 Found 256 hits with Last Name = 'maycock' and Initial = 'al'
Found 256 hits with Last Name = 'maycock' and Initial = 'al' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50029699
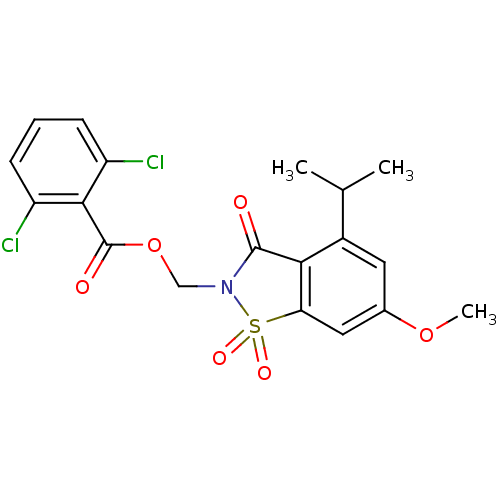
(2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)cccc3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C19H17Cl2NO6S/c1-10(2)12-7-11(27-3)8-15-16(12)18(23)22(29(15,25)26)9-28-19(24)17-13(20)5-4-6-14(17)21/h4-8,10H,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50039631
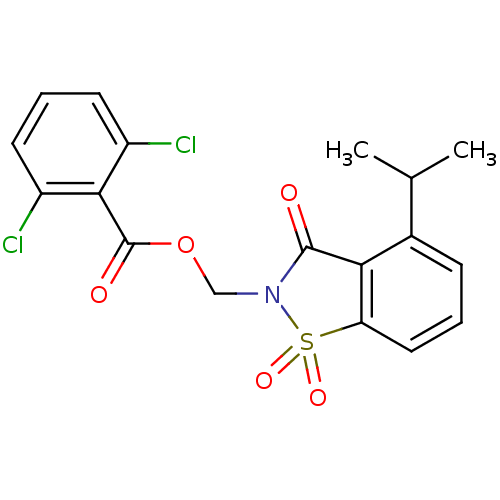
(2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...)Show SMILES CC(C)c1cccc2c1C(=O)N(COC(=O)c1c(Cl)cccc1Cl)S2(=O)=O Show InChI InChI=1S/C18H15Cl2NO5S/c1-10(2)11-5-3-8-14-15(11)17(22)21(27(14,24)25)9-26-18(23)16-12(19)6-4-7-13(16)20/h3-8,10H,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286326
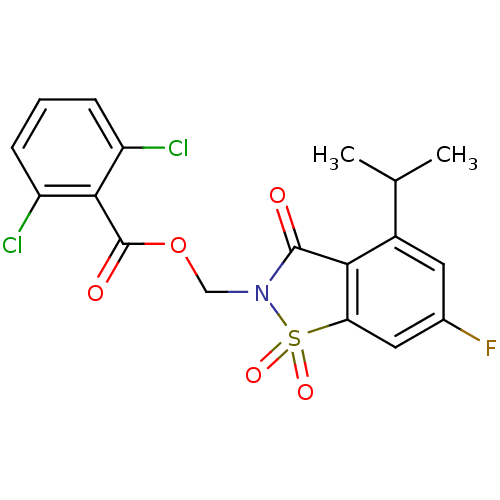
(2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...)Show SMILES CC(C)c1cc(F)cc2c1C(=O)N(COC(=O)c1c(Cl)cccc1Cl)S2(=O)=O Show InChI InChI=1S/C18H14Cl2FNO5S/c1-9(2)11-6-10(21)7-14-15(11)17(23)22(28(14,25)26)8-27-18(24)16-12(19)4-3-5-13(16)20/h3-7,9H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
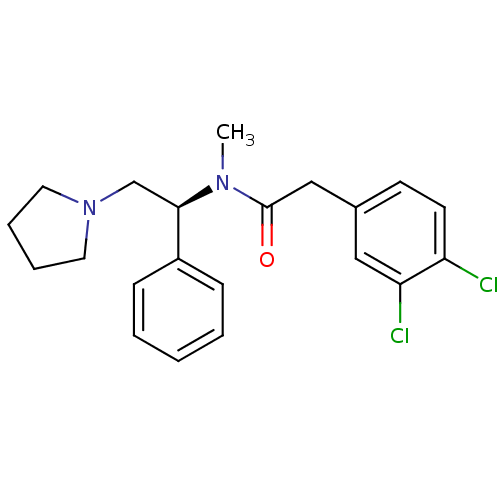
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344

((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965
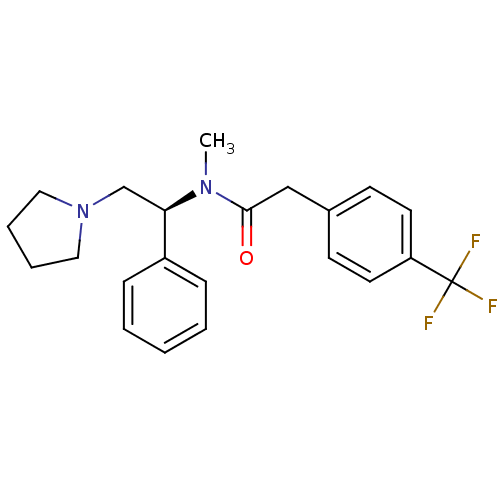
(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965

(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093964
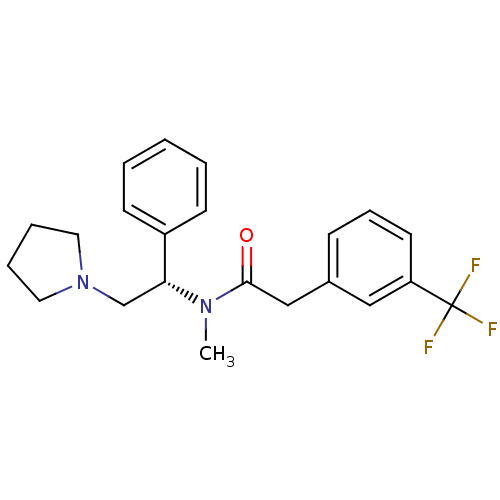
(CHEMBL313484 | N-Methyl-N-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-8-7-11-19(14-17)22(23,24)25)20(16-27-12-5-6-13-27)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093969
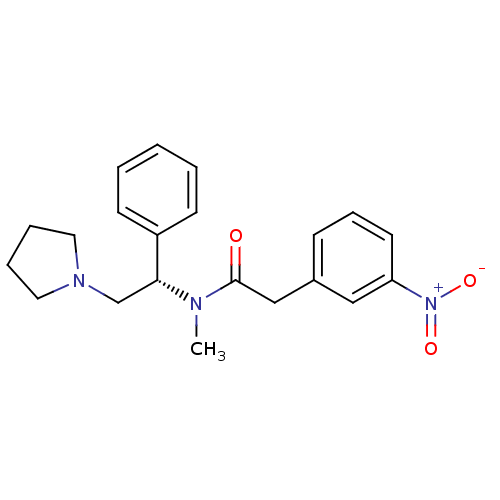
(CHEMBL82919 | N-Methyl-2-(3-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-8-7-11-19(14-17)24(26)27)20(16-23-12-5-6-13-23)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093958
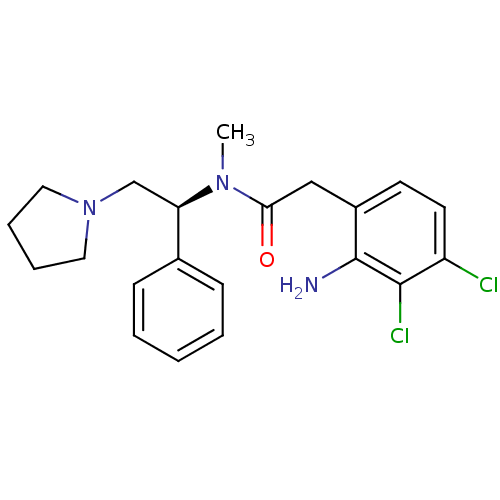
(2-(2-Amino-3,4-dichloro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N Show InChI InChI=1S/C21H25Cl2N3O/c1-25(19(27)13-16-9-10-17(22)20(23)21(16)24)18(14-26-11-5-6-12-26)15-7-3-2-4-8-15/h2-4,7-10,18H,5-6,11-14,24H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093966
(2-(3,4-Dichloro-2-nitro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1[N+]([O-])=O Show InChI InChI=1S/C21H23Cl2N3O3/c1-24(18(14-25-11-5-6-12-25)15-7-3-2-4-8-15)19(27)13-16-9-10-17(22)20(23)21(16)26(28)29/h2-4,7-10,18H,5-6,11-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093970
(2-(3,4-Dichloro-2-dimethanesulfonylamino-phenyl)-N...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H29Cl2N3O5S2/c1-26(20(16-27-13-7-8-14-27)17-9-5-4-6-10-17)21(29)15-18-11-12-19(24)22(25)23(18)28(34(2,30)31)35(3,32)33/h4-6,9-12,20H,7-8,13-16H2,1-3H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093962
(CHEMBL87306 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-18-11-5-6-12-19(18)22(23,24)25)20(16-27-13-7-8-14-27)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286333
(5,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...)Show SMILES CCCc1c2C(=O)N(CSc3nnnn3-c3ccccc3)S(=O)(=O)c2cc(OC)c1OC Show InChI InChI=1S/C20H21N5O5S2/c1-4-8-14-17-16(11-15(29-2)18(14)30-3)32(27,28)24(19(17)26)12-31-20-21-22-23-25(20)13-9-6-5-7-10-13/h5-7,9-11H,4,8,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50029703
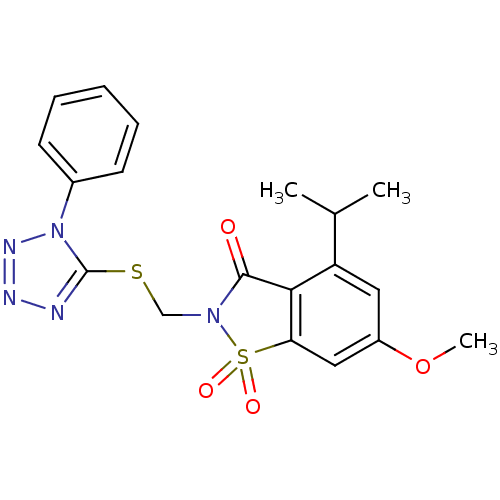
(4-Isopropyl-6-methoxy-1,1-dioxo-2-(1-phenyl-1H-tet...)Show SMILES COc1cc2c(C(=O)N(CSc3nnnn3-c3ccccc3)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C19H19N5O4S2/c1-12(2)15-9-14(28-3)10-16-17(15)18(25)23(30(16,26)27)11-29-19-20-21-22-24(19)13-7-5-4-6-8-13/h4-10,12H,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50282874
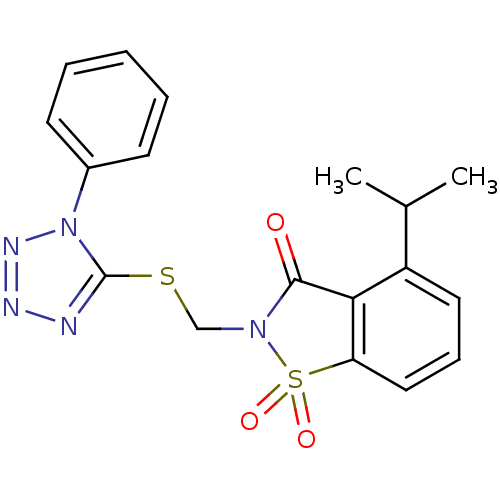
(4-Isopropyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...)Show SMILES CC(C)c1cccc2c1C(=O)N(CSc1nnnn1-c1ccccc1)S2(=O)=O Show InChI InChI=1S/C18H17N5O3S2/c1-12(2)14-9-6-10-15-16(14)17(24)22(28(15,25)26)11-27-18-19-20-21-23(18)13-7-4-3-5-8-13/h3-10,12H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160834
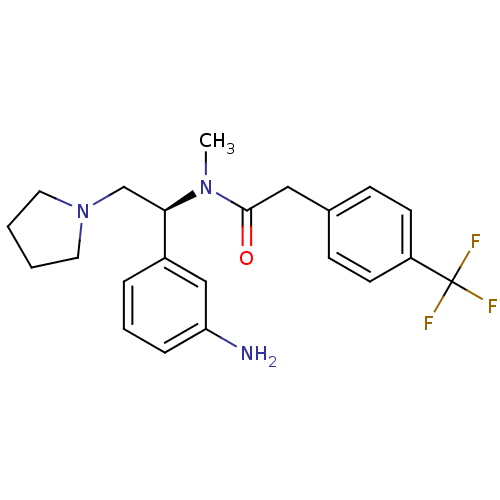
(CHEMBL183248 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O/c1-27(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-28-11-2-3-12-28)17-5-4-6-19(26)14-17/h4-10,14,20H,2-3,11-13,15,26H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160840
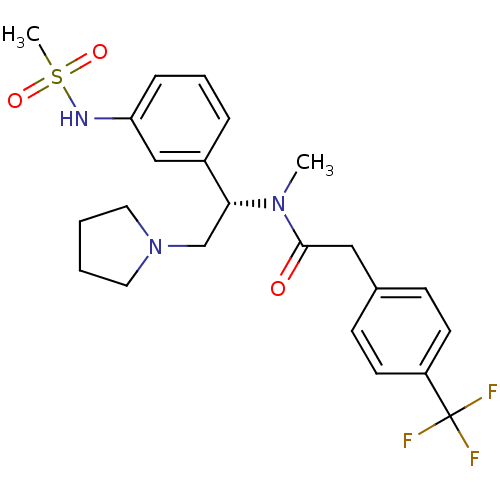
(CHEMBL365486 | N-[(S)-1-(3-Methanesulfonylamino-ph...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NS(C)(=O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H28F3N3O3S/c1-28(22(30)14-17-8-10-19(11-9-17)23(24,25)26)21(16-29-12-3-4-13-29)18-6-5-7-20(15-18)27-33(2,31)32/h5-11,15,21,27H,3-4,12-14,16H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093968
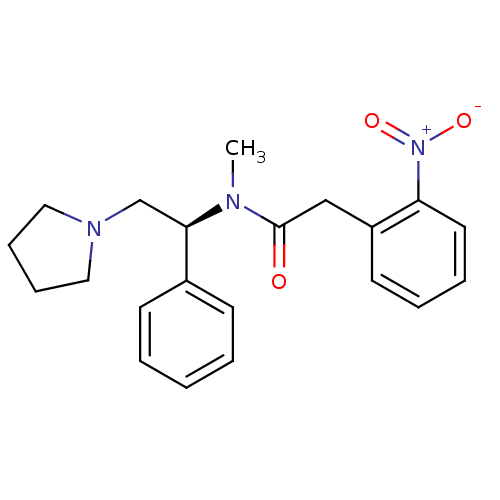
(CHEMBL87207 | N-Methyl-2-(2-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-18-11-5-6-12-19(18)24(26)27)20(16-23-13-7-8-14-23)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160832
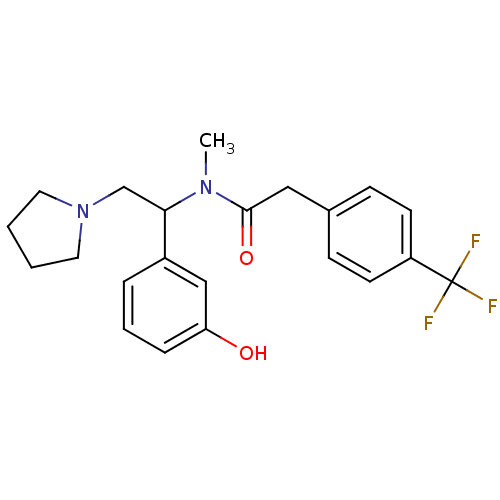
(CHEMBL181279 | N-[1-(3-Hydroxy-phenyl)-2-pyrrolidi...)Show SMILES CN(C(CN1CCCC1)c1cccc(O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O2/c1-26(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-27-11-2-3-12-27)17-5-4-6-19(28)14-17/h4-10,14,20,28H,2-3,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160842
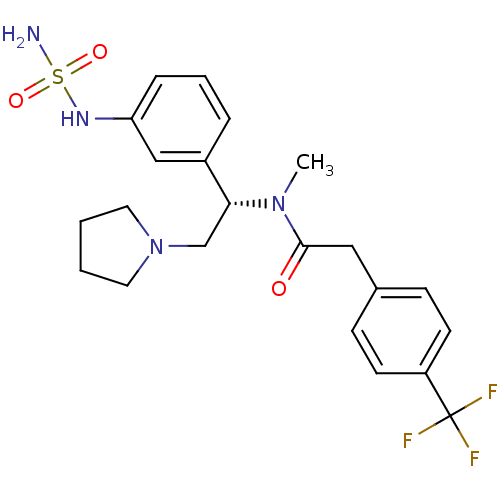
(CHEMBL362248 | N-[(S)-1-(3-aminosulfonylamino-phen...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NS(N)(=O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H27F3N4O3S/c1-28(21(30)13-16-7-9-18(10-8-16)22(23,24)25)20(15-29-11-2-3-12-29)17-5-4-6-19(14-17)27-33(26,31)32/h4-10,14,20,27H,2-3,11-13,15H2,1H3,(H2,26,31,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160836
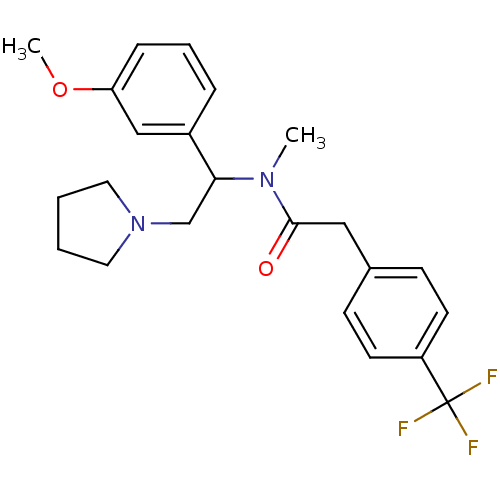
(CHEMBL180803 | N-[1-(3-Methoxy-phenyl)-2-pyrrolidi...)Show SMILES COc1cccc(c1)C(CN1CCCC1)N(C)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N2O2/c1-27(22(29)14-17-8-10-19(11-9-17)23(24,25)26)21(16-28-12-3-4-13-28)18-6-5-7-20(15-18)30-2/h5-11,15,21H,3-4,12-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093971
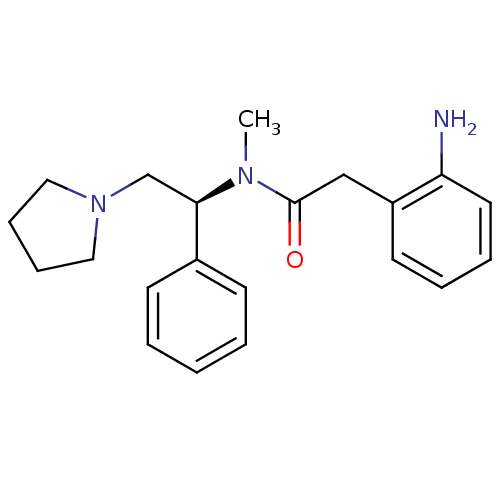
(2-(2-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-18-11-5-6-12-19(18)22)20(16-24-13-7-8-14-24)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50282873
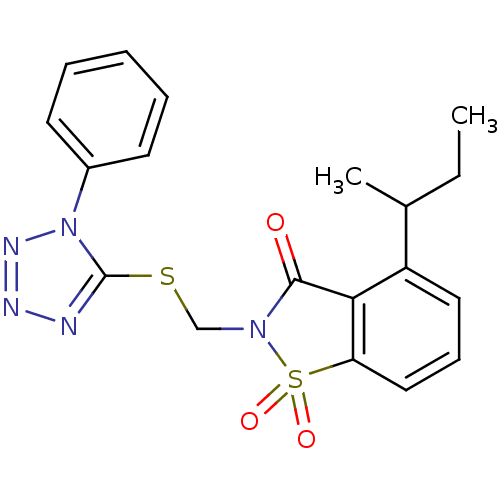
(4-sec-Butyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...)Show SMILES CCC(C)c1cccc2c1C(=O)N(CSc1nnnn1-c1ccccc1)S2(=O)=O Show InChI InChI=1S/C19H19N5O3S2/c1-3-13(2)15-10-7-11-16-17(15)18(25)23(29(16,26)27)12-28-19-20-21-22-24(19)14-8-5-4-6-9-14/h4-11,13H,3,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286327
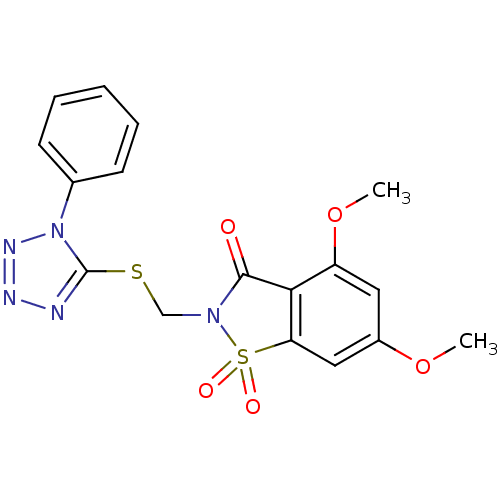
(4,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...)Show SMILES COc1cc2c(C(=O)N(CSc3nnnn3-c3ccccc3)S2(=O)=O)c(OC)c1 Show InChI InChI=1S/C17H15N5O5S2/c1-26-12-8-13(27-2)15-14(9-12)29(24,25)21(16(15)23)10-28-17-18-19-20-22(17)11-6-4-3-5-7-11/h3-9H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160838
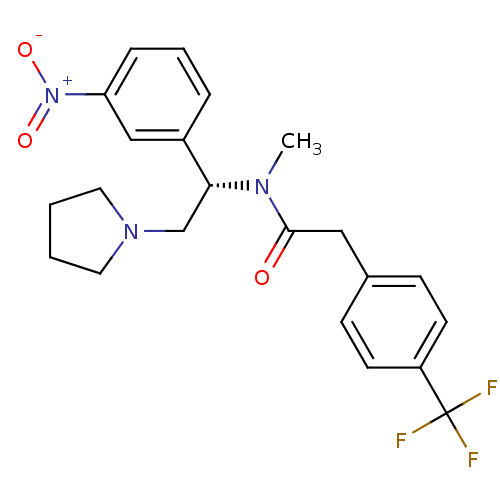
(CHEMBL365198 | N-Methyl-N-[(S)-1-(3-nitro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(c1)[N+]([O-])=O)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H24F3N3O3/c1-26(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-27-11-2-3-12-27)17-5-4-6-19(14-17)28(30)31/h4-10,14,20H,2-3,11-13,15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50282868
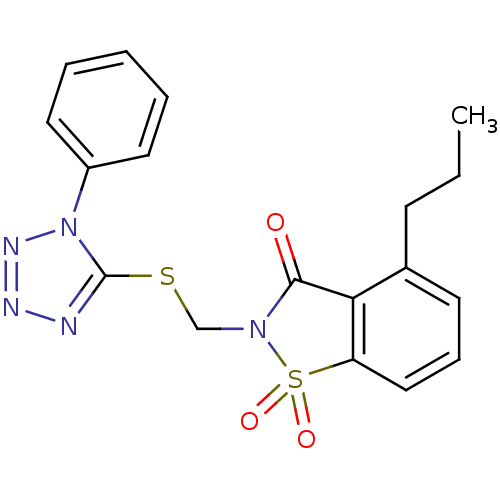
(1,1-Dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulfanylmeth...)Show SMILES CCCc1cccc2c1C(=O)N(CSc1nnnn1-c1ccccc1)S2(=O)=O Show InChI InChI=1S/C18H17N5O3S2/c1-2-7-13-8-6-11-15-16(13)17(24)22(28(15,25)26)12-27-18-19-20-21-23(18)14-9-4-3-5-10-14/h3-6,8-11H,2,7,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50161317
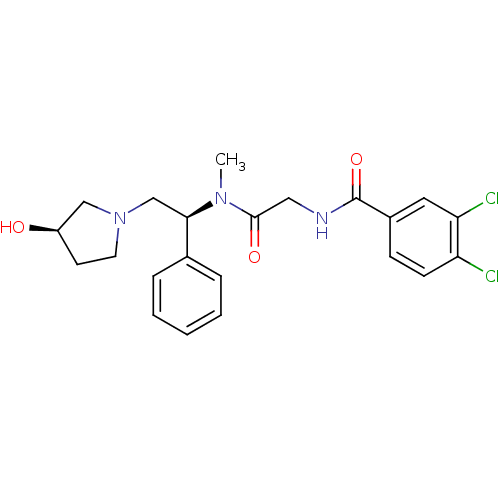
(3,4-Dichloro-N-({[(S)-2-((R)-3-hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@@H](O)C1)c1ccccc1)C(=O)CNC(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H25Cl2N3O3/c1-26(21(29)12-25-22(30)16-7-8-18(23)19(24)11-16)20(15-5-3-2-4-6-15)14-27-10-9-17(28)13-27/h2-8,11,17,20,28H,9-10,12-14H2,1H3,(H,25,30)/t17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093963
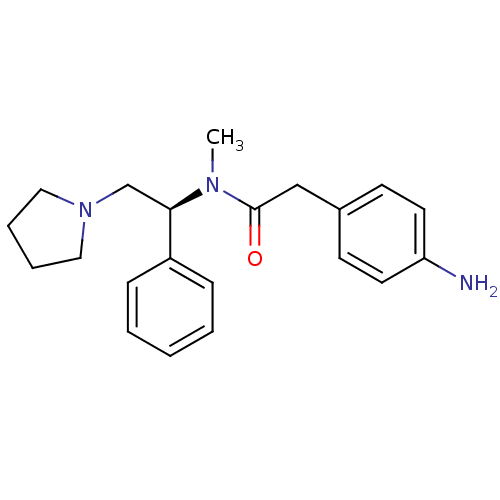
(2-(4-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(N)cc1 Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-17-9-11-19(22)12-10-17)20(16-24-13-5-6-14-24)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093967
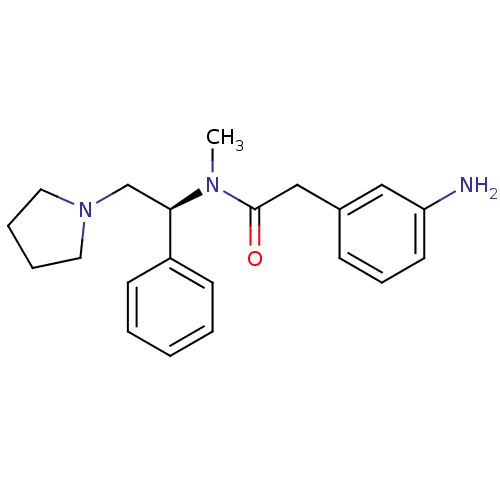
(2-(3-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(N)c1 Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-17-8-7-11-19(22)14-17)20(16-24-12-5-6-13-24)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286329
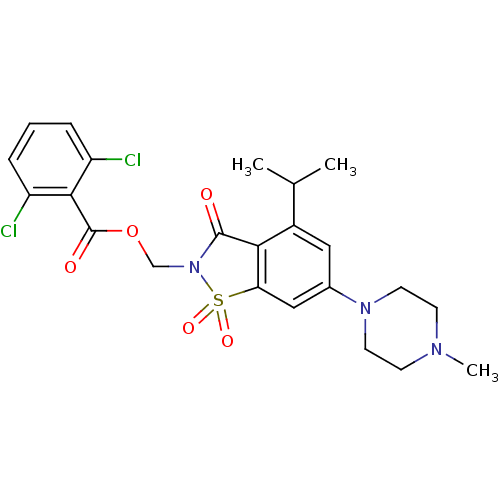
(2,6-Dichloro-benzoic acid 4-isopropyl-6-(4-methyl-...)Show SMILES CC(C)c1cc(cc2c1C(=O)N(COC(=O)c1c(Cl)cccc1Cl)S2(=O)=O)N1CCN(C)CC1 Show InChI InChI=1S/C23H25Cl2N3O5S/c1-14(2)16-11-15(27-9-7-26(3)8-10-27)12-19-20(16)22(29)28(34(19,31)32)13-33-23(30)21-17(24)5-4-6-18(21)25/h4-6,11-12,14H,7-10,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093972

(CHEMBL87986 | N-Methyl-2-(4-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-9-11-19(12-10-17)24(26)27)20(16-23-13-5-6-14-23)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50282866
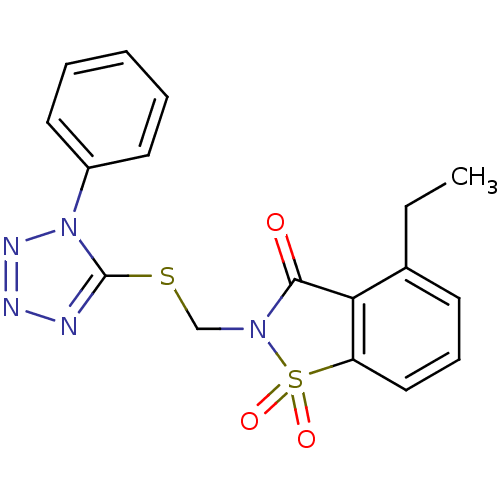
(4-Ethyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulf...)Show SMILES CCc1cccc2c1C(=O)N(CSc1nnnn1-c1ccccc1)S2(=O)=O Show InChI InChI=1S/C17H15N5O3S2/c1-2-12-7-6-10-14-15(12)16(23)21(27(14,24)25)11-26-17-18-19-20-22(17)13-8-4-3-5-9-13/h3-10H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50161315
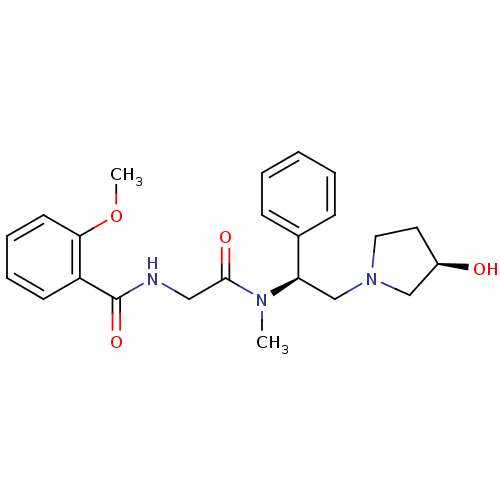
(CHEMBL179627 | N-({[(S)-2-((R)-3-Hydroxy-pyrrolidi...)Show SMILES COc1ccccc1C(=O)NCC(=O)N(C)[C@H](CN1CC[C@@H](O)C1)c1ccccc1 Show InChI InChI=1S/C23H29N3O4/c1-25(22(28)14-24-23(29)19-10-6-7-11-21(19)30-2)20(17-8-4-3-5-9-17)16-26-13-12-18(27)15-26/h3-11,18,20,27H,12-16H2,1-2H3,(H,24,29)/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286330
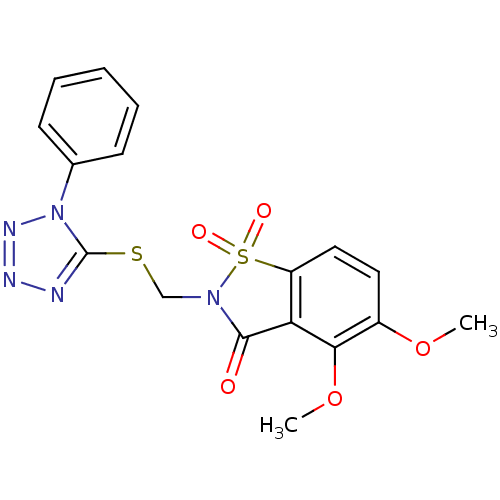
(4,5-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...)Show SMILES COc1ccc2c(C(=O)N(CSc3nnnn3-c3ccccc3)S2(=O)=O)c1OC Show InChI InChI=1S/C17H15N5O5S2/c1-26-12-8-9-13-14(15(12)27-2)16(23)21(29(13,24)25)10-28-17-18-19-20-22(17)11-6-4-3-5-7-11/h3-9H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286328

(2,6-Dichloro-benzoic acid 6-dimethylamino-4-isopro...)Show SMILES CC(C)c1cc(cc2c1C(=O)N(COC(=O)c1c(Cl)cccc1Cl)S2(=O)=O)N(C)C Show InChI InChI=1S/C20H20Cl2N2O5S/c1-11(2)13-8-12(23(3)4)9-16-17(13)19(25)24(30(16,27)28)10-29-20(26)18-14(21)6-5-7-15(18)22/h5-9,11H,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM83435
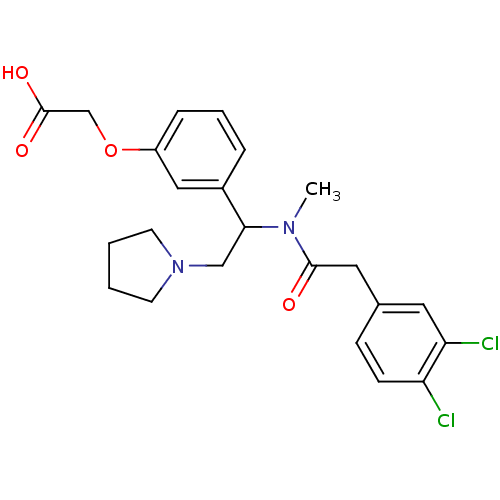
(2-[3-[1-[2-(3,4-dichlorophenyl)ethanoyl-methyl-ami...)Show SMILES CN(C(CN1CCCC1)c1cccc(OCC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C23H26Cl2N2O4/c1-26(22(28)12-16-7-8-19(24)20(25)11-16)21(14-27-9-2-3-10-27)17-5-4-6-18(13-17)31-15-23(29)30/h4-8,11,13,21H,2-3,9-10,12,14-15H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160833
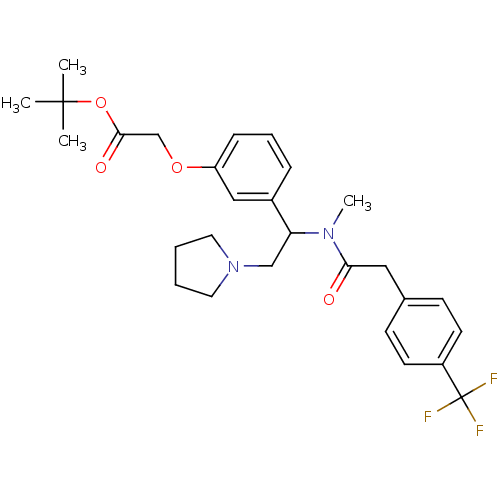
(CHEMBL360678 | [3-(1-{Methyl-[2-(4-trifluoromethyl...)Show SMILES CN(C(CN1CCCC1)c1cccc(OCC(=O)OC(C)(C)C)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H35F3N2O4/c1-27(2,3)37-26(35)19-36-23-9-7-8-21(17-23)24(18-33-14-5-6-15-33)32(4)25(34)16-20-10-12-22(13-11-20)28(29,30)31/h7-13,17,24H,5-6,14-16,18-19H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50161320

(3,4-Dichloro-N-{[methyl-((S)-1-phenyl-2-pyrrolidin...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)CNC(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H25Cl2N3O2/c1-26(20(15-27-11-5-6-12-27)16-7-3-2-4-8-16)21(28)14-25-22(29)17-9-10-18(23)19(24)13-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3,(H,25,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160835
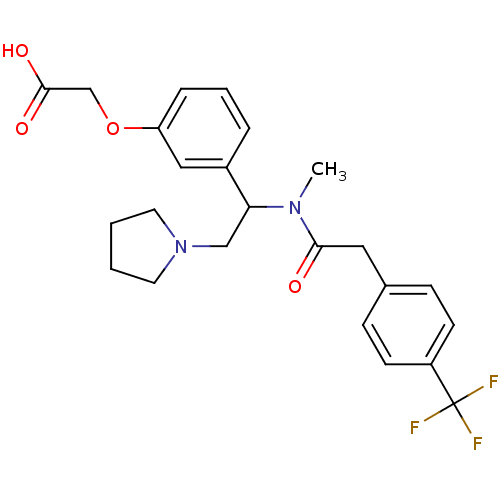
(CHEMBL185554 | [3-(1-{Methyl-[2-(4-trifluoromethyl...)Show SMILES CN(C(CN1CCCC1)c1cccc(OCC(O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C24H27F3N2O4/c1-28(22(30)13-17-7-9-19(10-8-17)24(25,26)27)21(15-29-11-2-3-12-29)18-5-4-6-20(14-18)33-16-23(31)32/h4-10,14,21H,2-3,11-13,15-16H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50161316
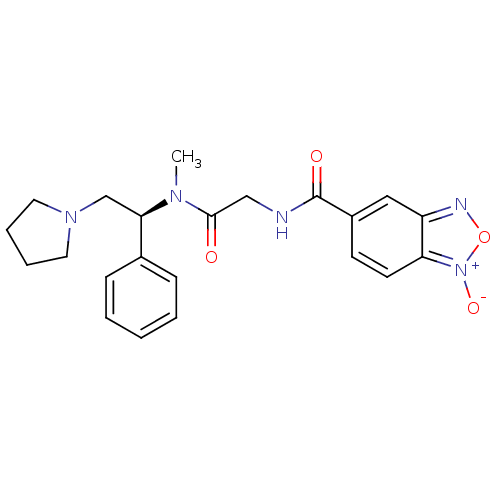
(1-Oxy-benzo[1,2,5]oxadiazole-5-carboxylic acid {[m...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)CNC(=O)c1ccc2[n+]([O-])onc2c1 Show InChI InChI=1S/C22H25N5O4/c1-25(20(15-26-11-5-6-12-26)16-7-3-2-4-8-16)21(28)14-23-22(29)17-9-10-19-18(13-17)24-31-27(19)30/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3,(H,23,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286332
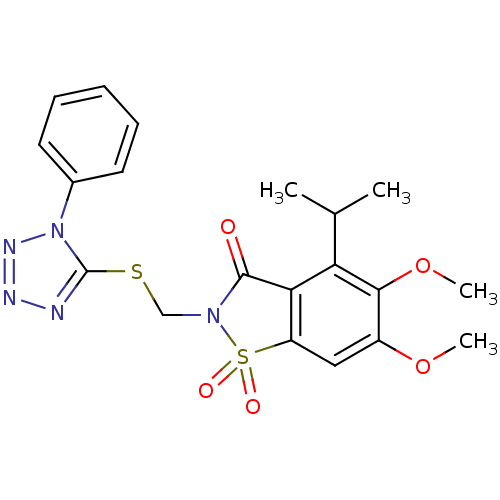
(4-Isopropyl-5,6-dimethoxy-1,1-dioxo-2-(1-phenyl-1H...)Show SMILES COc1cc2c(C(=O)N(CSc3nnnn3-c3ccccc3)S2(=O)=O)c(C(C)C)c1OC Show InChI InChI=1S/C20H21N5O5S2/c1-12(2)16-17-15(10-14(29-3)18(16)30-4)32(27,28)24(19(17)26)11-31-20-21-22-23-25(20)13-8-6-5-7-9-13/h5-10,12H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093961
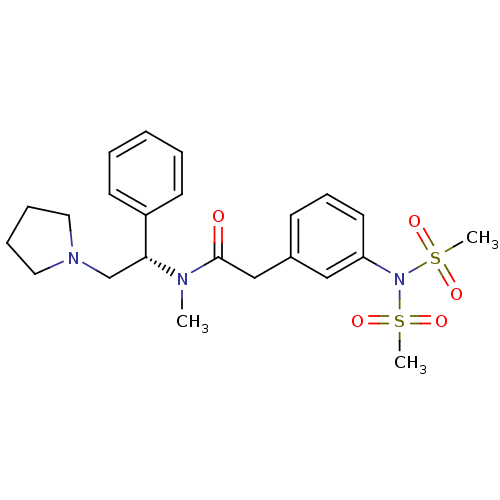
(2-(3-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-14-7-8-15-25)20-11-5-4-6-12-20)23(27)17-19-10-9-13-21(16-19)26(32(2,28)29)33(3,30)31/h4-6,9-13,16,22H,7-8,14-15,17-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50161313
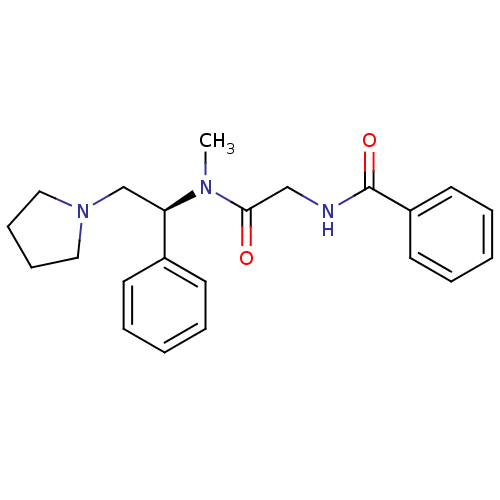
(CHEMBL178359 | N-{[Methyl-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)CNC(=O)c1ccccc1 Show InChI InChI=1S/C22H27N3O2/c1-24(21(26)16-23-22(27)19-12-6-3-7-13-19)20(17-25-14-8-9-15-25)18-10-4-2-5-11-18/h2-7,10-13,20H,8-9,14-17H2,1H3,(H,23,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093960
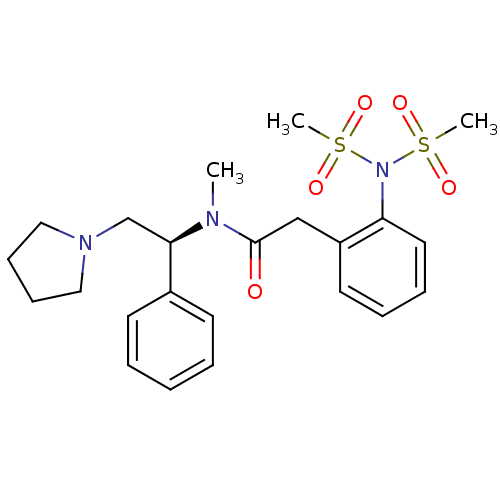
(2-(2-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-15-9-10-16-25)19-11-5-4-6-12-19)23(27)17-20-13-7-8-14-21(20)26(32(2,28)29)33(3,30)31/h4-8,11-14,22H,9-10,15-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160839
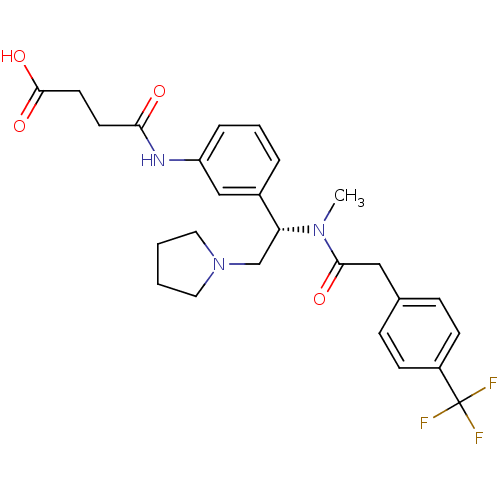
(CHEMBL181922 | N-[3-((S)-1-{Methyl-[2-(4-trifluoro...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)CCC(O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C26H30F3N3O4/c1-31(24(34)15-18-7-9-20(10-8-18)26(27,28)29)22(17-32-13-2-3-14-32)19-5-4-6-21(16-19)30-23(33)11-12-25(35)36/h4-10,16,22H,2-3,11-15,17H2,1H3,(H,30,33)(H,35,36)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50282878
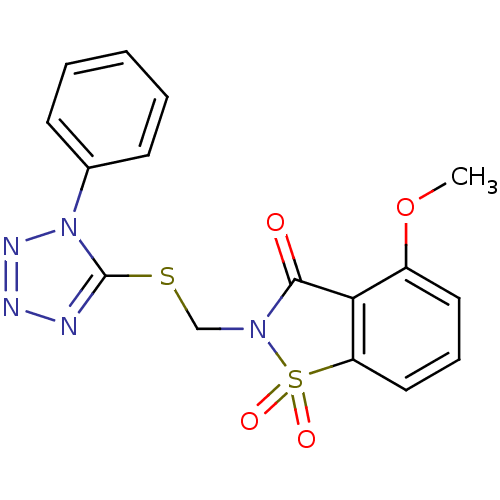
(4-Methoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-ylsu...)Show SMILES COc1cccc2c1C(=O)N(CSc1nnnn1-c1ccccc1)S2(=O)=O Show InChI InChI=1S/C16H13N5O4S2/c1-25-12-8-5-9-13-14(12)15(22)20(27(13,23)24)10-26-16-17-18-19-21(16)11-6-3-2-4-7-11/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036479
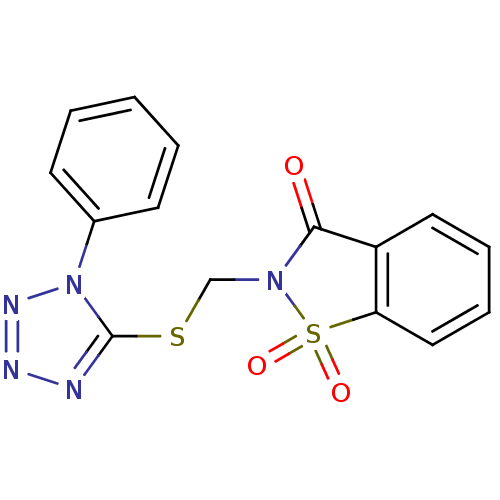
(1,1-Dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulfanylmeth...)Show SMILES O=C1N(CSc2nnnn2-c2ccccc2)S(=O)(=O)c2ccccc12 Show InChI InChI=1S/C15H11N5O3S2/c21-14-12-8-4-5-9-13(12)25(22,23)19(14)10-24-15-16-17-18-20(15)11-6-2-1-3-7-11/h1-9H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ratio of Koff to that of Kon was determined on human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286331
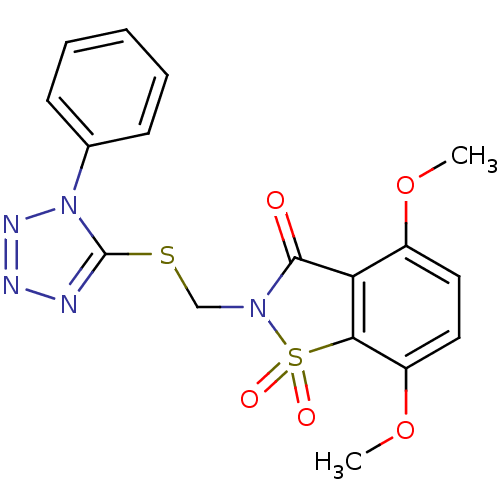
(4,7-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...)Show SMILES COc1ccc(OC)c2c1C(=O)N(CSc1nnnn1-c1ccccc1)S2(=O)=O Show InChI InChI=1S/C17H15N5O5S2/c1-26-12-8-9-13(27-2)15-14(12)16(23)21(29(15,24)25)10-28-17-18-19-20-22(17)11-6-4-3-5-7-11/h3-9H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50161314
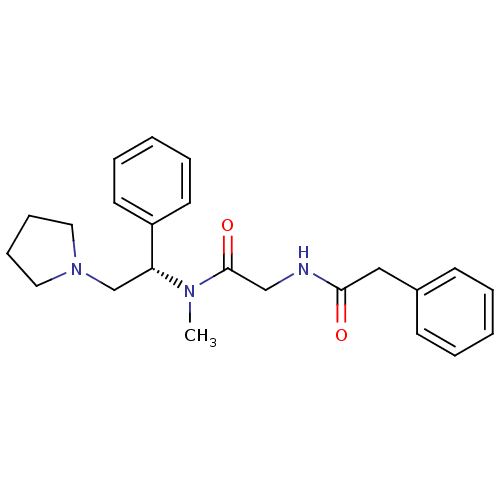
(CHEMBL359543 | N-{[Methyl-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)CNC(=O)Cc1ccccc1 Show InChI InChI=1S/C23H29N3O2/c1-25(23(28)17-24-22(27)16-19-10-4-2-5-11-19)21(18-26-14-8-9-15-26)20-12-6-3-7-13-20/h2-7,10-13,21H,8-9,14-18H2,1H3,(H,24,27)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data