 Found 75 hits with Last Name = 'milner' and Initial = 'ph'
Found 75 hits with Last Name = 'milner' and Initial = 'ph' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082226
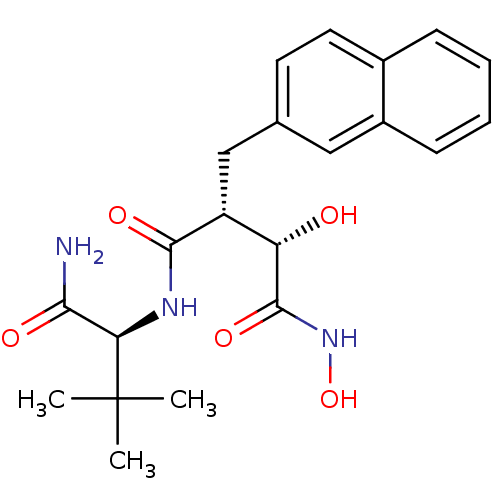
((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H27N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-10,15-17,25,29H,11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082218
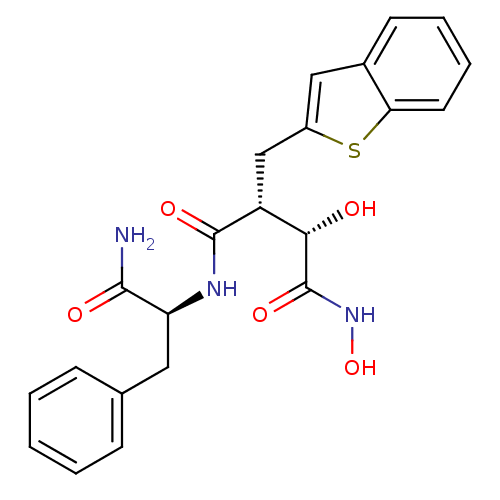
((2R,3S)-2-Benzo[b]thiophen-2-ylmethyl-N*1*-((S)-1-...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc2ccccc2s1)[C@H](O)C(=O)NO Show InChI InChI=1S/C22H23N3O5S/c23-20(27)17(10-13-6-2-1-3-7-13)24-21(28)16(19(26)22(29)25-30)12-15-11-14-8-4-5-9-18(14)31-15/h1-9,11,16-17,19,26,30H,10,12H2,(H2,23,27)(H,24,28)(H,25,29)/t16-,17+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082225
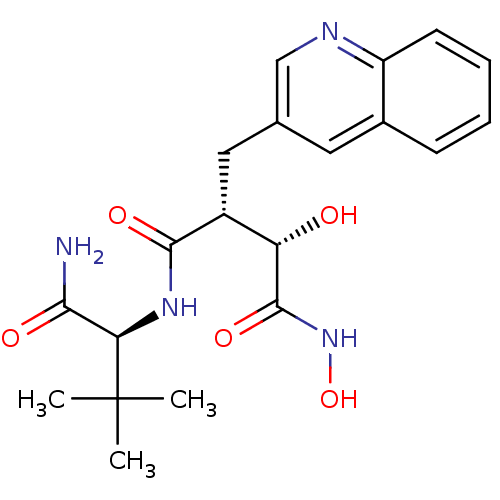
((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1cnc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C20H26N4O5/c1-20(2,3)16(17(21)26)23-18(27)13(15(25)19(28)24-29)9-11-8-12-6-4-5-7-14(12)22-10-11/h4-8,10,13,15-16,25,29H,9H2,1-3H3,(H2,21,26)(H,23,27)(H,24,28)/t13-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50403719

(CHEMBL2111687)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50403718
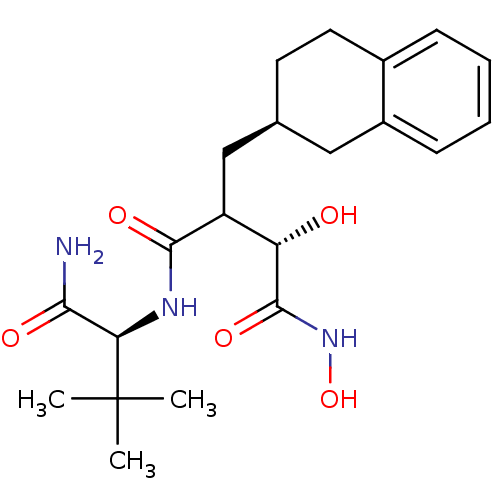
(CHEMBL2112477)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082221
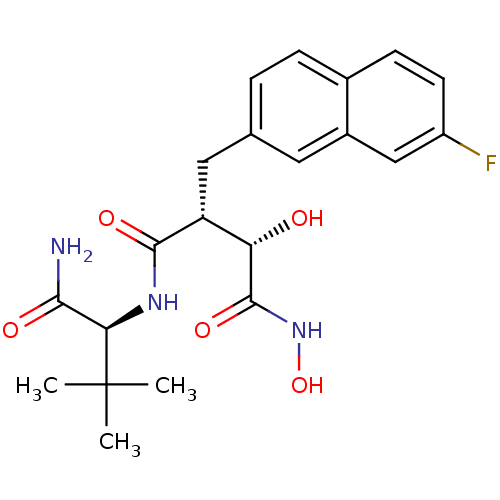
((2R,3S)-N*1*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccc(F)cc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H26FN3O5/c1-21(2,3)17(18(23)27)24-19(28)15(16(26)20(29)25-30)9-11-4-5-12-6-7-14(22)10-13(12)8-11/h4-8,10,15-17,26,30H,9H2,1-3H3,(H2,23,27)(H,24,28)(H,25,29)/t15-,16+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Proteus mirabilis) | BDBM50021959

(CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...)Show InChI InChI=1S/C8H9NO5/c10-2-1-4-7(8(12)13)9-5(11)3-6(9)14-4/h1,6-7,10H,2-3H2,(H,12,13)/b4-1-/t6-,7-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95.4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Proteus mirabilis C889 beta-lactamase assessed as inhibition of nitrocefin hydrolysis preincubated for 5 mins by microtiter plate assay |
J Nat Prod 57: 654-7 (1994)
Article DOI: 10.1021/np50107a016
BindingDB Entry DOI: 10.7270/Q2SQ935W |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082214
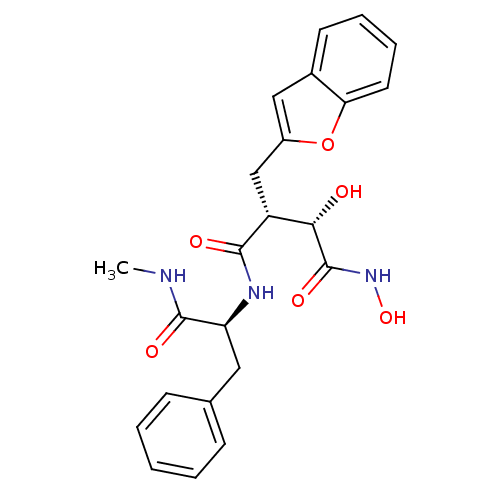
((2R,3S)-2-Benzofuran-2-ylmethyl-3,N*4*-dihydroxy-N...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc2ccccc2o1)[C@H](O)C(=O)NO Show InChI InChI=1S/C23H25N3O6/c1-24-22(29)18(11-14-7-3-2-4-8-14)25-21(28)17(20(27)23(30)26-31)13-16-12-15-9-5-6-10-19(15)32-16/h2-10,12,17-18,20,27,31H,11,13H2,1H3,(H,24,29)(H,25,28)(H,26,30)/t17-,18+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082216

((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(7...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccc(F)cc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H24FN3O5/c25-18-9-8-16-7-6-15(10-17(16)13-18)11-19(21(29)24(32)28-33)23(31)27-20(22(26)30)12-14-4-2-1-3-5-14/h1-10,13,19-21,29,33H,11-12H2,(H2,26,30)(H,27,31)(H,28,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082214

((2R,3S)-2-Benzofuran-2-ylmethyl-3,N*4*-dihydroxy-N...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc2ccccc2o1)[C@H](O)C(=O)NO Show InChI InChI=1S/C23H25N3O6/c1-24-22(29)18(11-14-7-3-2-4-8-14)25-21(28)17(20(27)23(30)26-31)13-16-12-15-9-5-6-10-19(15)32-16/h2-10,12,17-18,20,27,31H,11,13H2,1H3,(H,24,29)(H,25,28)(H,26,30)/t17-,18+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease (MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082218

((2R,3S)-2-Benzo[b]thiophen-2-ylmethyl-N*1*-((S)-1-...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc2ccccc2s1)[C@H](O)C(=O)NO Show InChI InChI=1S/C22H23N3O5S/c23-20(27)17(10-13-6-2-1-3-7-13)24-21(28)16(19(26)22(29)25-30)12-15-11-14-8-4-5-9-18(14)31-15/h1-9,11,16-17,19,26,30H,10,12H2,(H2,23,27)(H,24,28)(H,25,29)/t16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50021959

(CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...)Show InChI InChI=1S/C8H9NO5/c10-2-1-4-7(8(12)13)9-5(11)3-6(9)14-4/h1,6-7,10H,2-3H2,(H,12,13)/b4-1-/t6-,7-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli JT4 beta-lactamase TEM1 assessed as inhibition of nitrocefin hydrolysis preincubated for 5 mins by microtiter plate as... |
J Nat Prod 57: 654-7 (1994)
Article DOI: 10.1021/np50107a016
BindingDB Entry DOI: 10.7270/Q2SQ935W |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Staphylococcus aureus) | BDBM50021959

(CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...)Show InChI InChI=1S/C8H9NO5/c10-2-1-4-7(8(12)13)9-5(11)3-6(9)14-4/h1,6-7,10H,2-3H2,(H,12,13)/b4-1-/t6-,7-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus Russell beta-lactamase assessed as inhibition of nitrocefin hydrolysis pre-incubated for 5 mins by microtiter pla... |
J Nat Prod 57: 654-7 (1994)
Article DOI: 10.1021/np50107a016
BindingDB Entry DOI: 10.7270/Q2SQ935W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082225

((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1cnc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C20H26N4O5/c1-20(2,3)16(17(21)26)23-18(27)13(15(25)19(28)24-29)9-11-8-12-6-4-5-7-14(12)22-10-11/h4-8,10,13,15-16,25,29H,9H2,1-3H3,(H2,21,26)(H,23,27)(H,24,28)/t13-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-9) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082220
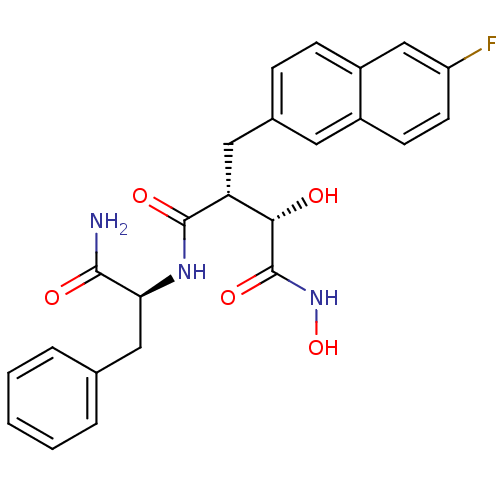
((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(6...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2cc(F)ccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H24FN3O5/c25-18-9-8-16-10-15(6-7-17(16)13-18)11-19(21(29)24(32)28-33)23(31)27-20(22(26)30)12-14-4-2-1-3-5-14/h1-10,13,19-21,29,33H,11-12H2,(H2,26,30)(H,27,31)(H,28,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082229
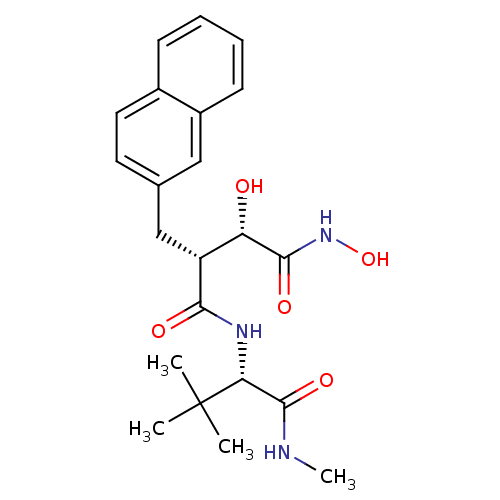
((2R,3S)-N*4*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C22H29N3O5/c1-22(2,3)18(21(29)23-4)24-19(27)16(17(26)20(28)25-30)12-13-9-10-14-7-5-6-8-15(14)11-13/h5-11,16-18,26,30H,12H2,1-4H3,(H,23,29)(H,24,27)(H,25,28)/t16-,17+,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50403718

(CHEMBL2112477)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase(MMP-3) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50403718

(CHEMBL2112477)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-9) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082227
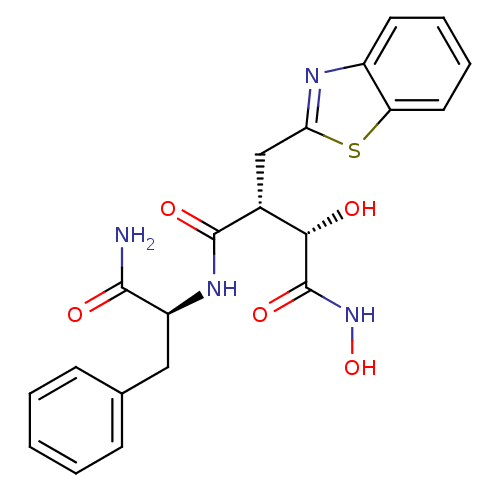
((2R,3S)-2-Benzothiazol-2-ylmethyl-N*1*-((S)-1-carb...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1nc2ccccc2s1)[C@H](O)C(=O)NO Show InChI InChI=1S/C21H22N4O5S/c22-19(27)15(10-12-6-2-1-3-7-12)24-20(28)13(18(26)21(29)25-30)11-17-23-14-8-4-5-9-16(14)31-17/h1-9,13,15,18,26,30H,10-11H2,(H2,22,27)(H,24,28)(H,25,29)/t13-,15+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082217
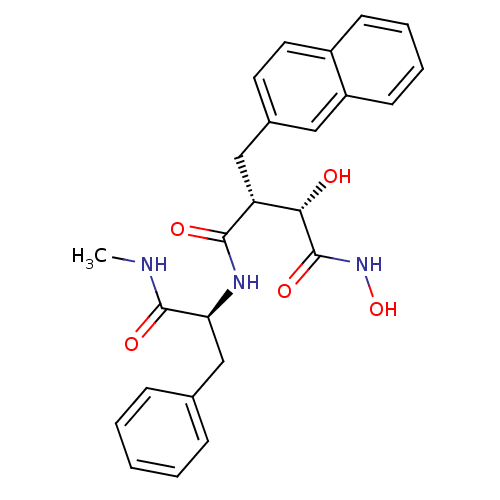
((2R,3S)-2,N*1*-Dihydroxy-N*4*-((S)-1-methylcarbamo...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C25H27N3O5/c1-26-24(31)21(15-16-7-3-2-4-8-16)27-23(30)20(22(29)25(32)28-33)14-17-11-12-18-9-5-6-10-19(18)13-17/h2-13,20-22,29,33H,14-15H2,1H3,(H,26,31)(H,27,30)(H,28,32)/t20-,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50403719

(CHEMBL2111687)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-9) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082227

((2R,3S)-2-Benzothiazol-2-ylmethyl-N*1*-((S)-1-carb...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1nc2ccccc2s1)[C@H](O)C(=O)NO Show InChI InChI=1S/C21H22N4O5S/c22-19(27)15(10-12-6-2-1-3-7-12)24-20(28)13(18(26)21(29)25-30)11-17-23-14-8-4-5-9-16(14)31-17/h1-9,13,15,18,26,30H,10-11H2,(H2,22,27)(H,24,28)(H,25,29)/t13-,15+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082215

((2R,3S)-N*4*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2,N*...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H25N3O5/c25-22(29)20(14-15-6-2-1-3-7-15)26-23(30)19(21(28)24(31)27-32)13-16-10-11-17-8-4-5-9-18(17)12-16/h1-12,19-21,28,32H,13-14H2,(H2,25,29)(H,26,30)(H,27,31)/t19-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082226

((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H27N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-10,15-17,25,29H,11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-9) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082224
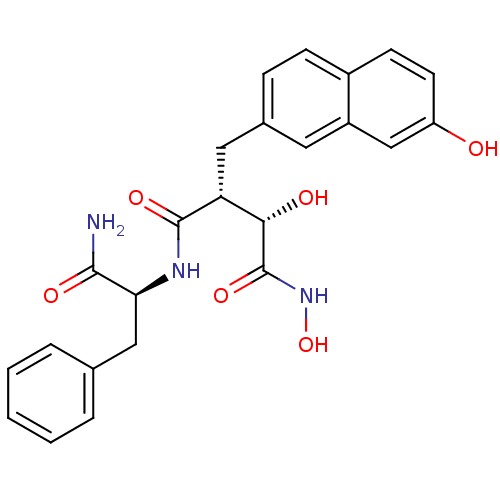
((2R,3S)-N*4*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2,N*...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccc(O)cc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H25N3O6/c25-22(30)20(12-14-4-2-1-3-5-14)26-23(31)19(21(29)24(32)27-33)11-15-6-7-16-8-9-18(28)13-17(16)10-15/h1-10,13,19-21,28-29,33H,11-12H2,(H2,25,30)(H,26,31)(H,27,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50403718

(CHEMBL2112477)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082220

((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(6...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2cc(F)ccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H24FN3O5/c25-18-9-8-16-10-15(6-7-17(16)13-18)11-19(21(29)24(32)28-33)23(31)27-20(22(26)30)12-14-4-2-1-3-5-14/h1-10,13,19-21,29,33H,11-12H2,(H2,26,30)(H,27,31)(H,28,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-9) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082217

((2R,3S)-2,N*1*-Dihydroxy-N*4*-((S)-1-methylcarbamo...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C25H27N3O5/c1-26-24(31)21(15-16-7-3-2-4-8-16)27-23(30)20(22(29)25(32)28-33)14-17-11-12-18-9-5-6-10-19(18)13-17/h2-13,20-22,29,33H,14-15H2,1H3,(H,26,31)(H,27,30)(H,28,32)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069587
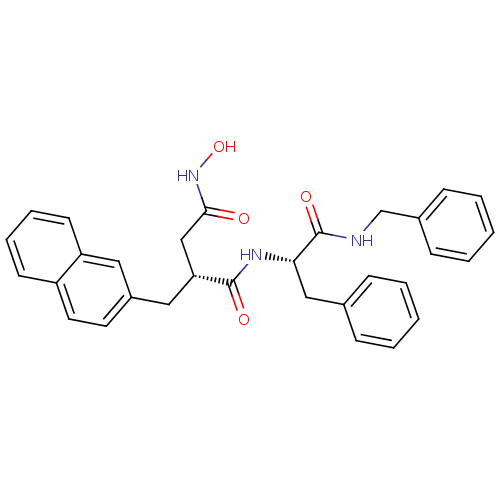
((R)-N*1*-((S)-1-Benzylcarbamoyl-2-phenyl-ethyl)-N*...)Show SMILES ONC(=O)C[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H31N3O4/c35-29(34-38)20-27(18-24-15-16-25-13-7-8-14-26(25)17-24)30(36)33-28(19-22-9-3-1-4-10-22)31(37)32-21-23-11-5-2-6-12-23/h1-17,27-28,38H,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082230
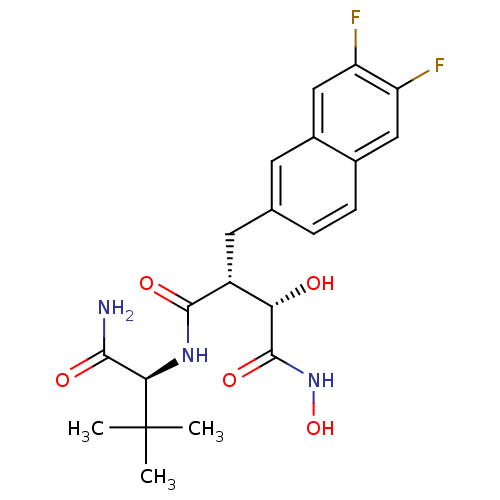
((2R,3S)-N*1*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2cc(F)c(F)cc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H25F2N3O5/c1-21(2,3)17(18(24)28)25-19(29)13(16(27)20(30)26-31)7-10-4-5-11-8-14(22)15(23)9-12(11)6-10/h4-6,8-9,13,16-17,27,31H,7H2,1-3H3,(H2,24,28)(H,25,29)(H,26,30)/t13-,16+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082231
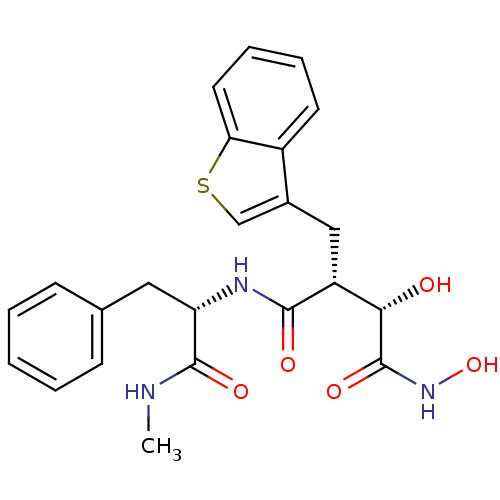
((2R,3S)-2-Benzo[b]thiophen-3-ylmethyl-3,N*4*-dihyd...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1csc2ccccc12)[C@H](O)C(=O)NO Show InChI InChI=1S/C23H25N3O5S/c1-24-22(29)18(11-14-7-3-2-4-8-14)25-21(28)17(20(27)23(30)26-31)12-15-13-32-19-10-6-5-9-16(15)19/h2-10,13,17-18,20,27,31H,11-12H2,1H3,(H,24,29)(H,25,28)(H,26,30)/t17-,18+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069587

((R)-N*1*-((S)-1-Benzylcarbamoyl-2-phenyl-ethyl)-N*...)Show SMILES ONC(=O)C[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H31N3O4/c35-29(34-38)20-27(18-24-15-16-25-13-7-8-14-26(25)17-24)30(36)33-28(19-22-9-3-1-4-10-22)31(37)32-21-23-11-5-2-6-12-23/h1-17,27-28,38H,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082229

((2R,3S)-N*4*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C22H29N3O5/c1-22(2,3)18(21(29)23-4)24-19(27)16(17(26)20(28)25-30)12-13-9-10-14-7-5-6-8-15(14)11-13/h5-11,16-18,26,30H,12H2,1-4H3,(H,23,29)(H,24,27)(H,25,28)/t16-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082228
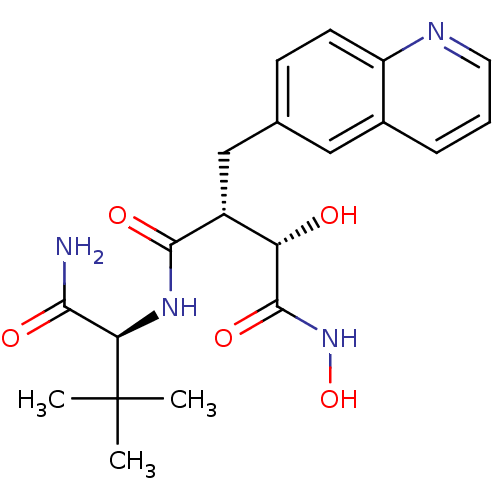
((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ncccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C20H26N4O5/c1-20(2,3)16(17(21)26)23-18(27)13(15(25)19(28)24-29)10-11-6-7-14-12(9-11)5-4-8-22-14/h4-9,13,15-16,25,29H,10H2,1-3H3,(H2,21,26)(H,23,27)(H,24,28)/t13-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50403719

(CHEMBL2111687)Show SMILES CC(C)(C)[C@H](NC(=O)C(C[C@@H]1CCc2ccccc2C1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H31N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-7,12,15-17,25,29H,8-11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t12-,15?,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263562
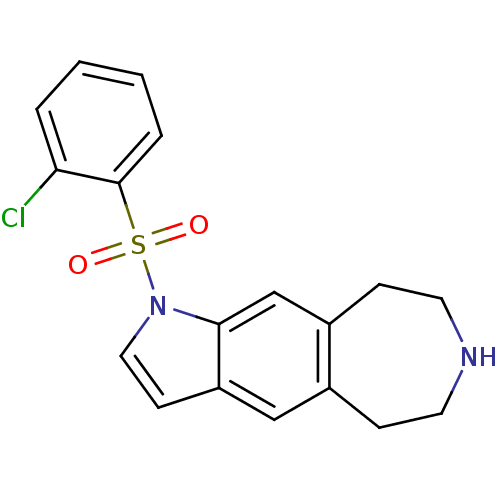
(1-(2-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-3-1-2-4-18(16)24(22,23)21-10-7-15-11-13-5-8-20-9-6-14(13)12-17(15)21/h1-4,7,10-12,20H,5-6,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082220

((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(6...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2cc(F)ccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H24FN3O5/c25-18-9-8-16-10-15(6-7-17(16)13-18)11-19(21(29)24(32)28-33)23(31)27-20(22(26)30)12-14-4-2-1-3-5-14/h1-10,13,19-21,29,33H,11-12H2,(H2,26,30)(H,27,31)(H,28,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase(MMP-3) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082225

((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1cnc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C20H26N4O5/c1-20(2,3)16(17(21)26)23-18(27)13(15(25)19(28)24-29)9-11-8-12-6-4-5-7-14(12)22-10-11/h4-8,10,13,15-16,25,29H,9H2,1-3H3,(H2,21,26)(H,23,27)(H,24,28)/t13-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase(MMP-3) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263563

(1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-2-1-3-17(12-16)24(22,23)21-9-6-15-10-13-4-7-20-8-5-14(13)11-18(15)21/h1-3,6,9-12,20H,4-5,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263562

(1-(2-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-3-1-2-4-18(16)24(22,23)21-10-7-15-11-13-5-8-20-9-6-14(13)12-17(15)21/h1-4,7,10-12,20H,5-6,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082225

((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1cnc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C20H26N4O5/c1-20(2,3)16(17(21)26)23-18(27)13(15(25)19(28)24-29)9-11-8-12-6-4-5-7-14(12)22-10-11/h4-8,10,13,15-16,25,29H,9H2,1-3H3,(H2,21,26)(H,23,27)(H,24,28)/t13-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263563

(1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-2-1-3-17(12-16)24(22,23)21-9-6-15-10-13-4-7-20-8-5-14(13)11-18(15)21/h1-3,6,9-12,20H,4-5,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263617
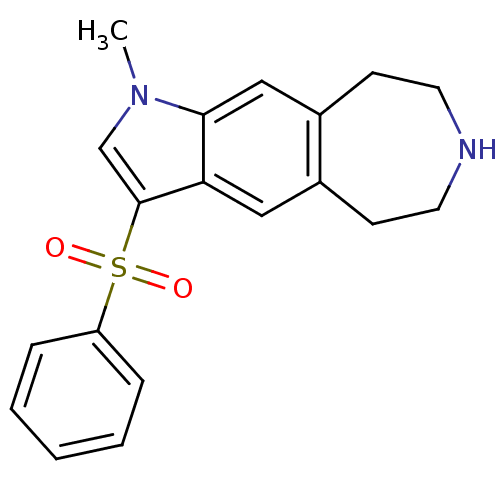
(1-methyl-3-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C19H20N2O2S/c1-21-13-19(24(22,23)16-5-3-2-4-6-16)17-11-14-7-9-20-10-8-15(14)12-18(17)21/h2-6,11-13,20H,7-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263566
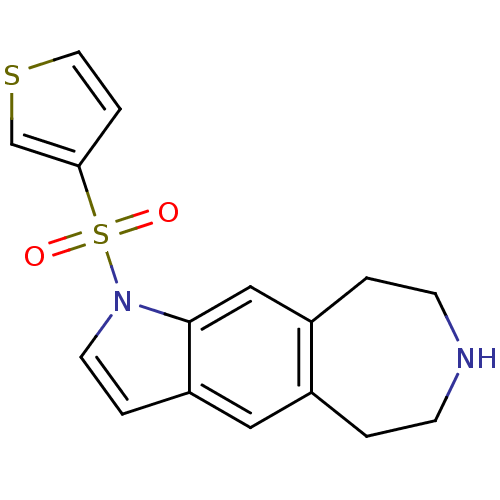
(1-(thiophen-3-ylsulfonyl)-1,5,6,7,8,9-hexahydroaze...)Show InChI InChI=1S/C16H16N2O2S2/c19-22(20,15-4-8-21-11-15)18-7-3-14-9-12-1-5-17-6-2-13(12)10-16(14)18/h3-4,7-11,17H,1-2,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082216

((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(7...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccc(F)cc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H24FN3O5/c25-18-9-8-16-7-6-15(10-17(16)13-18)11-19(21(29)24(32)28-33)23(31)27-20(22(26)30)12-14-4-2-1-3-5-14/h1-10,13,19-21,29,33H,11-12H2,(H2,26,30)(H,27,31)(H,28,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082226

((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H27N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-10,15-17,25,29H,11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase(MMP-3) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082224

((2R,3S)-N*4*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2,N*...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2ccc(O)cc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H25N3O6/c25-22(30)20(12-14-4-2-1-3-5-14)26-23(31)19(21(29)24(32)27-33)11-15-6-7-16-8-9-18(28)13-17(16)10-15/h1-10,13,19-21,28-29,33H,11-12H2,(H2,25,30)(H,26,31)(H,27,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082220

((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(6...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc2cc(F)ccc2c1)[C@H](O)C(=O)NO Show InChI InChI=1S/C24H24FN3O5/c25-18-9-8-16-10-15(6-7-17(16)13-18)11-19(21(29)24(32)28-33)23(31)27-20(22(26)30)12-14-4-2-1-3-5-14/h1-10,13,19-21,29,33H,11-12H2,(H2,26,30)(H,27,31)(H,28,32)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50082226

((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H27N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-10,15-17,25,29H,11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase(MMP-1) |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082212
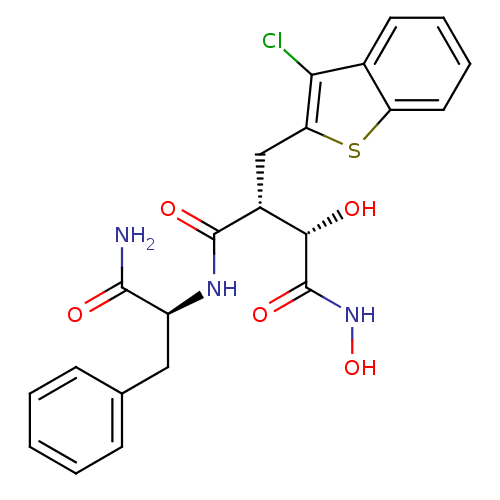
((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-2-(3...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1sc2ccccc2c1Cl)[C@H](O)C(=O)NO Show InChI InChI=1S/C22H22ClN3O5S/c23-18-13-8-4-5-9-16(13)32-17(18)11-14(19(27)22(30)26-31)21(29)25-15(20(24)28)10-12-6-2-1-3-7-12/h1-9,14-15,19,27,31H,10-11H2,(H2,24,28)(H,25,29)(H,26,30)/t14-,15+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data