

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304826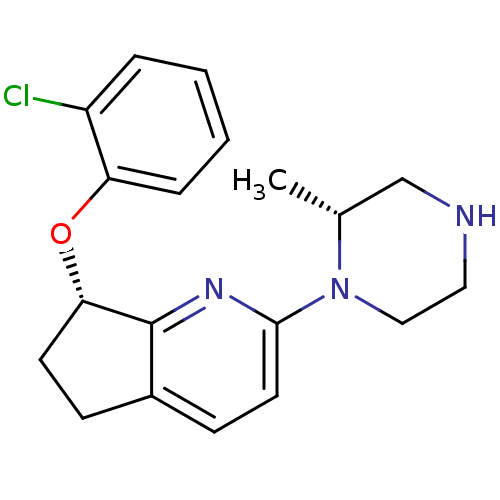 ((S)-7-(2-chlorophenoxy)-2-((R)-2-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human 5HT2C receptor expressed in mouse 3T3 cells by scintillation counting | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304805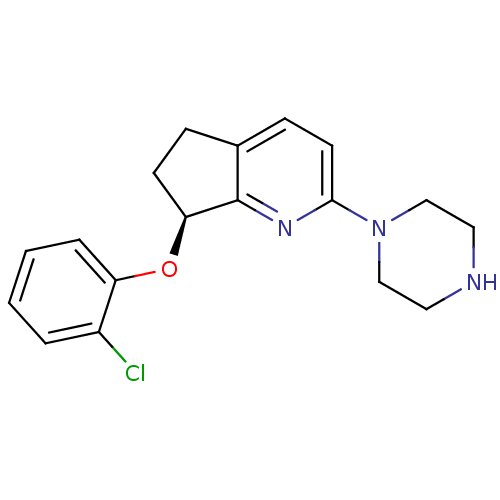 ((S)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304802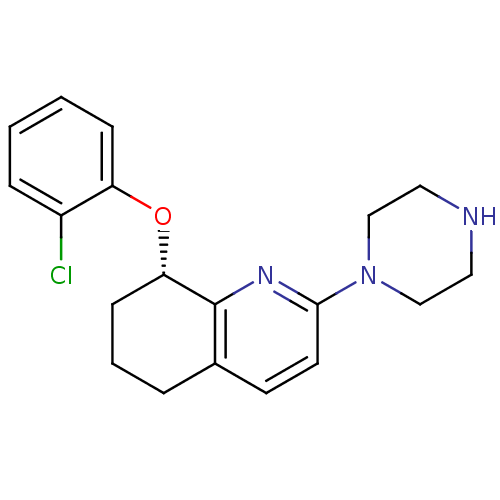 ((S)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304804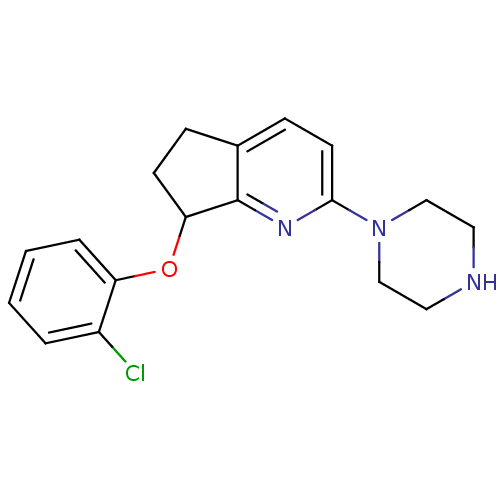 (7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304805 ((S)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304808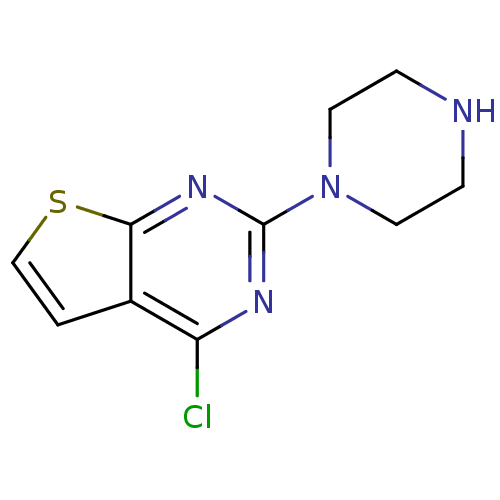 (4-chloro-2-(piperazin-1-yl)thieno[2,3-d]pyrimidine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342721 (CHEMBL1771259 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123737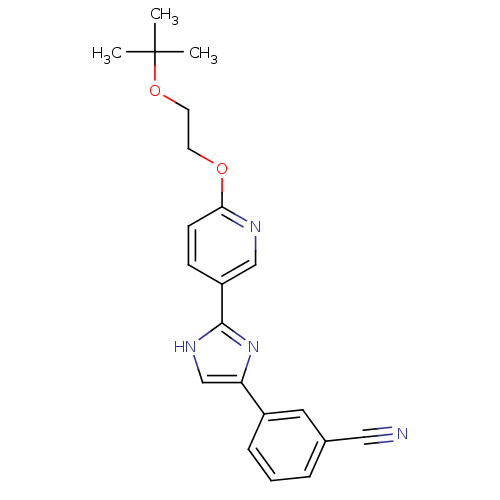 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the human Neuropeptide Y receptor type 5 was determined using [125I]- [PYY] as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304807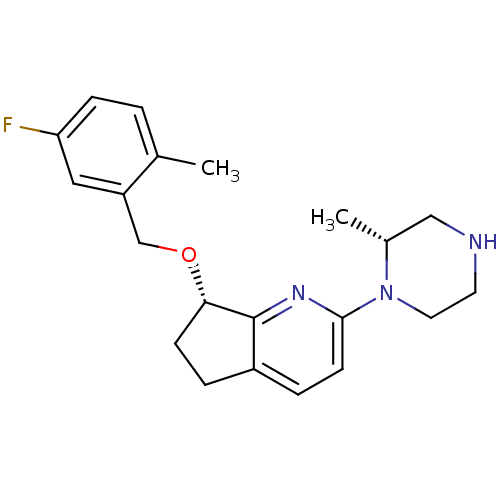 ((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304802 ((S)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133761 (3-[5-(3-Trifluoromethyl-phenyl)-1H-imidazol-2-yl]-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50097905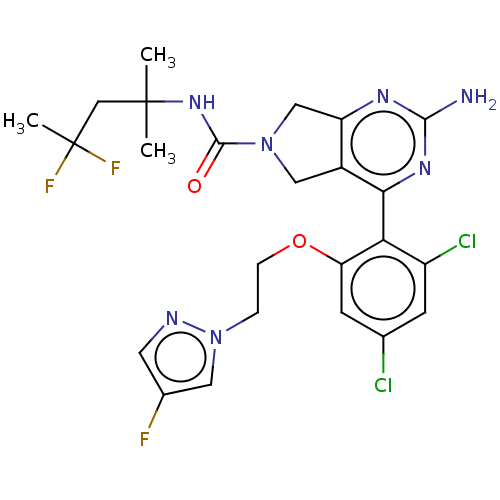 (CHEMBL3589960) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Inhibition of human recombinant HSP90 by scintillation proximity competition binding assay | J Med Chem 58: 5691-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00201 BindingDB Entry DOI: 10.7270/Q2ZP47W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50123737 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the rat Neuropeptide Y receptor type 5 was determined using [125I]- [Leu31,Pro34]PYY as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304803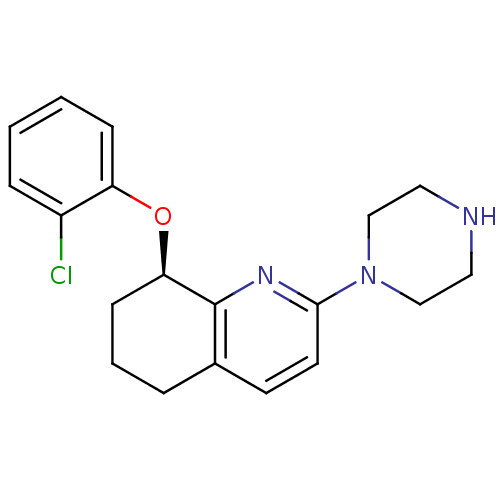 ((R)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50097903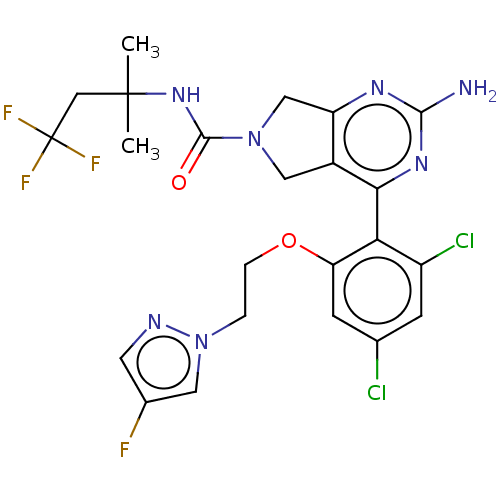 (CHEMBL3589958) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Inhibition of human recombinant HSP90 by scintillation proximity competition binding assay | J Med Chem 58: 5691-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00201 BindingDB Entry DOI: 10.7270/Q2ZP47W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304827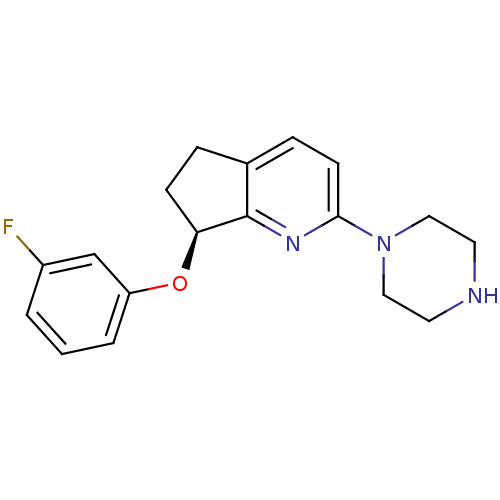 ((S)-7-(3-fluorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human 5HT2C receptor expressed in mouse 3T3 cells by scintillation counting | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342728 (CHEMBL1771260 | N1-(3-(2,6-dichloro-4-ethoxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50097907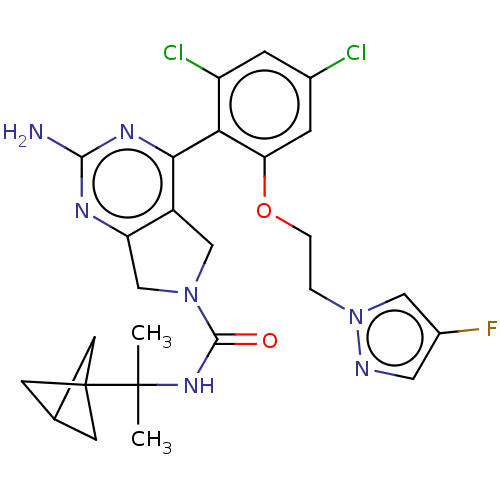 (CHEMBL3589962) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Inhibition of human recombinant HSP90 by scintillation proximity competition binding assay | J Med Chem 58: 5691-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00201 BindingDB Entry DOI: 10.7270/Q2ZP47W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (RAT) | BDBM50342721 (CHEMBL1771259 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125]sauvagin from rat recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133775 (4-[5-(3,4-Dichloro-phenyl)-1H-imidazol-2-yl]-pyrid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304804 (7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133780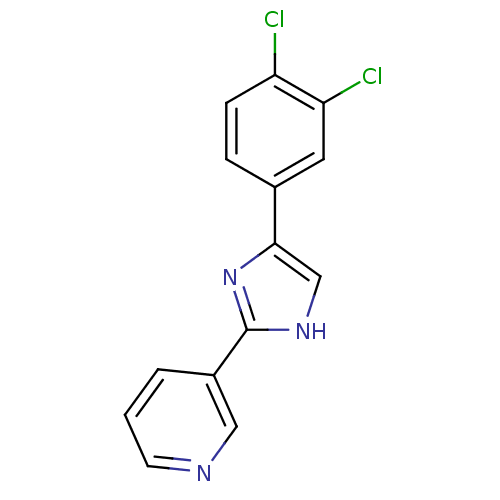 (3-[5-(3,4-Dichloro-phenyl)-1H-imidazol-2-yl]-pyrid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304809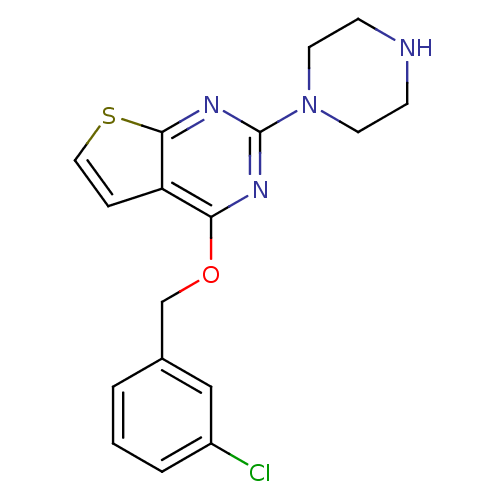 (4-(3-chlorobenzyloxy)-2-(piperazin-1-yl)thieno[2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304807 ((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133762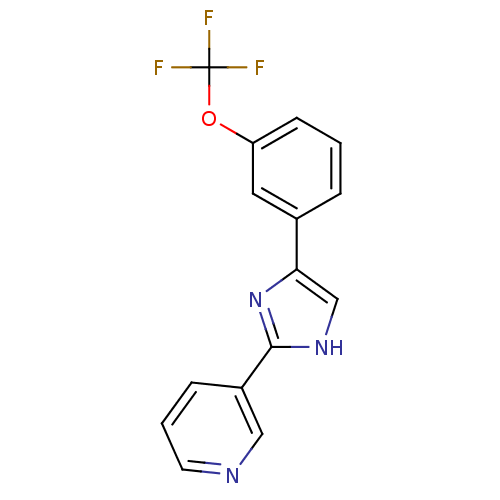 (3-[5-(3-Trifluoromethoxy-phenyl)-1H-imidazol-2-yl]...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342726 (CHEMBL1771262 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342724 (CHEMBL1771264 | N1-(3-(2,6-dichloro-4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50097904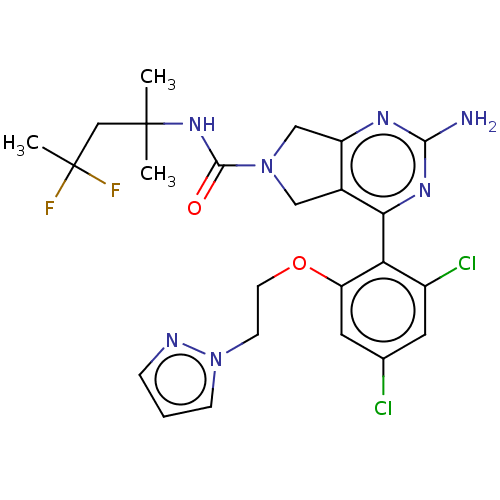 (CHEMBL3589959) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Inhibition of human recombinant HSP90 by scintillation proximity competition binding assay | J Med Chem 58: 5691-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00201 BindingDB Entry DOI: 10.7270/Q2ZP47W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342729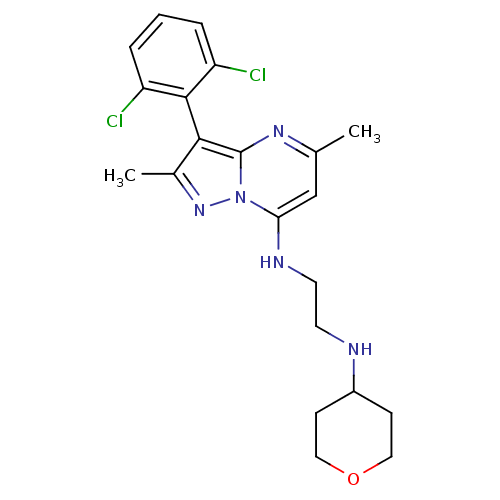 (CHEMBL1771258 | N1-(3-(2,6-dichlorophenyl)-2,5-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304801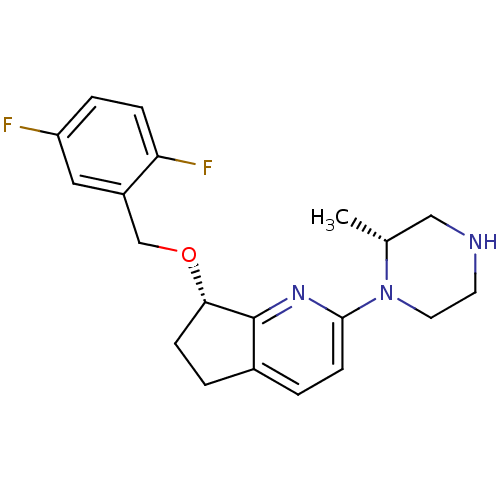 ((S)-7-(2,5-difluorobenzyloxy)-2-((R)-2-methylpiper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50097906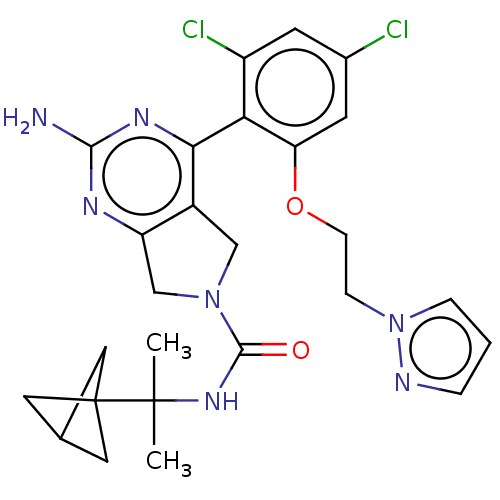 (CHEMBL3589961) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Inhibition of human recombinant HSP90 by scintillation proximity competition binding assay | J Med Chem 58: 5691-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00201 BindingDB Entry DOI: 10.7270/Q2ZP47W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50304807 ((S)-7-(5-fluoro-2-methylbenzyloxy)-2-((R)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human 5HT2B receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342727 (CHEMBL1771261 | N1-(3-(2,6-dichloro-4-propoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133779 (3-[5-(3-Chloro-phenyl)-1H-imidazol-2-yl]-pyridine ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha/90-beta (Homo sapiens (Human)) | BDBM50097902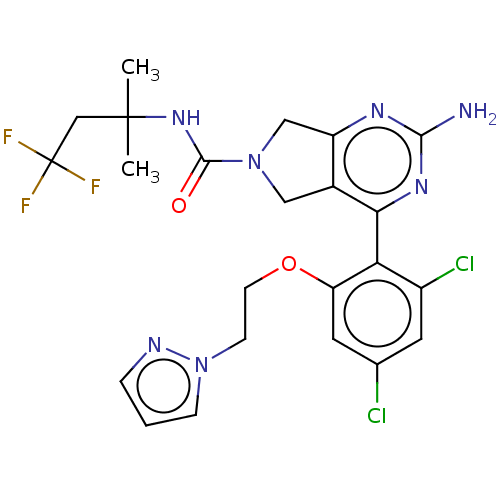 (CHEMBL3589957) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Inhibition of human recombinant HSP90 by scintillation proximity competition binding assay | J Med Chem 58: 5691-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00201 BindingDB Entry DOI: 10.7270/Q2ZP47W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218165 ((R)-1-(pyridin-3-yl)-2-(2-(4-(thiazol-4-yl)phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218166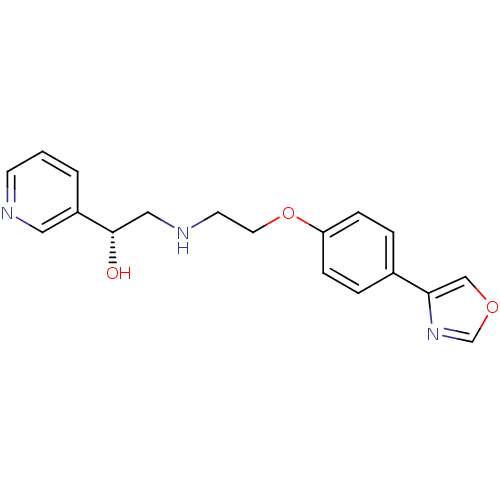 ((R)-2-(2-(4-(oxazol-4-yl)phenoxy)ethylamino)-1-(py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304803 ((R)-8-(2-chlorophenoxy)-2-(piperazin-1-yl)-5,6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50016970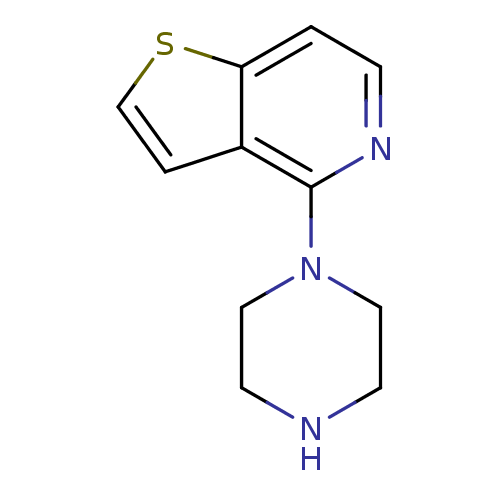 (4-Piperazin-1-yl-thieno[3,2-c]pyridine; hydrochlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133769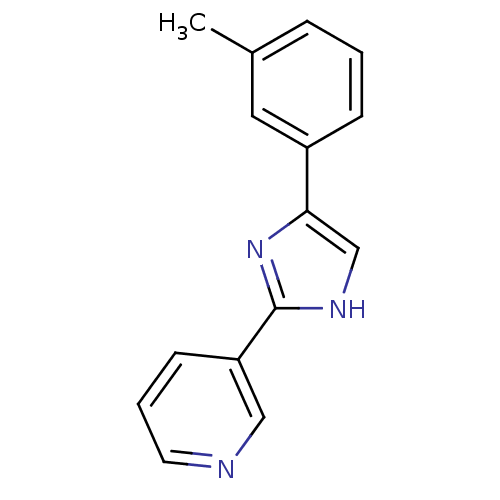 (3-(5-m-Tolyl-1H-imidazol-2-yl)-pyridine | CHEMBL33...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304800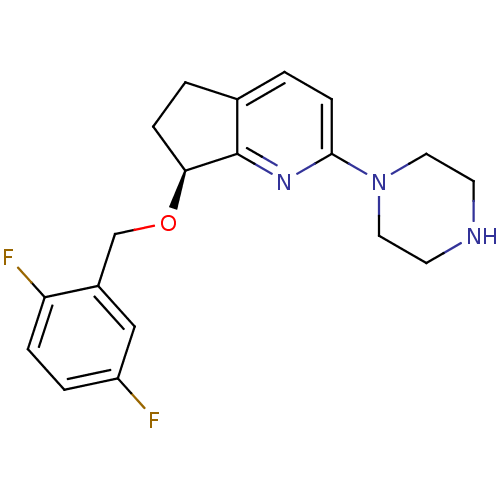 ((S)-7-(2,5-difluorobenzyloxy)-2-(piperazin-1-yl)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304801 ((S)-7-(2,5-difluorobenzyloxy)-2-((R)-2-methylpiper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133782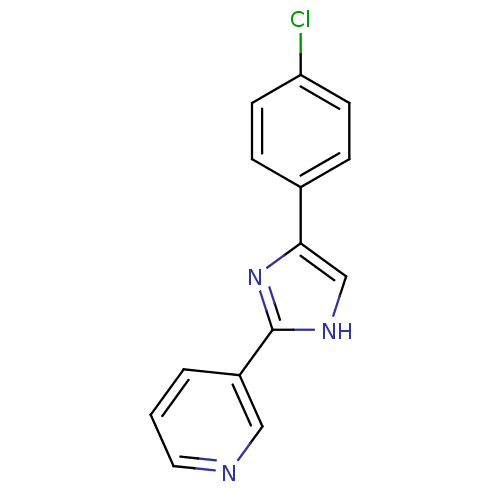 (3-[5-(4-Chloro-phenyl)-1H-imidazol-2-yl]-pyridine ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133773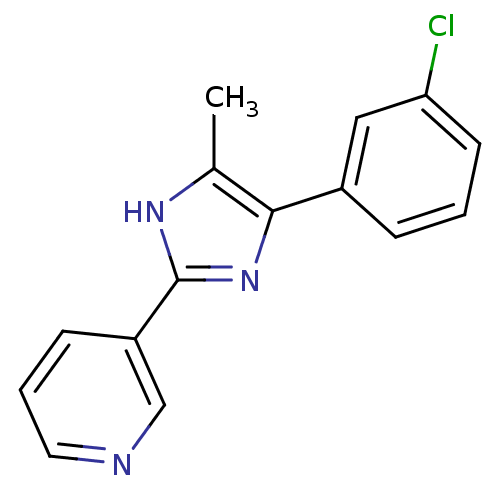 (3-[5-(3-Chloro-phenyl)-4-methyl-1H-imidazol-2-yl]-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50304806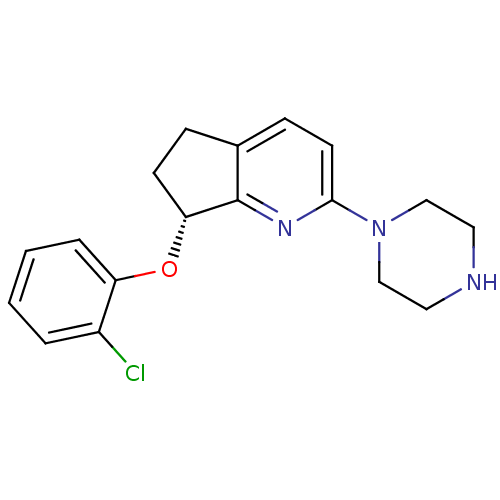 ((R)-7-(2-chlorophenoxy)-2-(piperazin-1-yl)-6,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50342731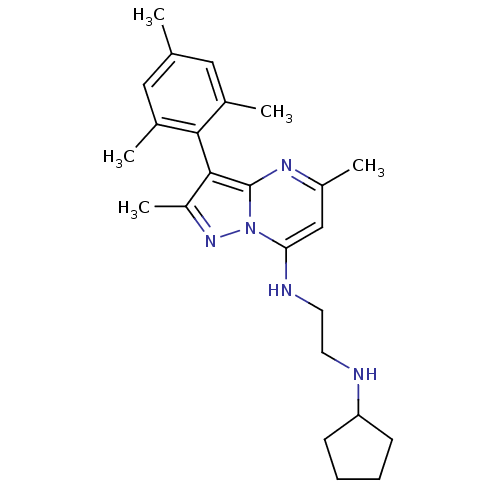 (CHEMBL1771256 | N1-cyclopentyl-N2-(3-mesityl-2,5-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-PYY from human recombinant NPY Y1 receptor overexpressed in membrane of Sf9 cells | Bioorg Med Chem Lett 21: 2641-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.116 BindingDB Entry DOI: 10.7270/Q2BP0336 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133767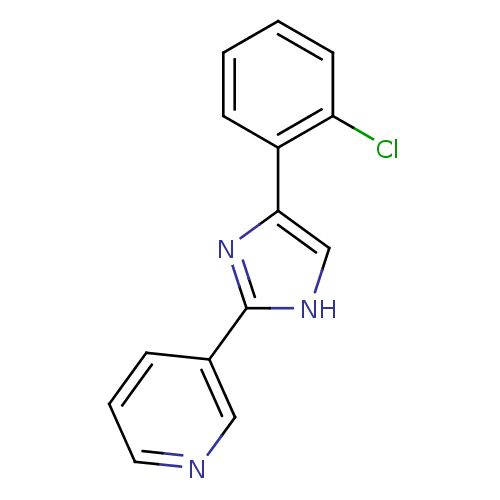 (3-[5-(2-Chloro-phenyl)-1H-imidazol-2-yl]-pyridine ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50304820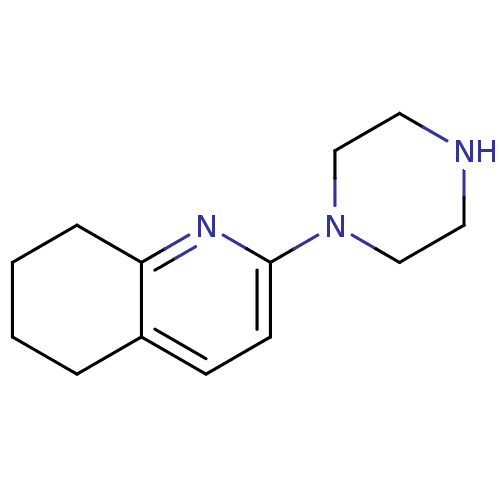 (2-(piperazin-1-yl)-5,6,7,8-tetrahydroquinoline | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]DOI from rat 5HT2A receptor expressed in mouse 3T3 cells | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218173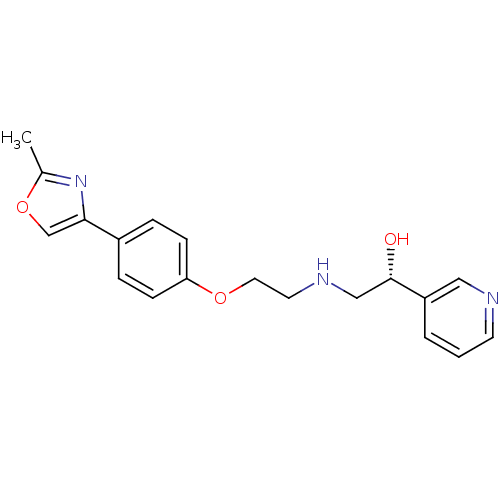 ((R)-2-(2-(4-(2-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50016972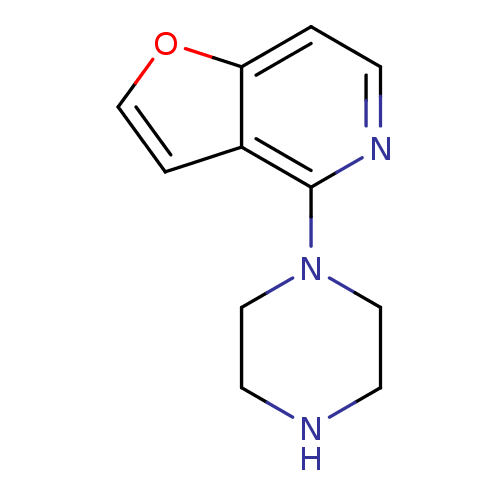 (4-Piperazin-1-yl-furo[3,2-c]pyridine; hydrochlorid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity against human 5HT2C receptor by FLIPR assay relative to 5HT | Bioorg Med Chem Lett 20: 266-71 (2010) Article DOI: 10.1016/j.bmcl.2009.10.112 BindingDB Entry DOI: 10.7270/Q2154H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 335 total ) | Next | Last >> |