

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orotidine 5'-phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50199178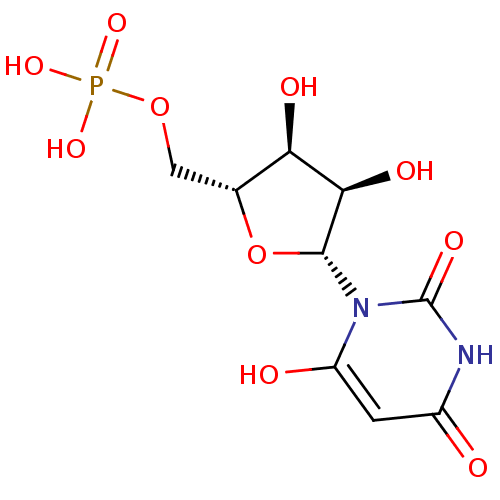 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase after overnight incubation at room temperature by VP-ITC microcalorimetry | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060602 (2-[3-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 1 nM) calculated for the high affinity components of the [3H]spiroperidol b... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060601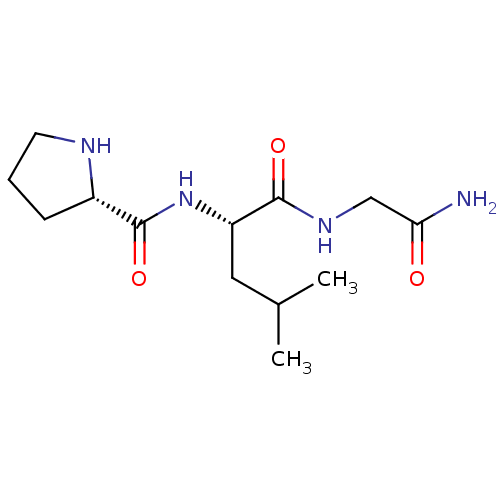 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 1 uM) calculated for the high affinity components of the [3H]spiroperidol b... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093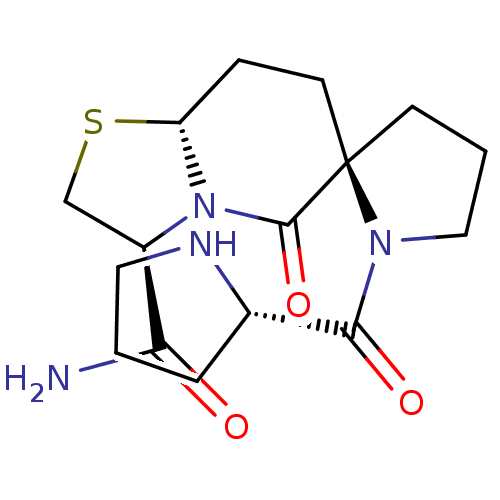 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603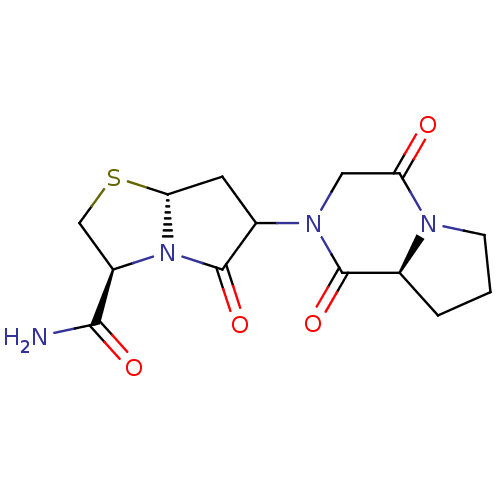 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Percentage of receptor in the low affinity form for the compound to Dopamine receptor D2 in absence of Gpp(NH)p (pre treated with 100 nM) | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 100 nM) calculated for the high affinity components of the [3H]spiroperidol... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Percentage of receptor in the high affinity form for the compound to Dopamine receptor D2 in presence of Gpp(NH)p (pretreated with 100 nM) | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060602 (2-[3-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p (Pre treated with 100 nM) calculated for the high affinity components of the [3H]spiroperidol... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in absence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recept... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060600 ((S)-2-((S)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074092 (6'-oxo-1-[(2S)-tetrahydro-1H-2-pyrrolylcarbonyl]-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060601 ((E)-6-(3-oxo-3-phenylprop-1-enyl)pyrimidine-2,4(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060603 ((3S,7aR)-6-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50060602 (2-[3-((2R,8aS)-1,4-Dioxo-hexahydro-pyrrolo[1,2-a]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of compound in presence of Gpp(NH)p calculated for the high affinity components of the [3H]spiroperidol binding to Dopamine recep... | J Med Chem 40: 3594-600 (1997) Article DOI: 10.1021/jm970328b BindingDB Entry DOI: 10.7270/Q2HX1DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50074093 ((2R,3'S,8a'R)-5'-oxo-1-((S)-pyrrolidine-2-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of... | J Med Chem 42: 628-37 (1999) Article DOI: 10.1021/jm980525q BindingDB Entry DOI: 10.7270/Q27080MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189013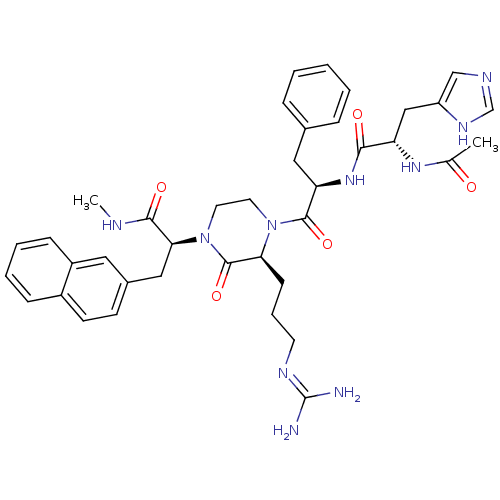 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189024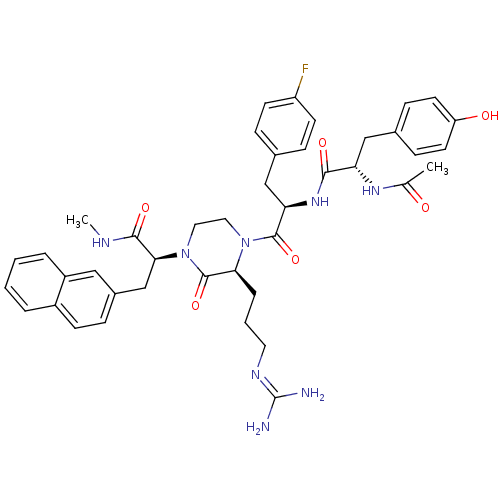 ((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189010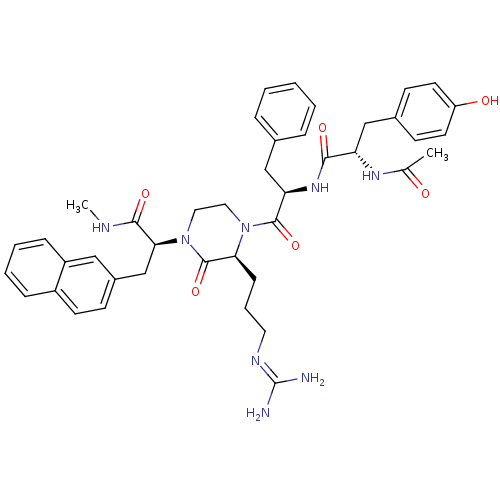 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189013 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189013 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189010 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189010 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189025 ((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189024 ((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50034090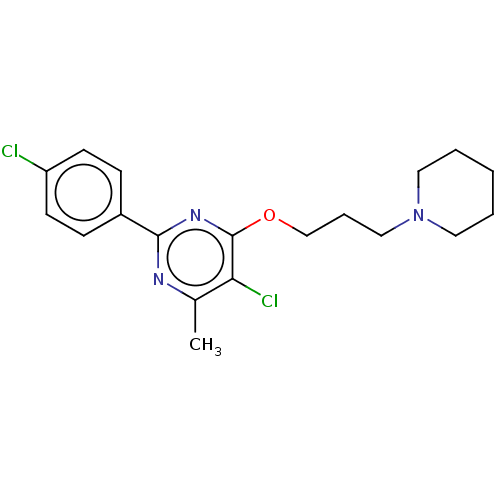 (CHEMBL3359239) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from recombinant human sigma1 receptor expressed in HEK293 cell membranes after 120 mins by microbeta scintillat... | Bioorg Med Chem 27: 1824-1835 (2019) Article DOI: 10.1016/j.bmc.2019.03.030 BindingDB Entry DOI: 10.7270/Q21839XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189023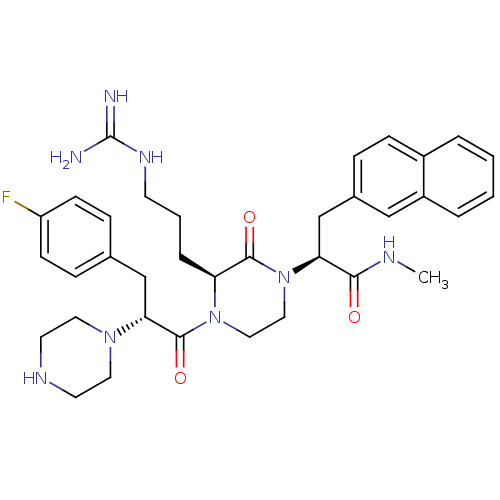 ((S)-2-((S)-4-((R)-3-(4-fluorophenyl)-2-(piperazin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189014 ((S)-2-((S)-4-((R)-2-acetamido-3-(4-fluorophenyl)pr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50253660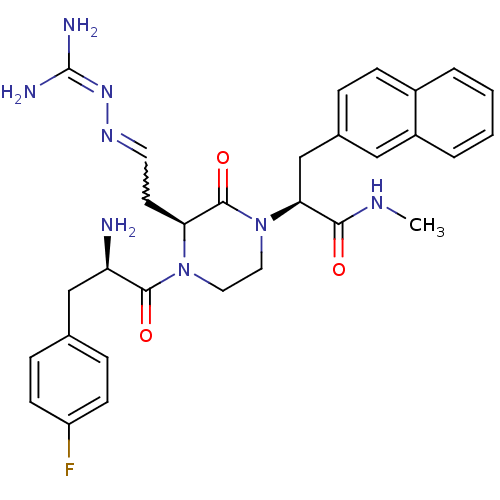 (2-{3-(2-Amino-ethyl-guanidino)-4-[2-amino-3-(4-flu...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells | J Med Chem 51: 6055-66 (2008) Article DOI: 10.1021/jm800525p BindingDB Entry DOI: 10.7270/Q2DV1JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189022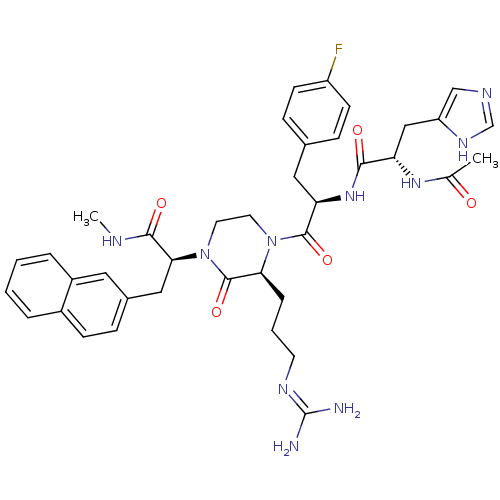 ((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189025 ((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50035131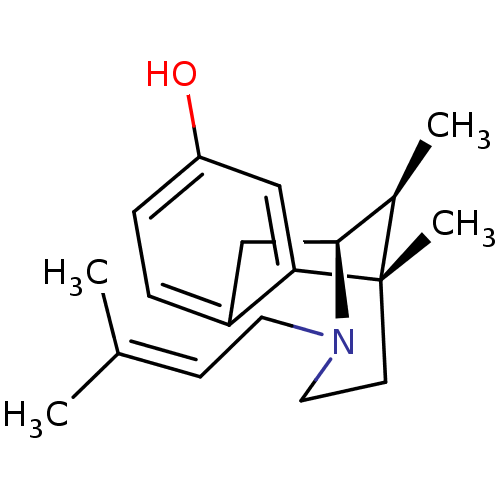 ((+)-(6R,11S)-6,11-dimethyl-3-(3-methyl-but-2-enyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from recombinant human sigma1 receptor expressed in HEK293 cell membranes after 120 mins by microbeta scintillat... | Bioorg Med Chem 27: 1824-1835 (2019) Article DOI: 10.1016/j.bmc.2019.03.030 BindingDB Entry DOI: 10.7270/Q21839XN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189025 ((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50253738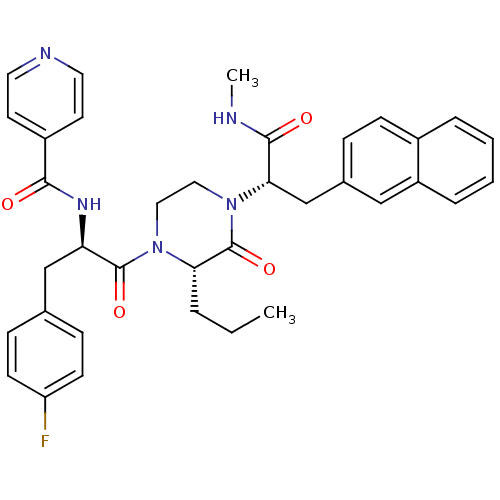 (CHEMBL449131 | N-((R)-3-(4-Fluorophenyl)-1-((S)-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells | J Med Chem 51: 6055-66 (2008) Article DOI: 10.1021/jm800525p BindingDB Entry DOI: 10.7270/Q2DV1JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50253736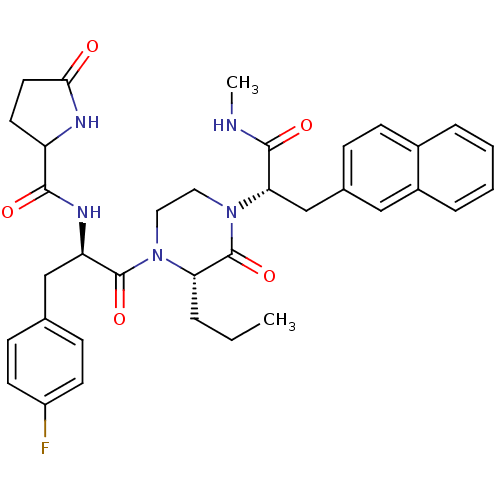 (CHEMBL506272 | N-((R)-3-(4-Fluorophenyl)-1-((S)-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells | J Med Chem 51: 6055-66 (2008) Article DOI: 10.1021/jm800525p BindingDB Entry DOI: 10.7270/Q2DV1JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50400738 (CHEMBL2203551) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from recombinant human sigma1 receptor expressed in HEK293 cell membranes after 120 mins by microbeta scintillat... | Bioorg Med Chem 27: 1824-1835 (2019) Article DOI: 10.1016/j.bmc.2019.03.030 BindingDB Entry DOI: 10.7270/Q21839XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189023 ((S)-2-((S)-4-((R)-3-(4-fluorophenyl)-2-(piperazin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50253745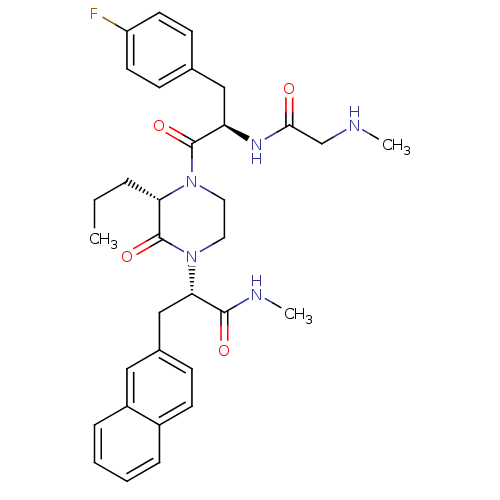 ((S)-2-((S)-4-((R)-3-(4-Fluorophenyl)-2-(2-(methyla...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells | J Med Chem 51: 6055-66 (2008) Article DOI: 10.1021/jm800525p BindingDB Entry DOI: 10.7270/Q2DV1JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1089 total ) | Next | Last >> |