

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699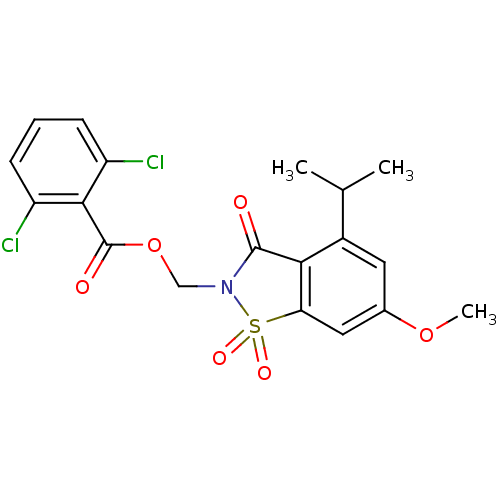 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286326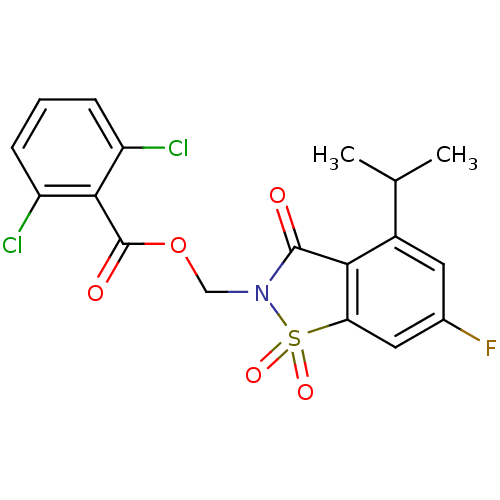 (2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631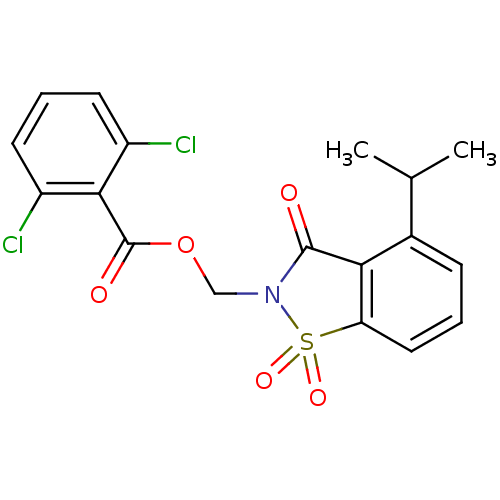 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50007344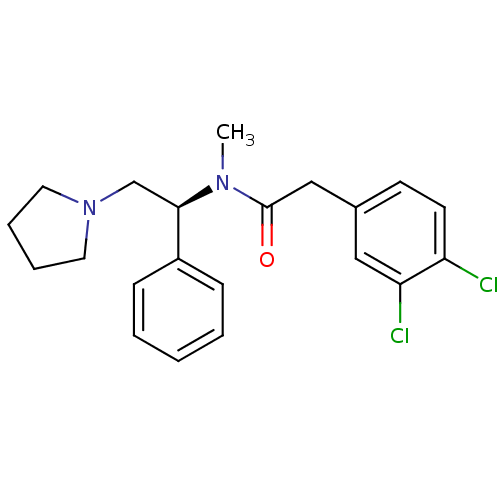 ((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand | Bioorg Med Chem Lett 15: 1279-82 (2005) Article DOI: 10.1016/j.bmcl.2005.01.038 BindingDB Entry DOI: 10.7270/Q2QF8SDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50007344 ((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093965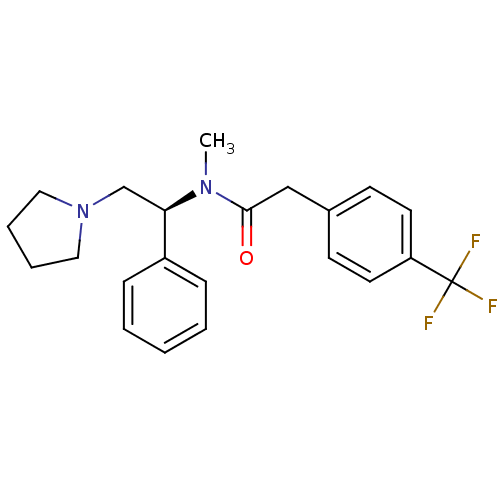 (CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093965 (CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093964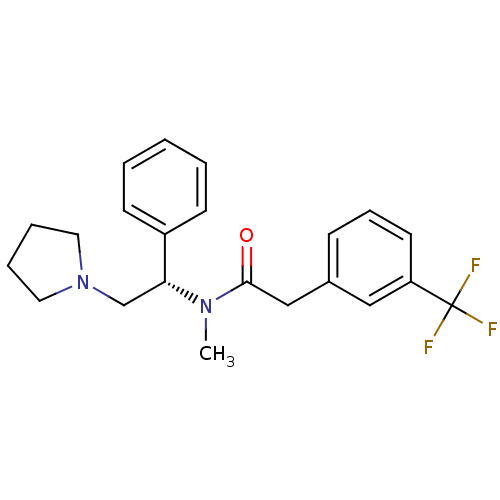 (CHEMBL313484 | N-Methyl-N-((S)-1-phenyl-2-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093969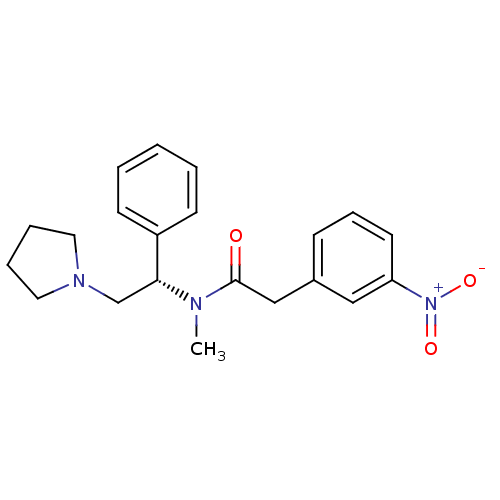 (CHEMBL82919 | N-Methyl-2-(3-nitro-phenyl)-N-((S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090002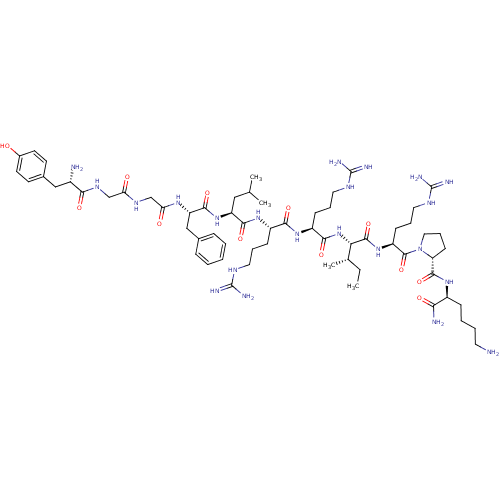 (CHEMBL411003 | Dynorphin A (1-11)-NH2H-Tyr-Gly-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards cloned human Opioid receptor kappa 1 in CHO cell membranes. | J Med Chem 43: 2698-702 (2000) BindingDB Entry DOI: 10.7270/Q25D8SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093958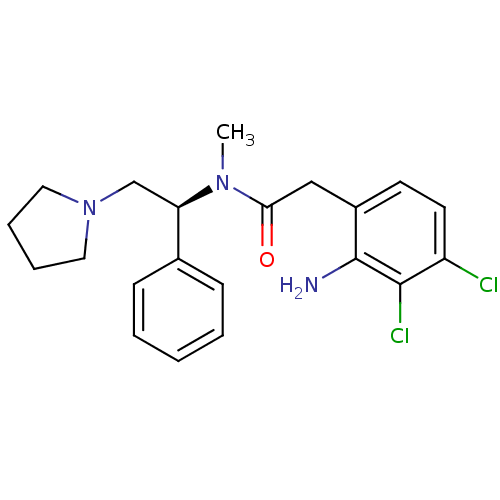 (2-(2-Amino-3,4-dichloro-phenyl)-N-methyl-N-((S)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093966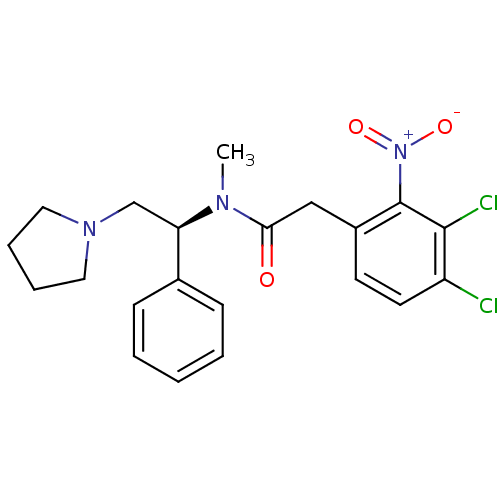 (2-(3,4-Dichloro-2-nitro-phenyl)-N-methyl-N-((S)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093970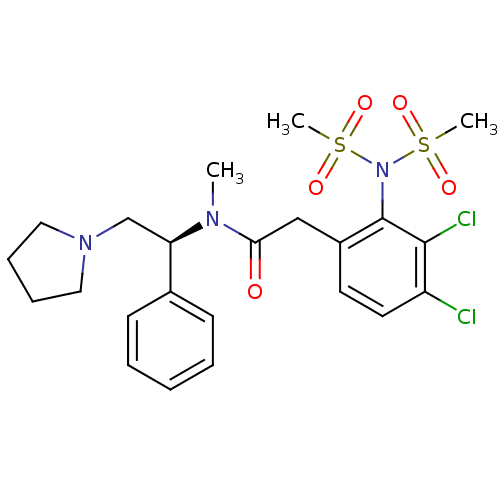 (2-(3,4-Dichloro-2-dimethanesulfonylamino-phenyl)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093962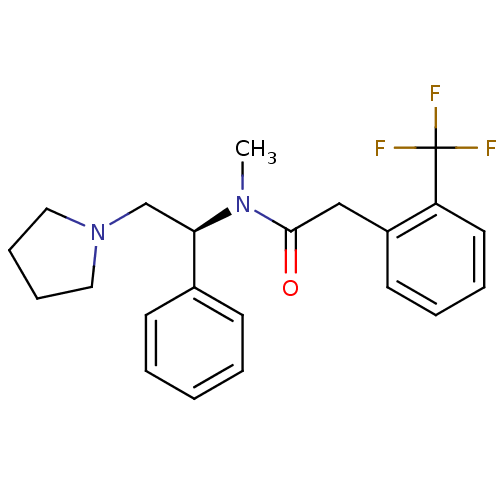 (CHEMBL87306 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090003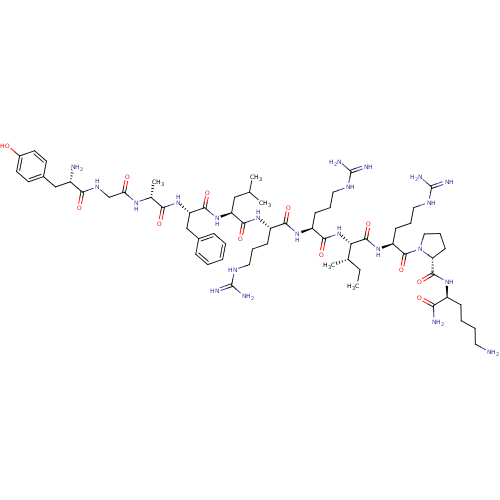 (CHEMBL405190 | Tyr-Gly-[D-Ala]-Phe-Leu-Arg-Arg-Ile...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Human Opioid receptor kappa 1 mediated stimulation of [35S]- GTPgammaS binding in CHO cells (Agonist potency). | J Med Chem 43: 2698-702 (2000) BindingDB Entry DOI: 10.7270/Q25D8SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286333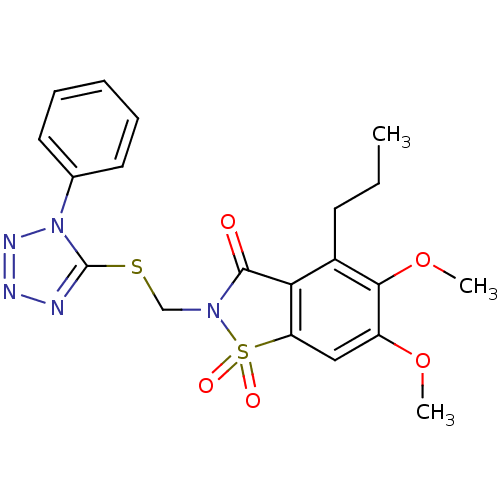 (5,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029703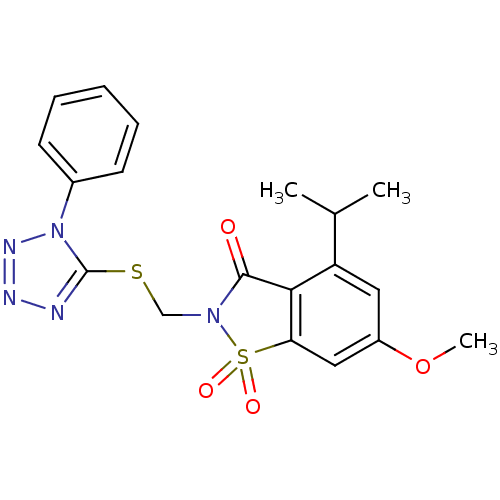 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(1-phenyl-1H-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282874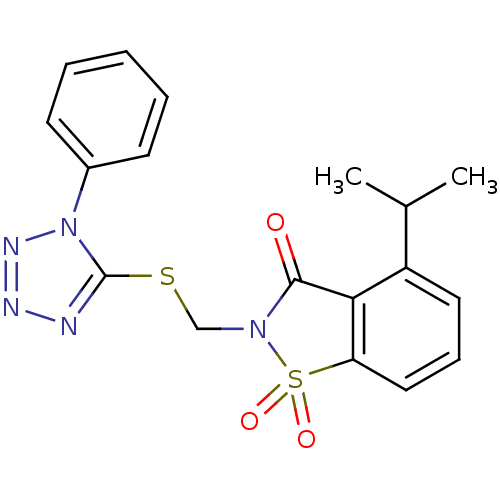 (4-Isopropyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160834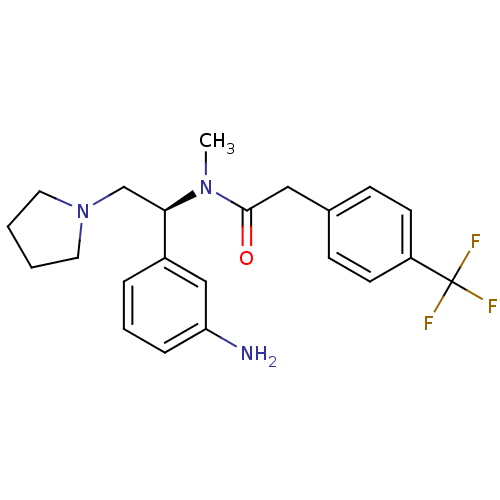 (CHEMBL183248 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551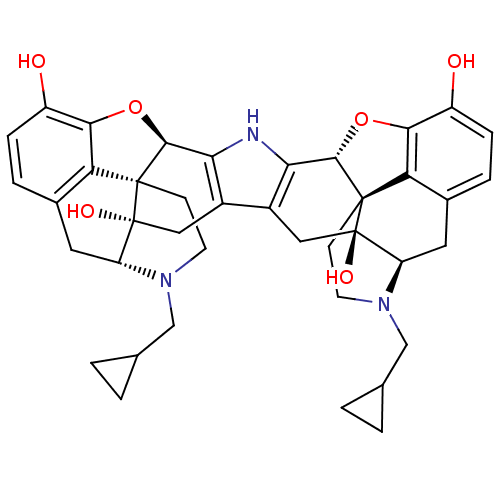 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Human Opioid receptor kappa 1 mediated stimulation of [35S]- GTPgammaS binding in CHO cells (Agonist potency). | J Med Chem 43: 2698-702 (2000) BindingDB Entry DOI: 10.7270/Q25D8SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160840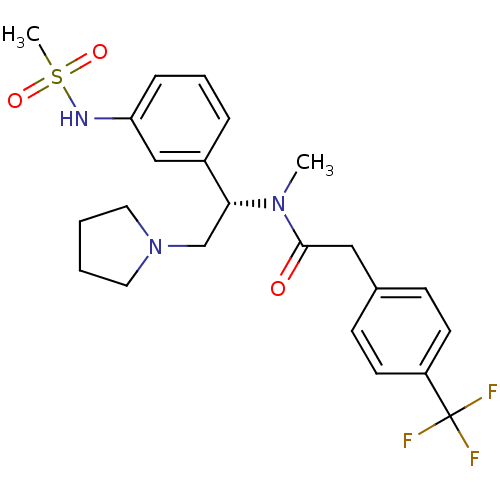 (CHEMBL365486 | N-[(S)-1-(3-Methanesulfonylamino-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093968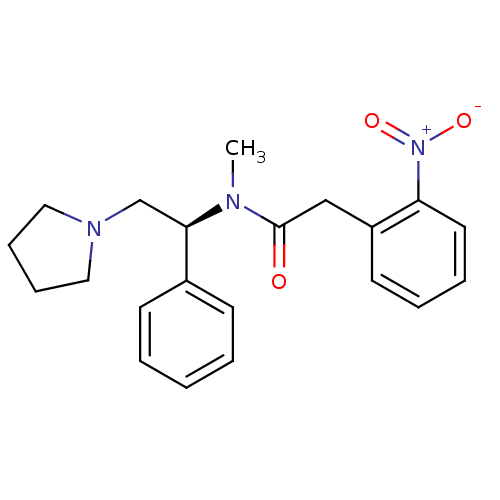 (CHEMBL87207 | N-Methyl-2-(2-nitro-phenyl)-N-((S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160832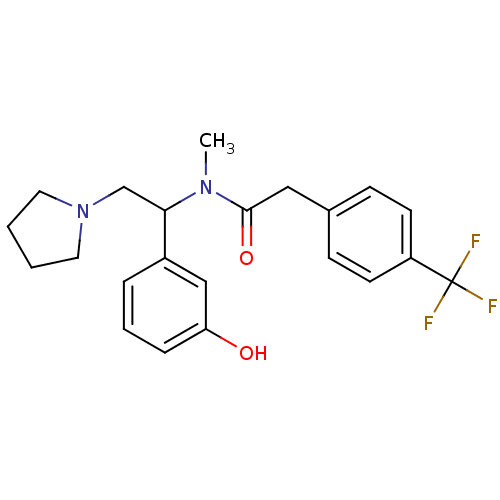 (CHEMBL181279 | N-[1-(3-Hydroxy-phenyl)-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160842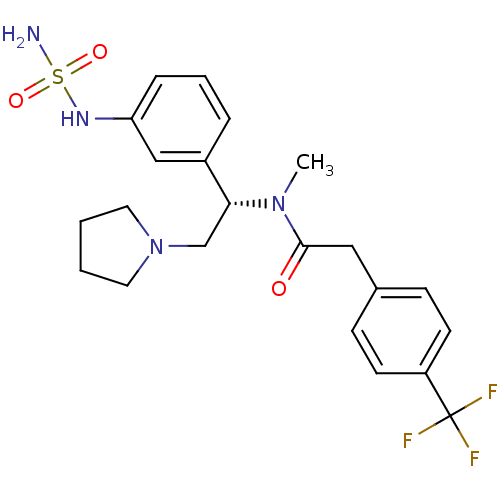 (CHEMBL362248 | N-[(S)-1-(3-aminosulfonylamino-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160836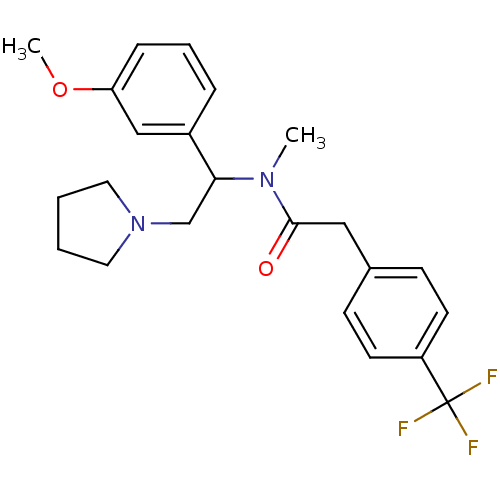 (CHEMBL180803 | N-[1-(3-Methoxy-phenyl)-2-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093971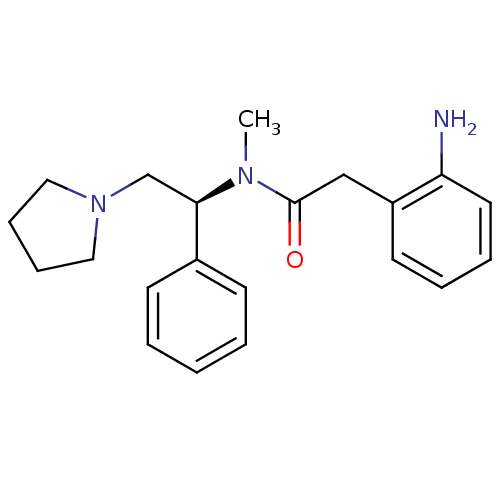 (2-(2-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282873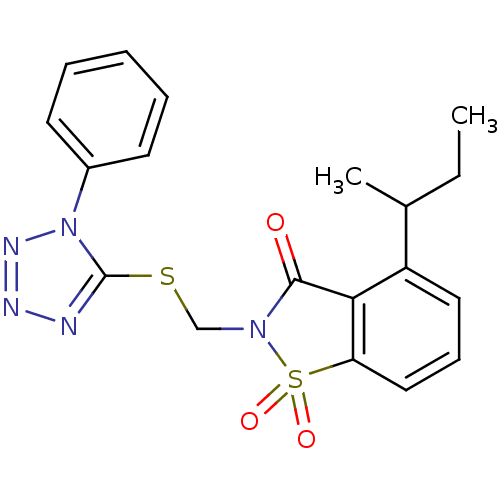 (4-sec-Butyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286327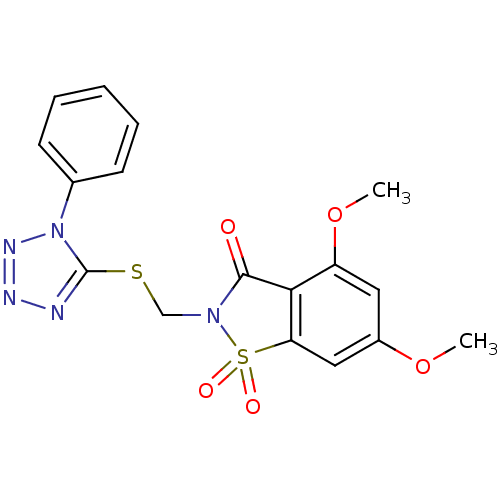 (4,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160838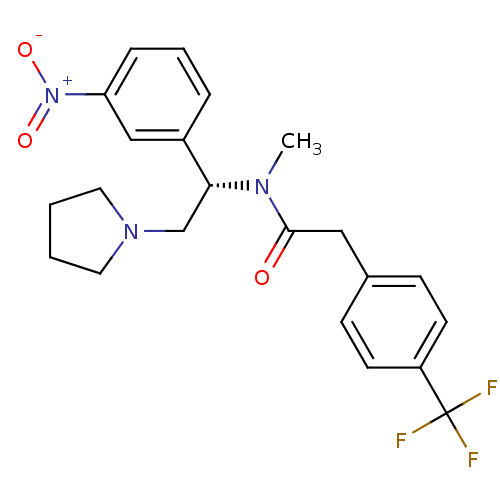 (CHEMBL365198 | N-Methyl-N-[(S)-1-(3-nitro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282868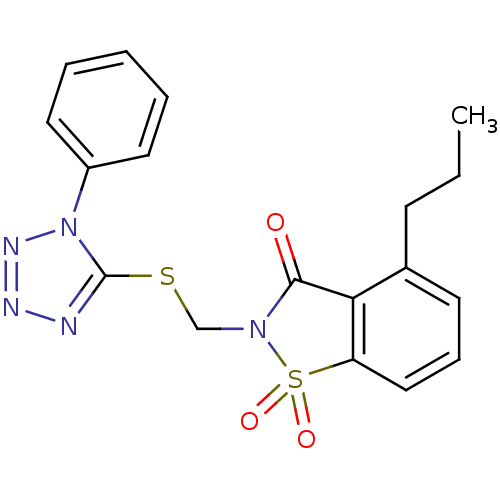 (1,1-Dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulfanylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50161317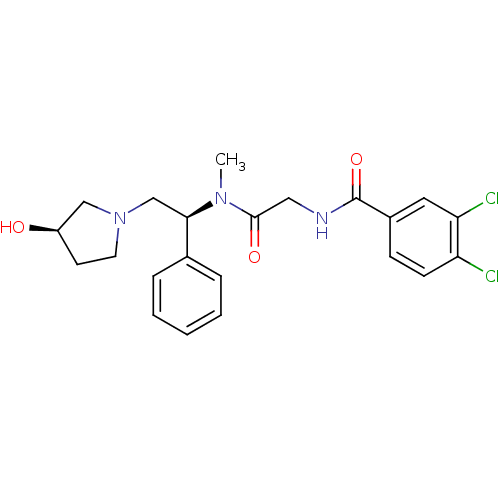 (3,4-Dichloro-N-({[(S)-2-((R)-3-hydroxy-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand | Bioorg Med Chem Lett 15: 1279-82 (2005) Article DOI: 10.1016/j.bmcl.2005.01.038 BindingDB Entry DOI: 10.7270/Q2QF8SDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093963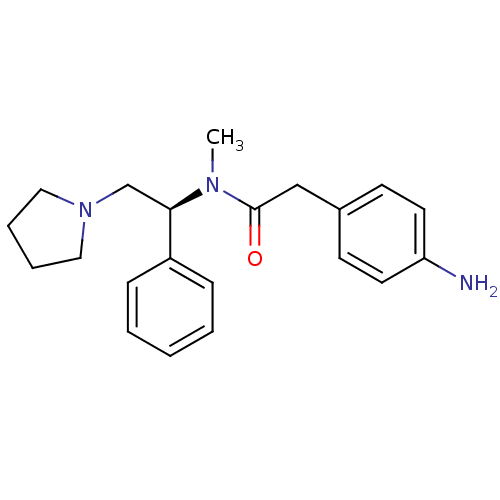 (2-(4-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093967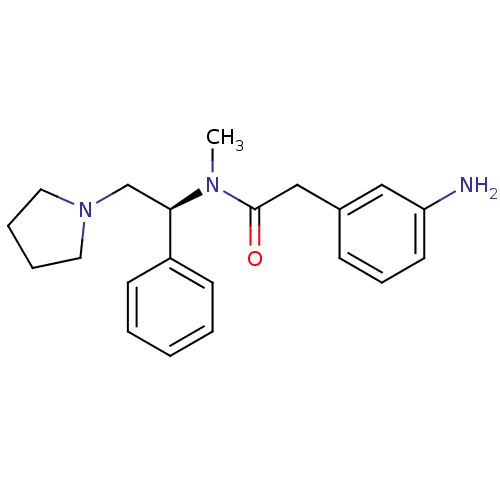 (2-(3-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093972 (CHEMBL87986 | N-Methyl-2-(4-nitro-phenyl)-N-((S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286329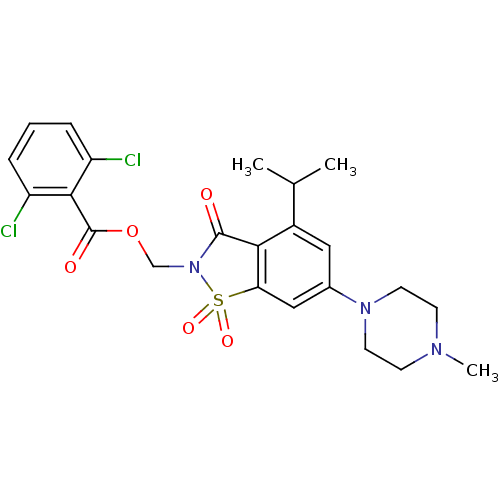 (2,6-Dichloro-benzoic acid 4-isopropyl-6-(4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282866 (4-Ethyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50161315 (CHEMBL179627 | N-({[(S)-2-((R)-3-Hydroxy-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand | Bioorg Med Chem Lett 15: 1279-82 (2005) Article DOI: 10.1016/j.bmcl.2005.01.038 BindingDB Entry DOI: 10.7270/Q2QF8SDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286330 (4,5-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286328 (2,6-Dichloro-benzoic acid 6-dimethylamino-4-isopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM83435 (2-[3-[1-[2-(3,4-dichlorophenyl)ethanoyl-methyl-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090006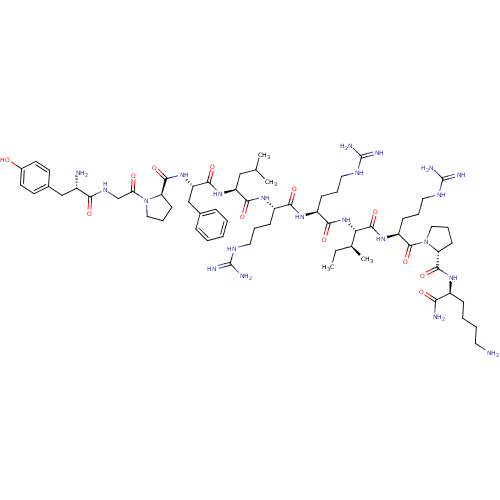 (CHEMBL407389 | [Pro3] Dynorphin A (1-11)-NH2H-Tyr-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Human Opioid receptor kappa 1 mediated stimulation of [35S]- GTPgammaS binding in CHO cells (Agonist potency). | J Med Chem 43: 2698-702 (2000) BindingDB Entry DOI: 10.7270/Q25D8SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50090002 (CHEMBL411003 | Dynorphin A (1-11)-NH2H-Tyr-Gly-Gly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards cloned human Opioid receptor delta 1 in CHO cell membranes. | J Med Chem 43: 2698-702 (2000) BindingDB Entry DOI: 10.7270/Q25D8SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160833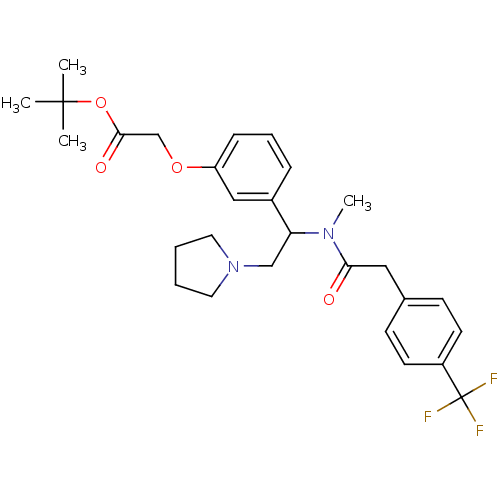 (CHEMBL360678 | [3-(1-{Methyl-[2-(4-trifluoromethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50161320 (3,4-Dichloro-N-{[methyl-((S)-1-phenyl-2-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand | Bioorg Med Chem Lett 15: 1279-82 (2005) Article DOI: 10.1016/j.bmcl.2005.01.038 BindingDB Entry DOI: 10.7270/Q2QF8SDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50160835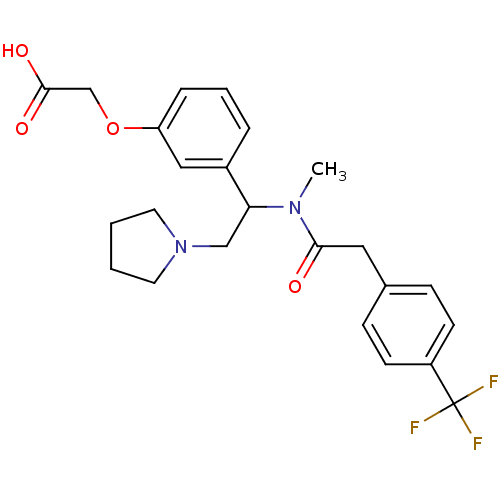 (CHEMBL185554 | [3-(1-{Methyl-[2-(4-trifluoromethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor | Bioorg Med Chem Lett 15: 1091-5 (2005) Article DOI: 10.1016/j.bmcl.2004.12.018 BindingDB Entry DOI: 10.7270/Q2JW8DCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50161316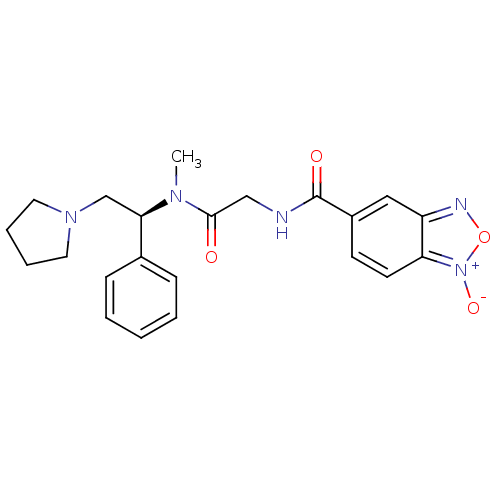 (1-Oxy-benzo[1,2,5]oxadiazole-5-carboxylic acid {[m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand | Bioorg Med Chem Lett 15: 1279-82 (2005) Article DOI: 10.1016/j.bmcl.2005.01.038 BindingDB Entry DOI: 10.7270/Q2QF8SDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286332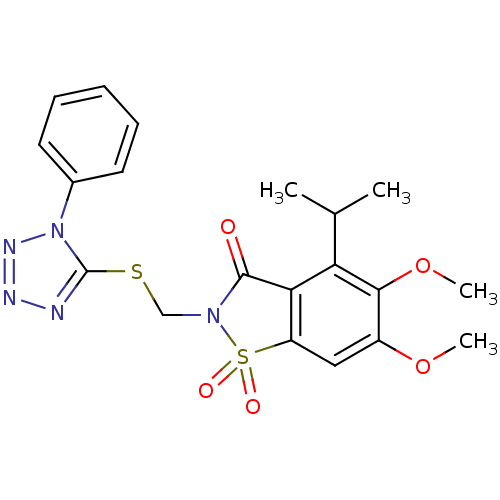 (4-Isopropyl-5,6-dimethoxy-1,1-dioxo-2-(1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093961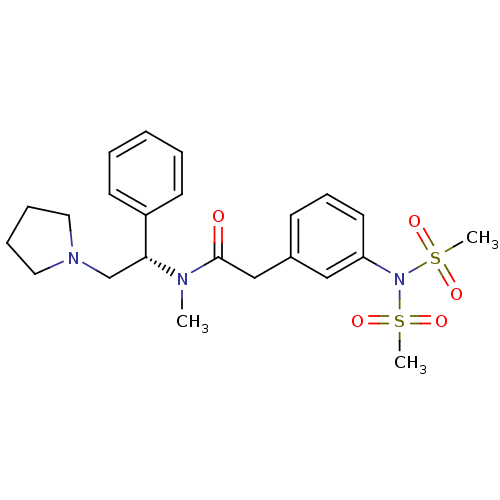 (2-(3-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 | Bioorg Med Chem Lett 10: 2567-70 (2001) BindingDB Entry DOI: 10.7270/Q26H4GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50161313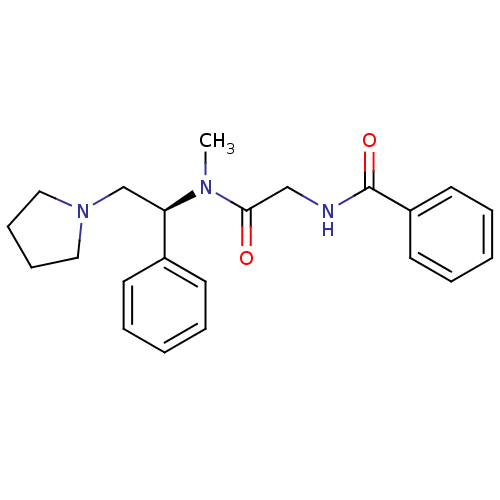 (CHEMBL178359 | N-{[Methyl-((S)-1-phenyl-2-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand | Bioorg Med Chem Lett 15: 1279-82 (2005) Article DOI: 10.1016/j.bmcl.2005.01.038 BindingDB Entry DOI: 10.7270/Q2QF8SDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090006 (CHEMBL407389 | [Pro3] Dynorphin A (1-11)-NH2H-Tyr-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor kappa 1 by to cloned human opioid receptors expressed in CHO cell membranes | J Med Chem 43: 2698-702 (2000) BindingDB Entry DOI: 10.7270/Q25D8SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 282 total ) | Next | Last >> |