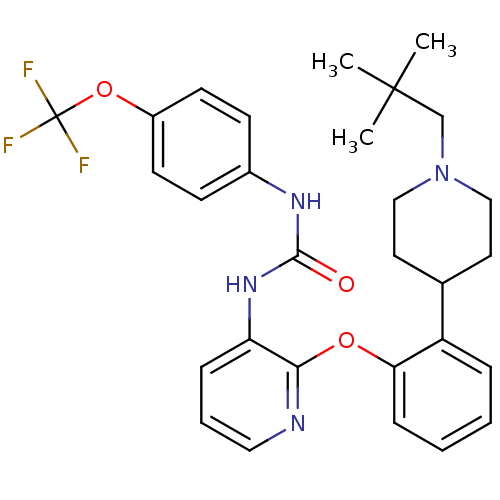 (CHEMBL3092631) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435807 (CHEMBL2393206) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435819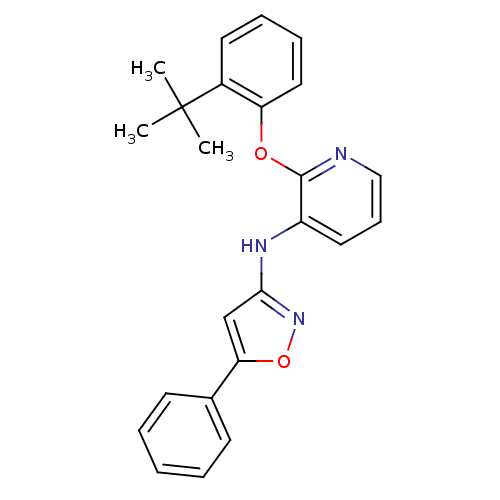 (CHEMBL2393219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14150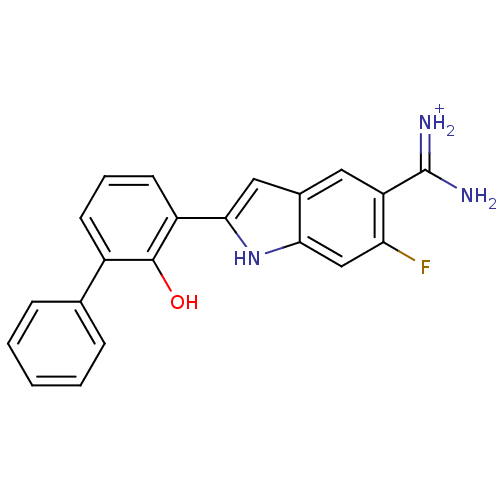 (APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435806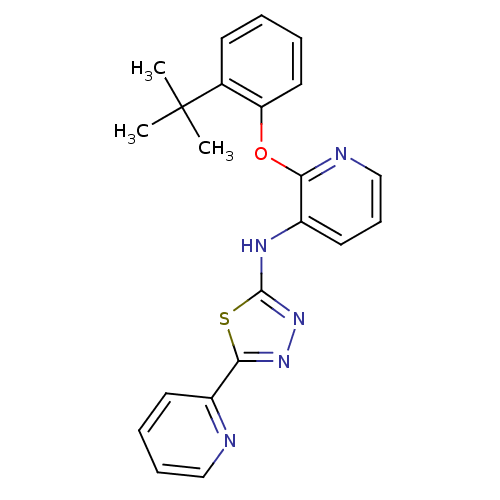 (CHEMBL2393207) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435818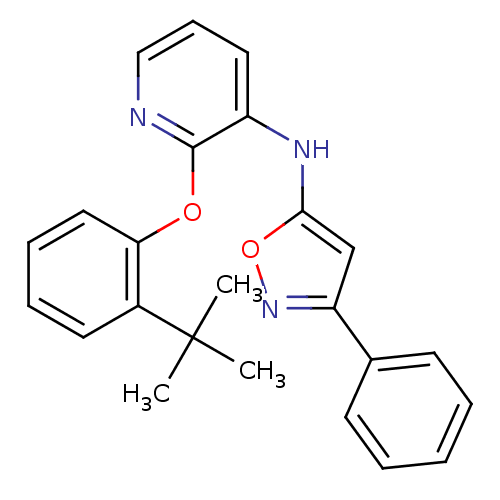 (CHEMBL2393220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14149 (6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435824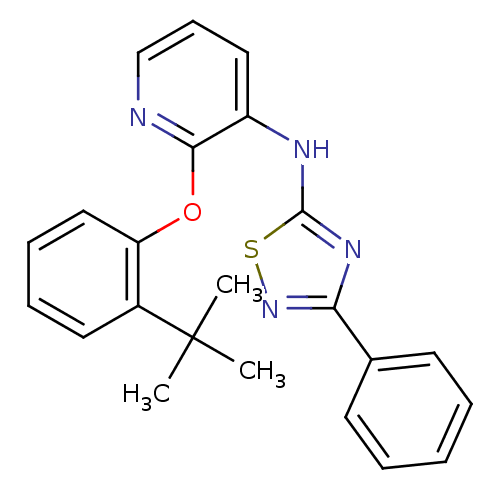 (CHEMBL2393214) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14145 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435811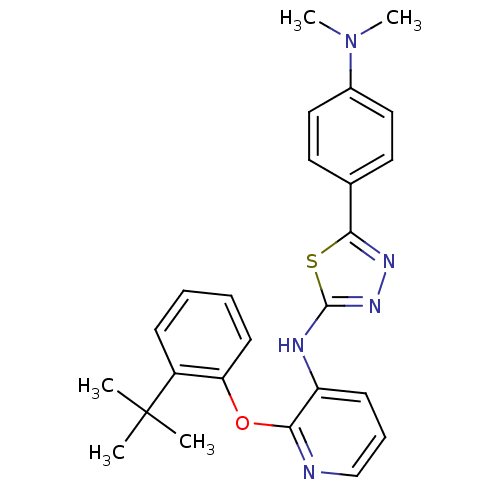 (CHEMBL2393202) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444486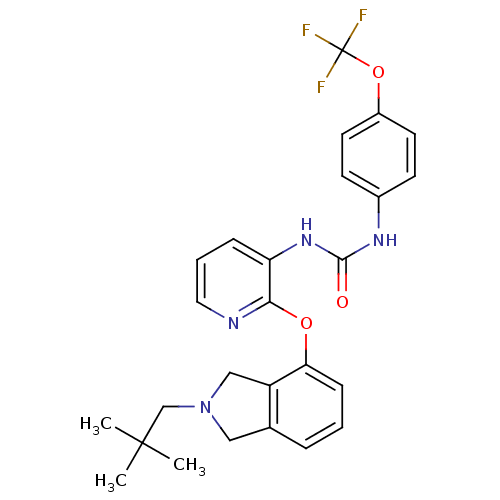 (CHEMBL3091475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115869 (6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-cyclohexylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115871 (6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-cyclopentylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM14142 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inihibtion of Human Serine Protease tissue type Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102767 (2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50115874 (2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of tissue-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101866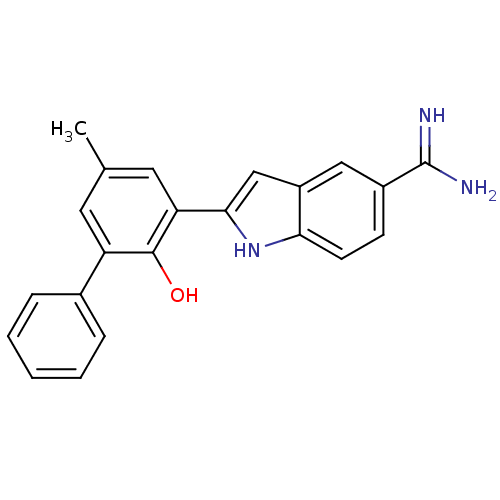 (2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435827 (CHEMBL2393210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102786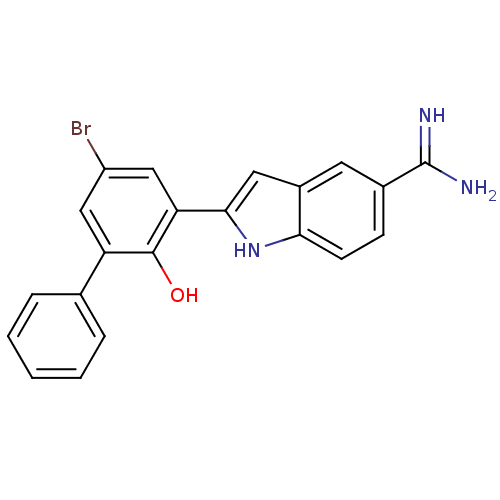 (2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102767 (2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435817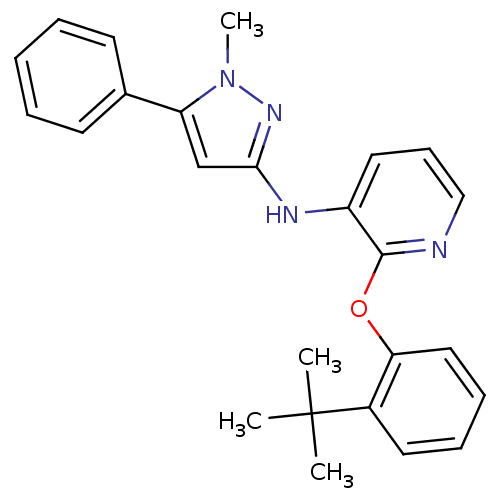 (CHEMBL2393196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50101873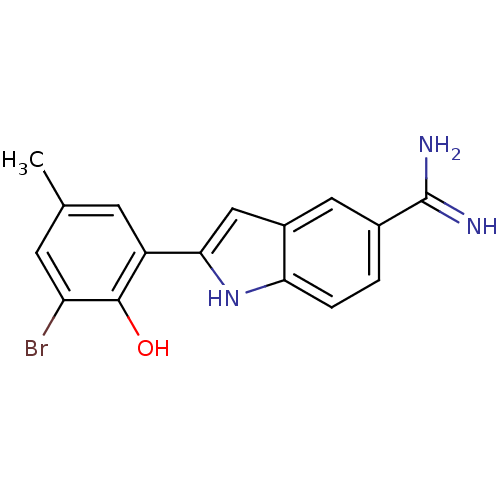 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444485 (CHEMBL3092620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435810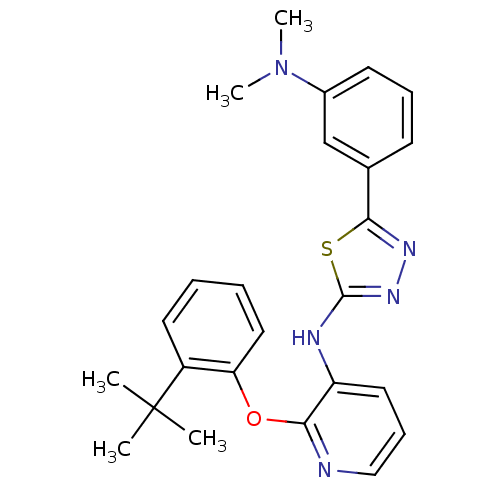 (CHEMBL2393203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102778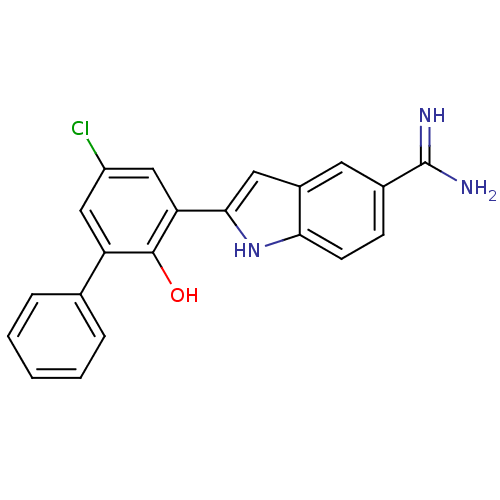 (2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50101873 (2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444484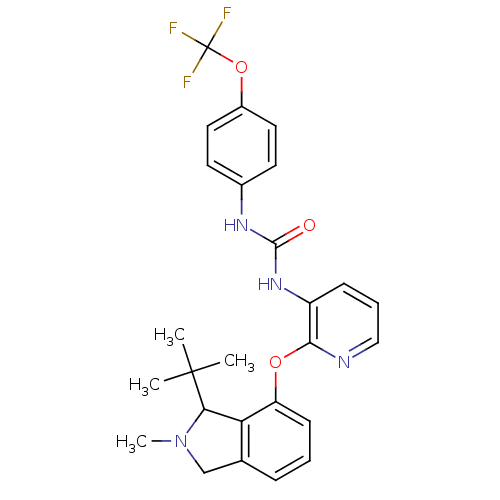 (CHEMBL3092629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435826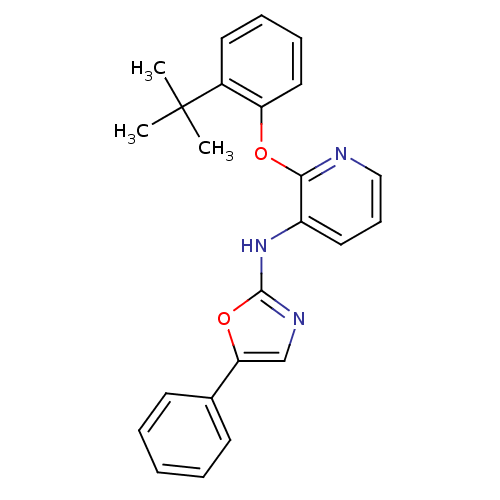 (CHEMBL2393212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444483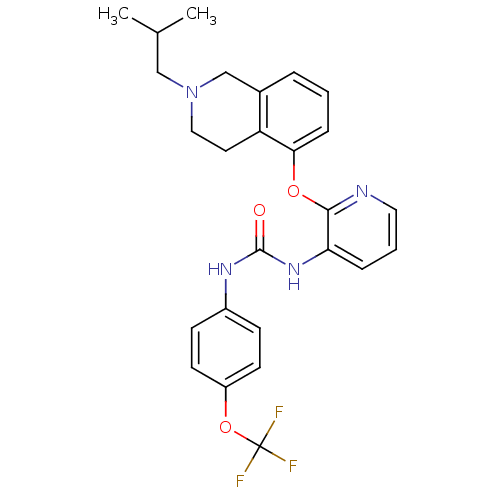 (CHEMBL3092614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50444482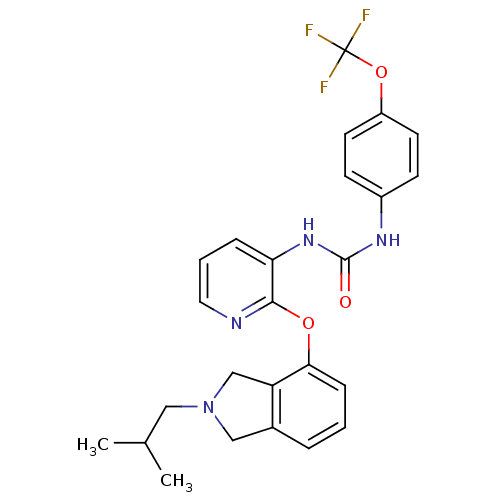 (CHEMBL3092628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 23: 6825-8 (2013) Article DOI: 10.1016/j.bmcl.2013.10.009 BindingDB Entry DOI: 10.7270/Q2TQ630T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14146 (APC-9008 | {amino[2-(3,5-dichloro-2-hydroxyphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435822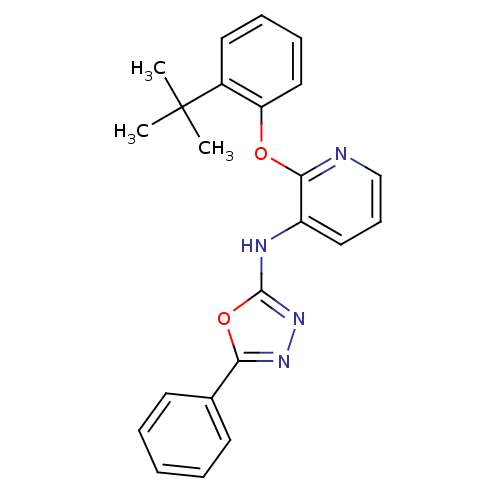 (CHEMBL2393216) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor expressed in HEK293 cells after 1 hr by scintillation counting analysis | Bioorg Med Chem Lett 23: 3519-22 (2013) Article DOI: 10.1016/j.bmcl.2013.04.041 BindingDB Entry DOI: 10.7270/Q28S4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease Trypsin. | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14156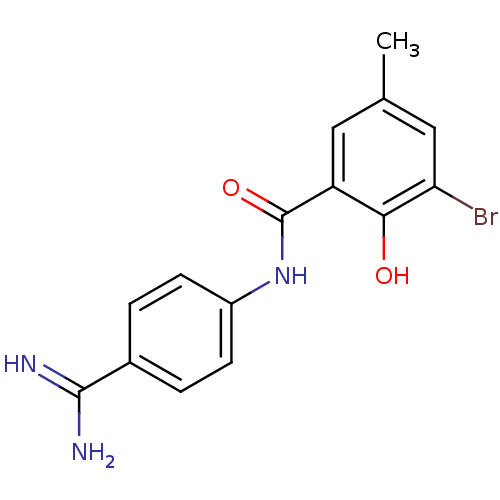 (3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2176 total ) | Next | Last >> |