 Found 175 hits with Last Name = 'mitani' and Initial = 'h'
Found 175 hits with Last Name = 'mitani' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348228
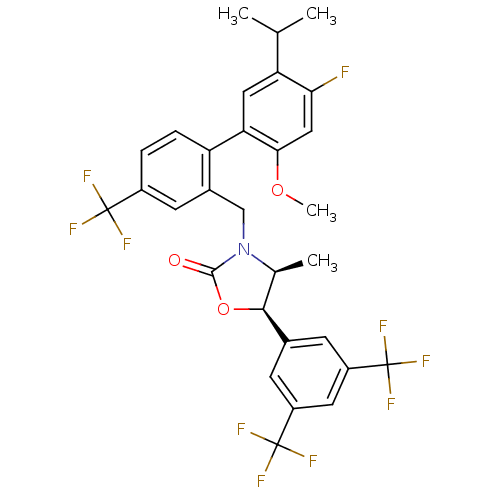
(CHEMBL1800807)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50312718
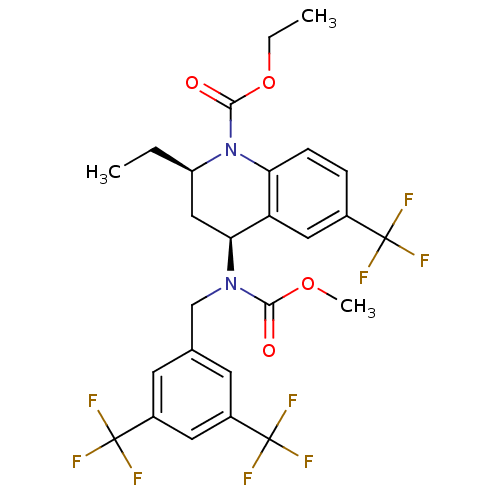
(CHEMBL479527 | torcetrapib)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)C(=O)OC)c2cc(ccc12)C(F)(F)F |r| Show InChI InChI=1S/C26H25F9N2O4/c1-4-18-12-21(19-11-15(24(27,28)29)6-7-20(19)37(18)23(39)41-5-2)36(22(38)40-3)13-14-8-16(25(30,31)32)10-17(9-14)26(33,34)35/h6-11,18,21H,4-5,12-13H2,1-3H3/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250371
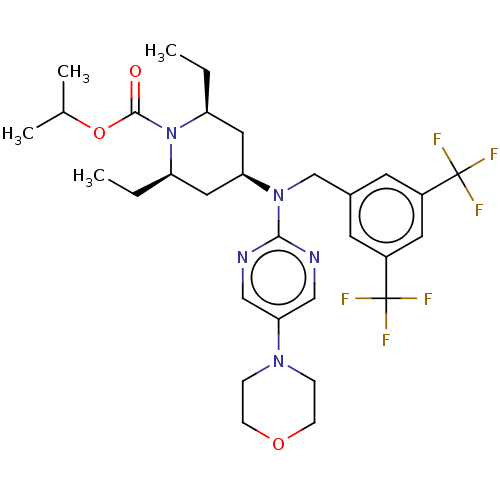
(CHEMBL4064793)Show SMILES CC[C@H]1C[C@H](C[C@@H](CC)N1C(=O)OC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)N1CCOCC1 |r| Show InChI InChI=1S/C30H39F6N5O3/c1-5-23-14-25(15-24(6-2)41(23)28(42)44-19(3)4)40(27-37-16-26(17-38-27)39-7-9-43-10-8-39)18-20-11-21(29(31,32)33)13-22(12-20)30(34,35)36/h11-13,16-17,19,23-25H,5-10,14-15,18H2,1-4H3/t23-,24+,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250366
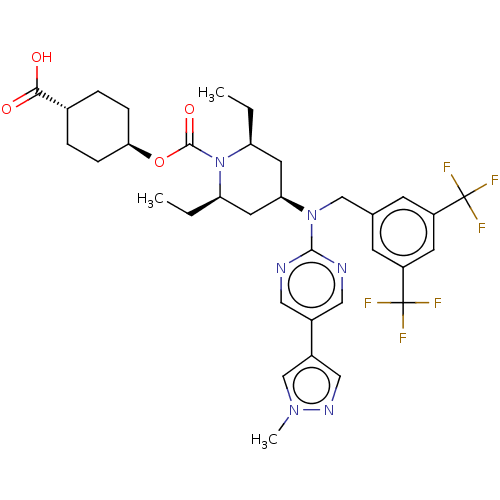
(CHEMBL4075773)Show SMILES CC[C@H]1C[C@H](C[C@@H](CC)N1C(=O)O[C@H]1CC[C@@H](CC1)C(O)=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r,wU:4.23,6.6,2.1,13.13,wD:16.20,(37.57,-12.9,;38.91,-13.68,;40.24,-12.9,;40.24,-11.36,;41.57,-10.59,;42.9,-11.36,;42.9,-12.9,;44.24,-13.68,;45.57,-12.9,;41.57,-13.67,;41.57,-15.21,;40.24,-15.97,;42.91,-15.97,;42.91,-17.51,;44.24,-18.28,;44.23,-19.82,;42.89,-20.59,;41.56,-19.82,;41.57,-18.28,;42.89,-22.13,;41.55,-22.9,;44.22,-22.91,;41.57,-9.05,;40.24,-8.27,;40.24,-6.73,;41.58,-5.97,;41.58,-4.43,;40.24,-3.66,;38.9,-4.44,;38.91,-5.98,;37.57,-3.67,;37.56,-2.13,;36.23,-4.45,;36.23,-2.9,;42.91,-3.66,;44.25,-4.44,;42.91,-2.12,;44.24,-2.89,;42.9,-8.28,;44.24,-9.05,;45.57,-8.28,;45.57,-6.74,;44.23,-5.97,;42.9,-6.74,;46.9,-5.97,;48.3,-6.59,;49.34,-5.45,;48.56,-4.11,;49.19,-2.71,;47.05,-4.44,)| Show InChI InChI=1S/C34H40F6N6O4/c1-4-26-13-28(14-27(5-2)46(26)32(49)50-29-8-6-21(7-9-29)30(47)48)45(31-41-15-22(16-42-31)23-17-43-44(3)19-23)18-20-10-24(33(35,36)37)12-25(11-20)34(38,39)40/h10-12,15-17,19,21,26-29H,4-9,13-14,18H2,1-3H3,(H,47,48)/t21-,26-,27+,28+,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250363
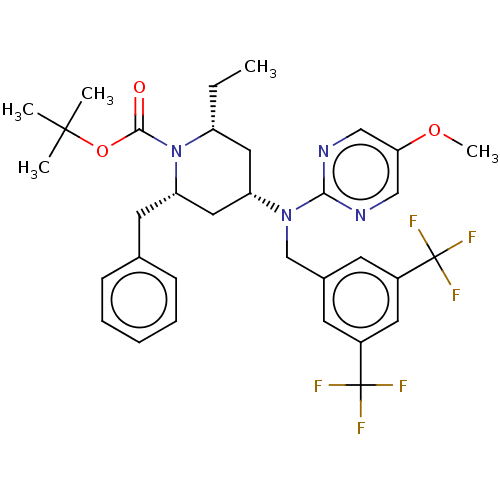
(CHEMBL4070653)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(OC)cn1 |r| Show InChI InChI=1S/C33H38F6N4O3/c1-6-25-16-26(17-27(14-21-10-8-7-9-11-21)43(25)30(44)46-31(2,3)4)42(29-40-18-28(45-5)19-41-29)20-22-12-23(32(34,35)36)15-24(13-22)33(37,38)39/h7-13,15,18-19,25-27H,6,14,16-17,20H2,1-5H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218471
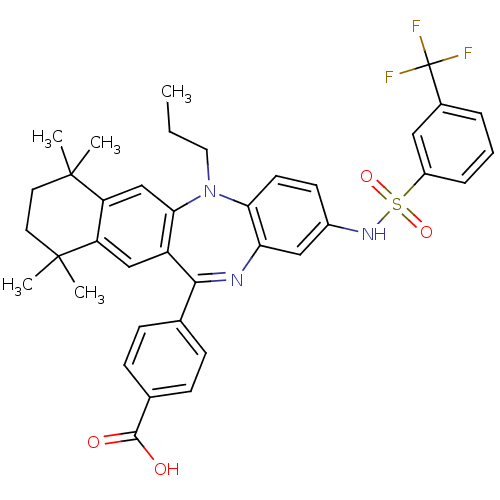
(4-[7,7,10,10-tetramethyl-5-propyl-2-(3-trifluorome...)Show SMILES CCCN1c2ccc(NS(=O)(=O)c3cccc(c3)C(F)(F)F)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:26| Show InChI InChI=1S/C38H38F3N3O4S/c1-6-18-44-32-15-14-26(43-49(47,48)27-9-7-8-25(19-27)38(39,40)41)20-31(32)42-34(23-10-12-24(13-11-23)35(45)46)28-21-29-30(22-33(28)44)37(4,5)17-16-36(29,2)3/h7-15,19-22,43H,6,16-18H2,1-5H3,(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218455

(4-[5-ethyl-7,7,10,10-tetramethyl-2-(3-trifluoromet...)Show SMILES CCN1c2ccc(NS(=O)(=O)c3cccc(c3)C(F)(F)F)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:25| Show InChI InChI=1S/C37H36F3N3O4S/c1-6-43-31-15-14-25(42-48(46,47)26-9-7-8-24(18-26)37(38,39)40)19-30(31)41-33(22-10-12-23(13-11-22)34(44)45)27-20-28-29(21-32(27)43)36(4,5)17-16-35(28,2)3/h7-15,18-21,42H,6,16-17H2,1-5H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50381415
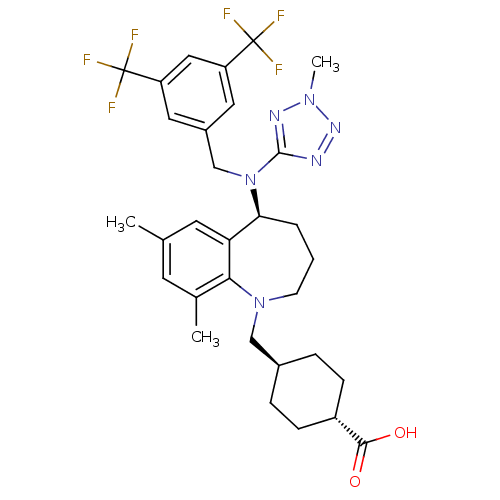
(EVACETRAPIB | LY2484595)Show SMILES Cc1cc(C)c2N(C[C@H]3CC[C@@H](CC3)C(O)=O)CCC[C@H](N(Cc3cc(cc(c3)C(F)(F)F)C(F)(F)F)c3nnn(C)n3)c2c1 |r,wU:20.21,11.14,wD:8.7,(-5.45,-18.22,;-4.12,-17.45,;-2.78,-18.22,;-1.45,-17.44,;-.11,-18.2,;-1.45,-15.91,;-.01,-15.34,;1.12,-16.38,;2.59,-15.92,;3.91,-16.71,;5.25,-15.96,;5.27,-14.42,;3.95,-13.63,;2.6,-14.38,;6.62,-13.68,;7.94,-14.47,;6.65,-12.14,;.44,-13.86,;-.45,-12.58,;-1.99,-12.47,;-3.03,-13.61,;-4.36,-12.84,;-4.37,-11.31,;-5.7,-10.54,;-5.7,-8.99,;-7.04,-8.22,;-8.37,-8.99,;-8.37,-10.54,;-7.04,-11.31,;-9.7,-11.31,;-11.04,-10.54,;-9.7,-12.85,;-11.05,-12.07,;-7.04,-6.69,;-5.71,-5.91,;-8.38,-5.92,;-7.06,-5.14,;-5.69,-13.62,;-7.1,-12.98,;-8.13,-14.13,;-7.35,-15.46,;-7.98,-16.87,;-5.85,-15.14,;-2.79,-15.14,;-4.12,-15.91,)| Show InChI InChI=1S/C31H36F6N6O2/c1-18-11-19(2)27-25(12-18)26(5-4-10-42(27)16-20-6-8-22(9-7-20)28(44)45)43(29-38-40-41(3)39-29)17-21-13-23(30(32,33)34)15-24(14-21)31(35,36)37/h11-15,20,22,26H,4-10,16-17H2,1-3H3,(H,44,45)/t20-,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218481
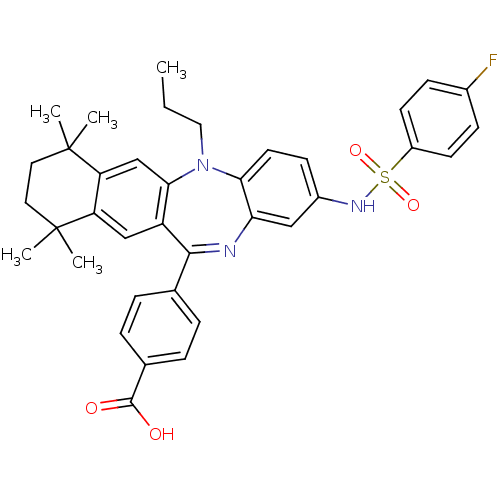
(4-[2-(4-fluoro-benzenesulfonylamino)-7,7,10,10-tet...)Show SMILES CCCN1c2ccc(NS(=O)(=O)c3ccc(F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:23| Show InChI InChI=1S/C37H38FN3O4S/c1-6-19-41-32-16-13-26(40-46(44,45)27-14-11-25(38)12-15-27)20-31(32)39-34(23-7-9-24(10-8-23)35(42)43)28-21-29-30(22-33(28)41)37(4,5)18-17-36(29,2)3/h7-16,20-22,40H,6,17-19H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218438
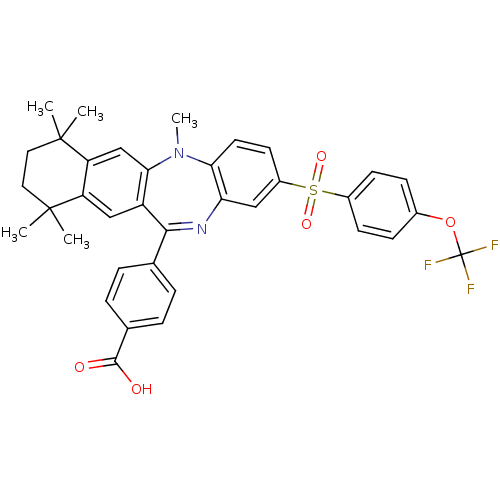
(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-b...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H33F3N2O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)47(44,45)24-12-10-23(11-13-24)46-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218461

(4-[2-(butane-1-sulfonylamino)-7,7,10,10-tetramethy...)Show SMILES CCCCS(=O)(=O)Nc1ccc2N(CCC)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:23| Show InChI InChI=1S/C35H43N3O4S/c1-7-9-19-43(41,42)37-25-14-15-30-29(20-25)36-32(23-10-12-24(13-11-23)33(39)40)26-21-27-28(22-31(26)38(30)18-8-2)35(5,6)17-16-34(27,3)4/h10-15,20-22,37H,7-9,16-19H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250365
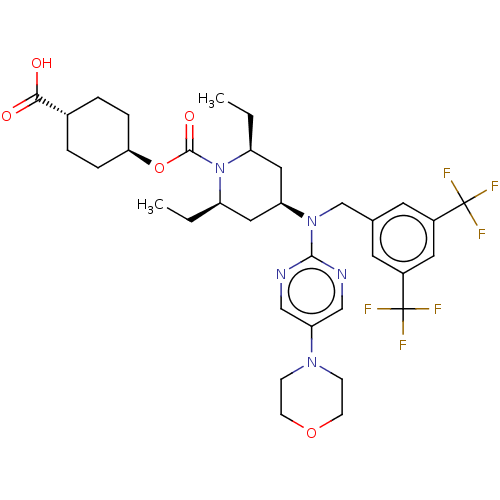
(CHEMBL4103344)Show SMILES CC[C@H]1C[C@H](C[C@@H](CC)N1C(=O)O[C@H]1CC[C@@H](CC1)C(O)=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)N1CCOCC1 |r,wU:4.23,6.6,2.1,13.13,wD:16.20,(20.99,-12.46,;22.31,-13.23,;23.65,-12.46,;23.65,-10.92,;24.98,-10.15,;26.31,-10.92,;26.31,-12.46,;27.64,-13.23,;28.98,-12.46,;24.98,-13.22,;24.98,-14.76,;23.64,-15.53,;26.31,-15.53,;26.31,-17.07,;27.65,-17.84,;27.64,-19.38,;26.3,-20.15,;24.97,-19.37,;24.97,-17.84,;26.3,-21.69,;24.96,-22.46,;27.63,-22.46,;24.98,-8.61,;23.64,-7.83,;23.64,-6.29,;24.98,-5.53,;24.99,-3.99,;23.65,-3.22,;22.31,-4,;22.32,-5.54,;20.98,-3.23,;20.98,-1.69,;19.65,-4.01,;19.64,-2.46,;26.32,-3.22,;27.65,-3.99,;26.32,-1.68,;27.65,-2.45,;26.31,-7.84,;27.65,-8.61,;28.98,-7.84,;28.98,-6.3,;27.64,-5.53,;26.31,-6.3,;30.31,-5.53,;31.63,-6.3,;32.96,-5.54,;32.97,-4,;31.63,-3.22,;30.3,-3.99,)| Show InChI InChI=1S/C34H43F6N5O5/c1-3-25-16-27(17-26(4-2)45(25)32(48)50-29-7-5-22(6-8-29)30(46)47)44(31-41-18-28(19-42-31)43-9-11-49-12-10-43)20-21-13-23(33(35,36)37)15-24(14-21)34(38,39)40/h13-15,18-19,22,25-27,29H,3-12,16-17,20H2,1-2H3,(H,46,47)/t22-,25-,26+,27+,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218455

(4-[5-ethyl-7,7,10,10-tetramethyl-2-(3-trifluoromet...)Show SMILES CCN1c2ccc(NS(=O)(=O)c3cccc(c3)C(F)(F)F)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:25| Show InChI InChI=1S/C37H36F3N3O4S/c1-6-43-31-15-14-25(42-48(46,47)26-9-7-8-24(18-26)37(38,39)40)19-30(31)41-33(22-10-12-23(13-11-22)34(44)45)27-20-28-29(21-32(27)43)36(4,5)17-16-35(28,2)3/h7-15,18-21,42H,6,16-17H2,1-5H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of LG100268-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250355
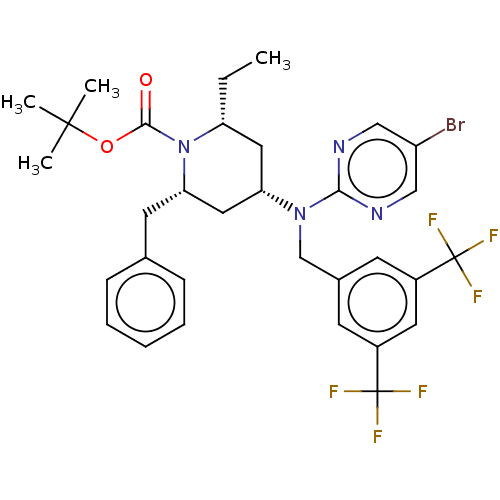
(CHEMBL4081265)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(Br)cn1 |r| Show InChI InChI=1S/C32H35BrF6N4O2/c1-5-25-15-26(16-27(13-20-9-7-6-8-10-20)43(25)29(44)45-30(2,3)4)42(28-40-17-24(33)18-41-28)19-21-11-22(31(34,35)36)14-23(12-21)32(37,38)39/h6-12,14,17-18,25-27H,5,13,15-16,19H2,1-4H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250355

(CHEMBL4081265)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(Br)cn1 |r| Show InChI InChI=1S/C32H35BrF6N4O2/c1-5-25-15-26(16-27(13-20-9-7-6-8-10-20)43(25)29(44)45-30(2,3)4)42(28-40-17-24(33)18-41-28)19-21-11-22(31(34,35)36)14-23(12-21)32(37,38)39/h6-12,14,17-18,25-27H,5,13,15-16,19H2,1-4H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250364
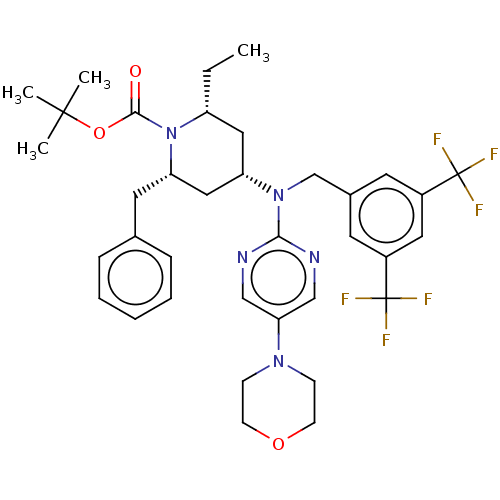
(CHEMBL4063323)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)N1CCOCC1 |r| Show InChI InChI=1S/C36H43F6N5O3/c1-5-28-19-29(20-30(17-24-9-7-6-8-10-24)47(28)33(48)50-34(2,3)4)46(32-43-21-31(22-44-32)45-11-13-49-14-12-45)23-25-15-26(35(37,38)39)18-27(16-25)36(40,41)42/h6-10,15-16,18,21-22,28-30H,5,11-14,17,19-20,23H2,1-4H3/t28-,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250364

(CHEMBL4063323)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)N1CCOCC1 |r| Show InChI InChI=1S/C36H43F6N5O3/c1-5-28-19-29(20-30(17-24-9-7-6-8-10-24)47(28)33(48)50-34(2,3)4)46(32-43-21-31(22-44-32)45-11-13-49-14-12-45)23-25-15-26(35(37,38)39)18-27(16-25)36(40,41)42/h6-10,15-16,18,21-22,28-30H,5,11-14,17,19-20,23H2,1-4H3/t28-,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218429
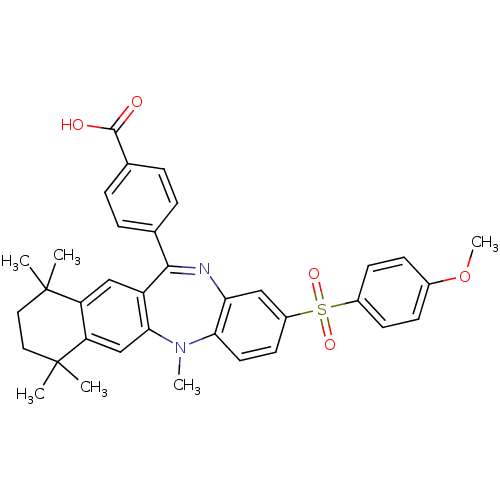
(4-[2-(4-methoxy-benzenesulfonyl)-5,7,7,10,10-penta...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:25| Show InChI InChI=1S/C36H36N2O5S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(39)40)37-30-19-26(15-16-31(30)38(32)5)44(41,42)25-13-11-24(43-6)12-14-25/h7-16,19-21H,17-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218471

(4-[7,7,10,10-tetramethyl-5-propyl-2-(3-trifluorome...)Show SMILES CCCN1c2ccc(NS(=O)(=O)c3cccc(c3)C(F)(F)F)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:26| Show InChI InChI=1S/C38H38F3N3O4S/c1-6-18-44-32-15-14-26(43-49(47,48)27-9-7-8-25(19-27)38(39,40)41)20-31(32)42-34(23-10-12-24(13-11-23)35(45)46)28-21-29-30(22-33(28)44)37(4,5)17-16-36(29,2)3/h7-15,19-22,43H,6,16-18H2,1-5H3,(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of LG100268-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218468
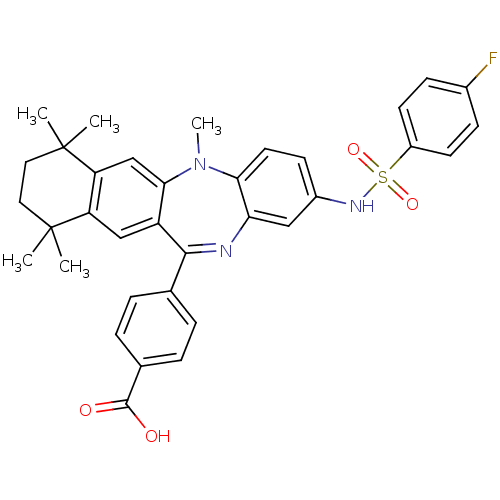
(4-[2-(4-fluoro-benzenesulfonylamino)-5,7,7,10,10-p...)Show SMILES CN1c2ccc(NS(=O)(=O)c3ccc(F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:21| Show InChI InChI=1S/C35H34FN3O4S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(40)41)37-29-18-24(12-15-30(29)39(31)5)38-44(42,43)25-13-10-23(36)11-14-25/h6-15,18-20,38H,16-17H2,1-5H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218448
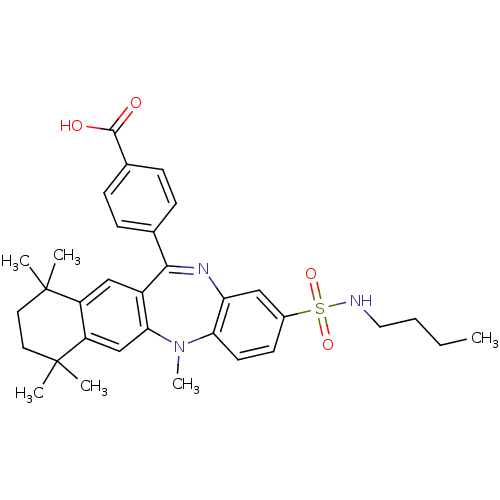
(4-(2-butylsulfamoyl-5,7,7,10,10-pentamethyl-7,8,9,...)Show SMILES CCCCNS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C33H39N3O4S/c1-7-8-17-34-41(39,40)23-13-14-28-27(18-23)35-30(21-9-11-22(12-10-21)31(37)38)24-19-25-26(20-29(24)36(28)6)33(4,5)16-15-32(25,2)3/h9-14,18-20,34H,7-8,15-17H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250370

(CHEMBL4083767)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(Br)cn1 |r| Show InChI InChI=1S/C31H33BrF6N4O2/c1-4-25-14-26(15-27(12-20-8-6-5-7-9-20)42(25)29(43)44-19(2)3)41(28-39-16-24(32)17-40-28)18-21-10-22(30(33,34)35)13-23(11-21)31(36,37)38/h5-11,13,16-17,19,25-27H,4,12,14-15,18H2,1-3H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218458
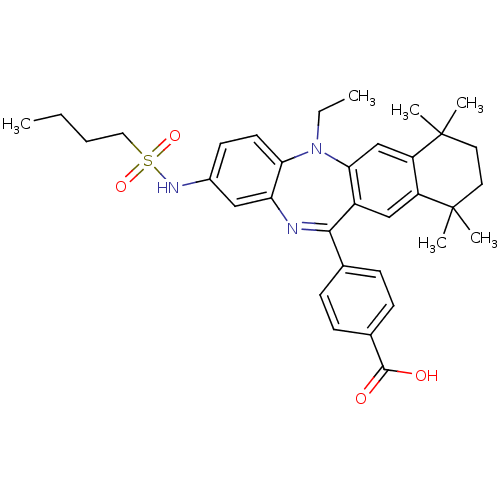
(4-[2-(butane-1-sulfonylamino)-5-ethyl-7,7,10,10-te...)Show SMILES CCCCS(=O)(=O)Nc1ccc2N(CC)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:22| Show InChI InChI=1S/C34H41N3O4S/c1-7-9-18-42(40,41)36-24-14-15-29-28(19-24)35-31(22-10-12-23(13-11-22)32(38)39)25-20-26-27(21-30(25)37(29)8-2)34(5,6)17-16-33(26,3)4/h10-15,19-21,36H,7-9,16-18H2,1-6H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218492

(4-[5-ethyl-2-(4-fluoro-benzenesulfonylamino)-7,7,1...)Show SMILES CCN1c2ccc(NS(=O)(=O)c3ccc(F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:22| Show InChI InChI=1S/C36H36FN3O4S/c1-6-40-31-16-13-25(39-45(43,44)26-14-11-24(37)12-15-26)19-30(31)38-33(22-7-9-23(10-8-22)34(41)42)27-20-28-29(21-32(27)40)36(4,5)18-17-35(28,2)3/h7-16,19-21,39H,6,17-18H2,1-5H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250357
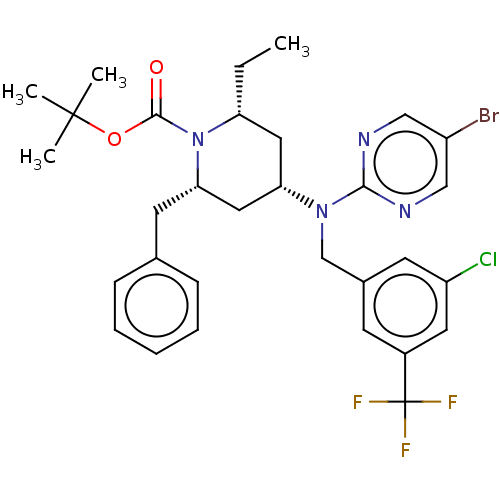
(CHEMBL4089133)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(Cl)cc(c1)C(F)(F)F)c1ncc(Br)cn1 |r| Show InChI InChI=1S/C31H35BrClF3N4O2/c1-5-25-15-26(16-27(13-20-9-7-6-8-10-20)40(25)29(41)42-30(2,3)4)39(28-37-17-23(32)18-38-28)19-21-11-22(31(34,35)36)14-24(33)12-21/h6-12,14,17-18,25-27H,5,13,15-16,19H2,1-4H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218438

(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-b...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H33F3N2O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)47(44,45)24-12-10-23(11-13-24)46-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218495
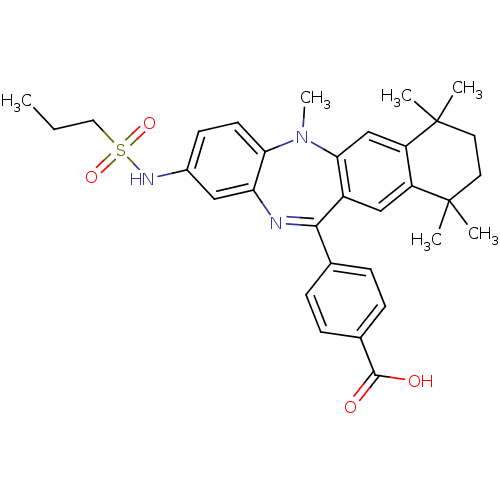
(4-[5,7,7,10,10-pentamethyl-2-(propane-1-sulfonylam...)Show SMILES CCCS(=O)(=O)Nc1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:20| Show InChI InChI=1S/C32H37N3O4S/c1-7-16-40(38,39)34-22-12-13-27-26(17-22)33-29(20-8-10-21(11-9-20)30(36)37)23-18-24-25(19-28(23)35(27)6)32(4,5)15-14-31(24,2)3/h8-13,17-19,34H,7,14-16H2,1-6H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218433
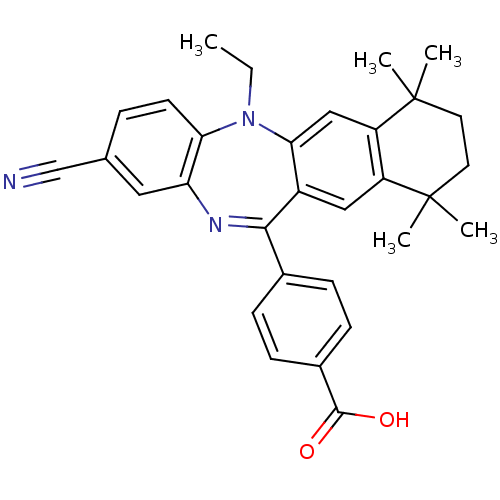
(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H31N3O2/c1-6-34-26-12-7-19(18-32)15-25(26)33-28(20-8-10-21(11-9-20)29(35)36)22-16-23-24(17-27(22)34)31(4,5)14-13-30(23,2)3/h7-12,15-17H,6,13-14H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218458

(4-[2-(butane-1-sulfonylamino)-5-ethyl-7,7,10,10-te...)Show SMILES CCCCS(=O)(=O)Nc1ccc2N(CC)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:22| Show InChI InChI=1S/C34H41N3O4S/c1-7-9-18-42(40,41)36-24-14-15-29-28(19-24)35-31(22-10-12-23(13-11-22)32(38)39)25-20-26-27(21-30(25)37(29)8-2)34(5,6)17-16-33(26,3)4/h10-15,19-21,36H,7-9,16-18H2,1-6H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of LG100268-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218454
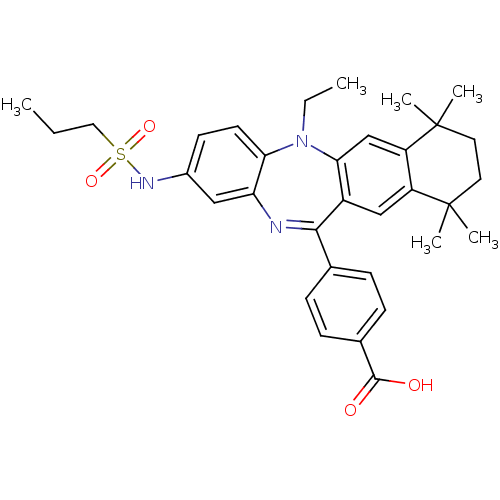
(4-[5-ethyl-7,7,10,10-tetramethyl-2-(propane-1-sulf...)Show SMILES CCCS(=O)(=O)Nc1ccc2N(CC)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C33H39N3O4S/c1-7-17-41(39,40)35-23-13-14-28-27(18-23)34-30(21-9-11-22(12-10-21)31(37)38)24-19-25-26(20-29(24)36(28)8-2)33(5,6)16-15-32(25,3)4/h9-14,18-20,35H,7-8,15-17H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218429

(4-[2-(4-methoxy-benzenesulfonyl)-5,7,7,10,10-penta...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:25| Show InChI InChI=1S/C36H36N2O5S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(39)40)37-30-19-26(15-16-31(30)38(32)5)44(41,42)25-13-11-24(43-6)12-14-25/h7-16,19-21H,17-18H2,1-6H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218469

(4-[2-(butane-1-sulfonylamino)-5,7,7,10,10-pentamet...)Show SMILES CCCCS(=O)(=O)Nc1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C33H39N3O4S/c1-7-8-17-41(39,40)35-23-13-14-28-27(18-23)34-30(21-9-11-22(12-10-21)31(37)38)24-19-25-26(20-29(24)36(28)6)33(4,5)16-15-32(25,2)3/h9-14,18-20,35H,7-8,15-17H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218434
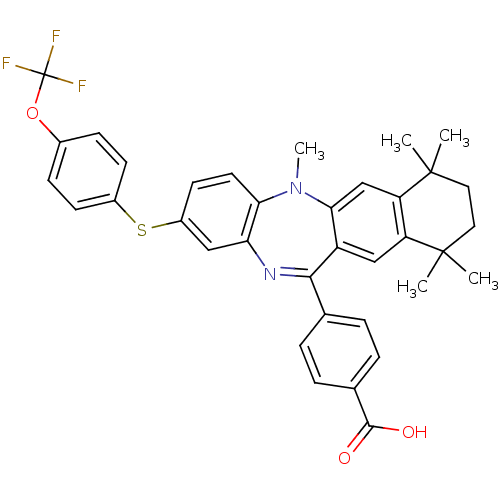
(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-p...)Show SMILES CN1c2ccc(Sc3ccc(OC(F)(F)F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:22| Show InChI InChI=1S/C36H33F3N2O3S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(42)43)40-29-18-25(14-15-30(29)41(31)5)45-24-12-10-23(11-13-24)44-36(37,38)39/h6-15,18-20H,16-17H2,1-5H3,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218496
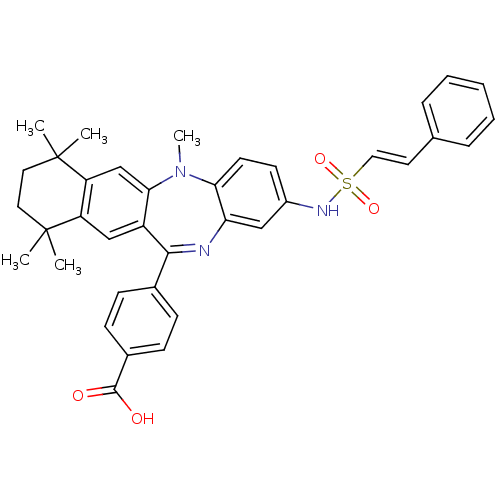
(4-[5,7,7,10,10-pentamethyl-2-((E)-2-phenyl-ethenes...)Show SMILES CN1c2ccc(NS(=O)(=O)\C=C\c3ccccc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:22| Show InChI InChI=1S/C37H37N3O4S/c1-36(2)18-19-37(3,4)30-23-33-28(22-29(30)36)34(25-11-13-26(14-12-25)35(41)42)38-31-21-27(15-16-32(31)40(33)5)39-45(43,44)20-17-24-9-7-6-8-10-24/h6-17,20-23,39H,18-19H2,1-5H3,(H,41,42)/b20-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218448

(4-(2-butylsulfamoyl-5,7,7,10,10-pentamethyl-7,8,9,...)Show SMILES CCCCNS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:21| Show InChI InChI=1S/C33H39N3O4S/c1-7-8-17-34-41(39,40)23-13-14-28-27(18-23)35-30(21-9-11-22(12-10-21)31(37)38)24-19-25-26(20-29(24)36(28)6)33(4,5)16-15-32(25,2)3/h9-14,18-20,34H,7-8,15-17H2,1-6H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218446
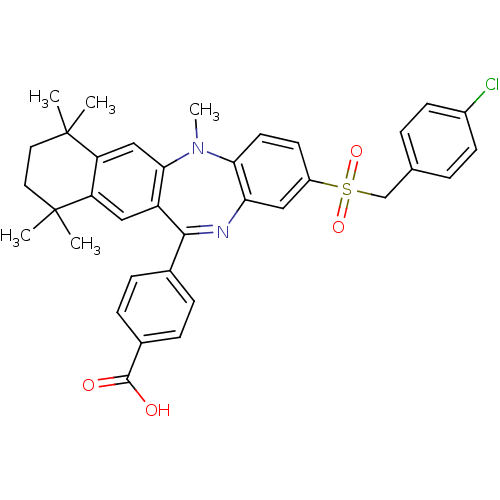
(4-[2-(4-chloro-phenylmethanesulfonyl)-5,7,7,10,10-...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)Cc1ccc(Cl)cc1 |t:9| Show InChI InChI=1S/C36H35ClN2O4S/c1-35(2)16-17-36(3,4)29-20-32-27(19-28(29)35)33(23-8-10-24(11-9-23)34(40)41)38-30-18-26(14-15-31(30)39(32)5)44(42,43)21-22-6-12-25(37)13-7-22/h6-15,18-20H,16-17,21H2,1-5H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218492

(4-[5-ethyl-2-(4-fluoro-benzenesulfonylamino)-7,7,1...)Show SMILES CCN1c2ccc(NS(=O)(=O)c3ccc(F)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:22| Show InChI InChI=1S/C36H36FN3O4S/c1-6-40-31-16-13-25(39-45(43,44)26-14-11-24(37)12-15-26)19-30(31)38-33(22-7-9-23(10-8-22)34(41)42)27-20-28-29(21-32(27)40)36(4,5)18-17-35(28,2)3/h7-16,19-21,39H,6,17-18H2,1-5H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of LG100268-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218436
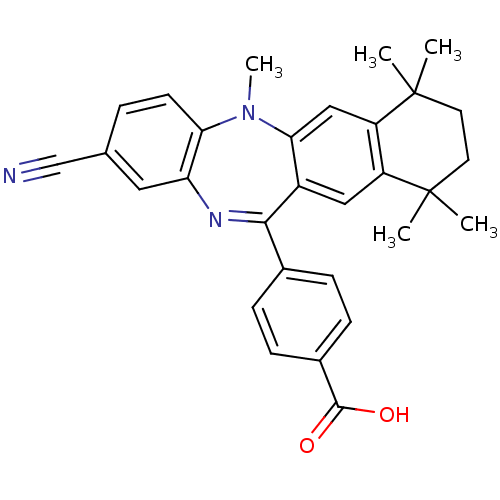
(4-(2-cyano-5,7,7,10,10-pentamethyl-7,8,9,10-tetrah...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:9| Show InChI InChI=1S/C30H29N3O2/c1-29(2)12-13-30(3,4)23-16-26-21(15-22(23)29)27(19-7-9-20(10-8-19)28(34)35)32-24-14-18(17-31)6-11-25(24)33(26)5/h6-11,14-16H,12-13H2,1-5H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218499
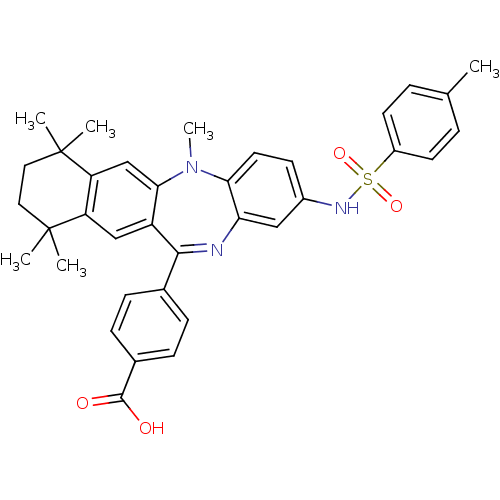
(4-[5,7,7,10,10-pentamethyl-2-(toluene-4-sulfonylam...)Show SMILES CN1c2ccc(NS(=O)(=O)c3ccc(C)cc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:21| Show InChI InChI=1S/C36H37N3O4S/c1-22-7-14-26(15-8-22)44(42,43)38-25-13-16-31-30(19-25)37-33(23-9-11-24(12-10-23)34(40)41)27-20-28-29(21-32(27)39(31)6)36(4,5)18-17-35(28,2)3/h7-16,19-21,38H,17-18H2,1-6H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218428
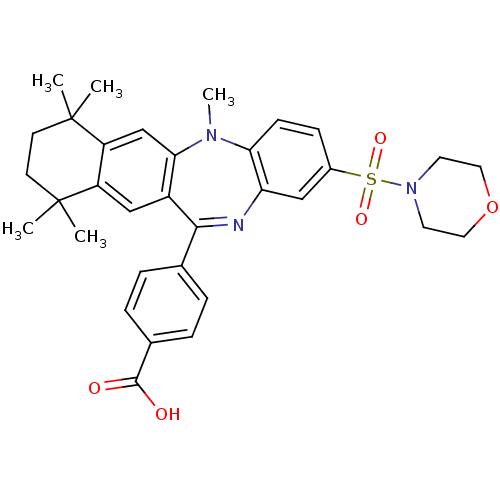
(4-[5,7,7,10,10-pentamethyl-2-(morpholine-4-sulfony...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)N1CCOCC1 |t:9| Show InChI InChI=1S/C33H37N3O5S/c1-32(2)12-13-33(3,4)26-20-29-24(19-25(26)32)30(21-6-8-22(9-7-21)31(37)38)34-27-18-23(10-11-28(27)35(29)5)42(39,40)36-14-16-41-17-15-36/h6-11,18-20H,12-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218444
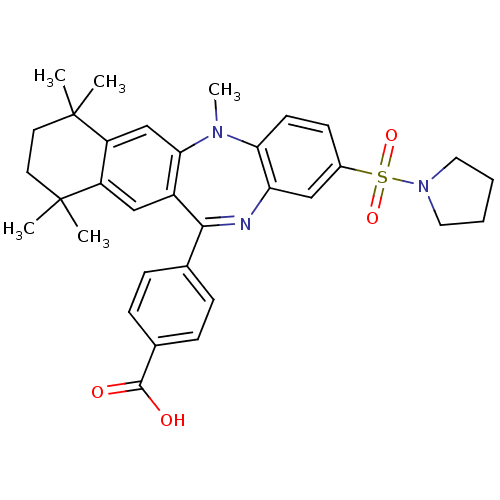
(4-[5,7,7,10,10-pentamethyl-2-(pyrrolidine-1-sulfon...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)N1CCCC1 |t:9| Show InChI InChI=1S/C33H37N3O4S/c1-32(2)14-15-33(3,4)26-20-29-24(19-25(26)32)30(21-8-10-22(11-9-21)31(37)38)34-27-18-23(12-13-28(27)35(29)5)41(39,40)36-16-6-7-17-36/h8-13,18-20H,6-7,14-17H2,1-5H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218453
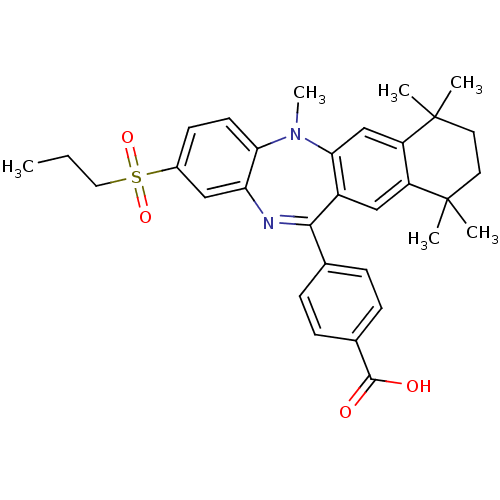
(4-[5,7,7,10,10-pentamethyl-2-(propane-1-sulfonyl)-...)Show SMILES CCCS(=O)(=O)c1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:19| Show InChI InChI=1S/C32H36N2O4S/c1-7-16-39(37,38)22-12-13-27-26(17-22)33-29(20-8-10-21(11-9-20)30(35)36)23-18-24-25(19-28(23)34(27)6)32(4,5)15-14-31(24,2)3/h8-13,17-19H,7,14-16H2,1-6H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218427
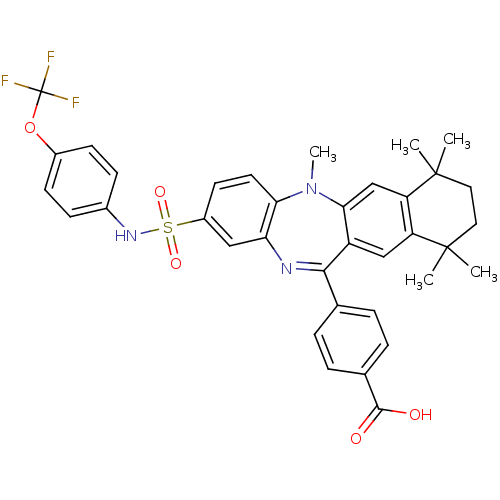
(4-[5,7,7,10,10-pentamethyl-2-(4-trifluoromethoxy-p...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)S(=O)(=O)Nc1ccc(OC(F)(F)F)cc1 |t:9| Show InChI InChI=1S/C36H34F3N3O5S/c1-34(2)16-17-35(3,4)28-20-31-26(19-27(28)34)32(21-6-8-22(9-7-21)33(43)44)40-29-18-25(14-15-30(29)42(31)5)48(45,46)41-23-10-12-24(13-11-23)47-36(37,38)39/h6-15,18-20,41H,16-17H2,1-5H3,(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218490

(4-[2-(4-methoxy-benzenesulfonylamino)-5,7,7,10,10-...)Show SMILES COc1ccc(cc1)S(=O)(=O)Nc1ccc2N(C)c3cc4c(cc3C(=Nc2c1)c1ccc(cc1)C(O)=O)C(C)(C)CCC4(C)C |c:26| Show InChI InChI=1S/C36H37N3O5S/c1-35(2)17-18-36(3,4)29-21-32-27(20-28(29)35)33(22-7-9-23(10-8-22)34(40)41)37-30-19-24(11-16-31(30)39(32)5)38-45(42,43)26-14-12-25(44-6)13-15-26/h7-16,19-21,38H,17-18H2,1-6H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218433

(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H31N3O2/c1-6-34-26-12-7-19(18-32)15-25(26)33-28(20-8-10-21(11-9-20)29(35)36)22-16-23-24(17-27(22)34)31(4,5)14-13-30(23,2)3/h7-12,15-17H,6,13-14H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LG100268-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218456

(4-[5,7,7,10,10-pentamethyl-2-(3-trifluoromethyl-be...)Show SMILES CN1c2ccc(NS(=O)(=O)c3cccc(c3)C(F)(F)F)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:24| Show InChI InChI=1S/C36H34F3N3O4S/c1-34(2)15-16-35(3,4)28-20-31-26(19-27(28)34)32(21-9-11-22(12-10-21)33(43)44)40-29-18-24(13-14-30(29)42(31)5)41-47(45,46)25-8-6-7-23(17-25)36(37,38)39/h6-14,17-20,41H,15-16H2,1-5H3,(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218424
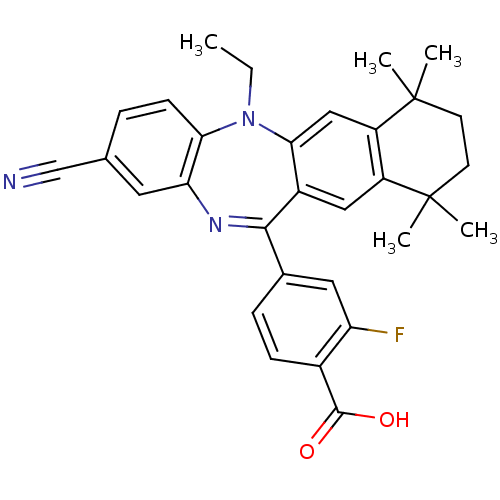
(4-(2-cyano-5-ethyl-7,7,10,10-tetramethyl-7,8,9,10-...)Show SMILES CCN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:10| Show InChI InChI=1S/C31H30FN3O2/c1-6-35-26-10-7-18(17-33)13-25(26)34-28(19-8-9-20(29(36)37)24(32)14-19)21-15-22-23(16-27(21)35)31(4,5)12-11-30(22,2)3/h7-10,13-16H,6,11-12H2,1-5H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218466

(4-(2-benzenesulfonylamino-5,7,7,10,10-pentamethyl-...)Show SMILES CN1c2ccc(NS(=O)(=O)c3ccccc3)cc2N=C(c2ccc(cc2)C(O)=O)c2cc3c(cc12)C(C)(C)CCC3(C)C |t:20| Show InChI InChI=1S/C35H35N3O4S/c1-34(2)17-18-35(3,4)28-21-31-26(20-27(28)34)32(22-11-13-23(14-12-22)33(39)40)36-29-19-24(15-16-30(29)38(31)5)37-43(41,42)25-9-7-6-8-10-25/h6-16,19-21,37H,17-18H2,1-5H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human RXRalpha expressed in EK293 cells assessed as inhibition of 9-cis-retinoic acid-induced transactivation |
Bioorg Med Chem Lett 17: 4808-11 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.079
BindingDB Entry DOI: 10.7270/Q2V40TWB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250359
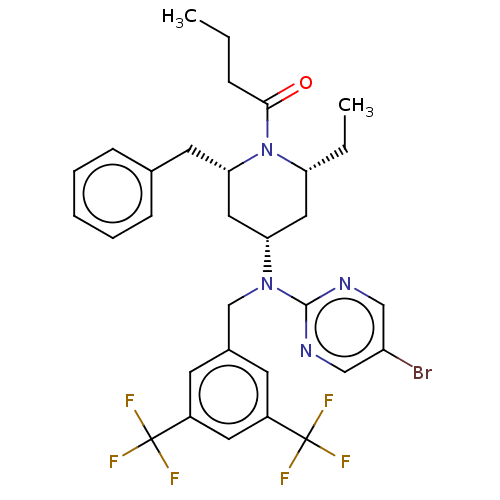
(CHEMBL4074770)Show SMILES CCCC(=O)N1[C@H](CC)C[C@@H](C[C@@H]1Cc1ccccc1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(Br)cn1 |r| Show InChI InChI=1S/C31H33BrF6N4O/c1-3-8-28(43)42-25(4-2)15-26(16-27(42)13-20-9-6-5-7-10-20)41(29-39-17-24(32)18-40-29)19-21-11-22(30(33,34)35)14-23(12-21)31(36,37)38/h5-7,9-12,14,17-18,25-27H,3-4,8,13,15-16,19H2,1-2H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50218432
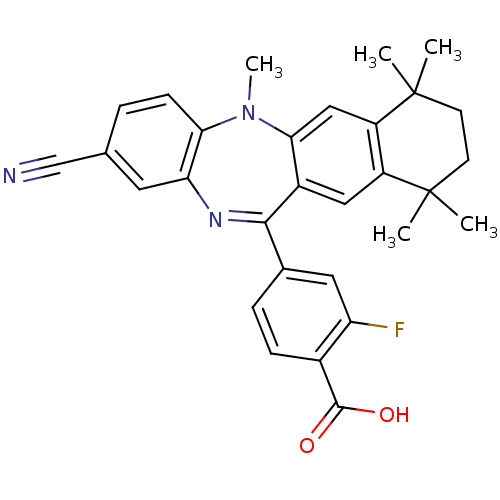
(4-(2-cyano-5,7,7,10,10-pentamethyl-7,8,9,10-tetrah...)Show SMILES CN1c2ccc(cc2N=C(c2ccc(C(O)=O)c(F)c2)c2cc3c(cc12)C(C)(C)CCC3(C)C)C#N |t:9| Show InChI InChI=1S/C30H28FN3O2/c1-29(2)10-11-30(3,4)22-15-26-20(14-21(22)29)27(18-7-8-19(28(35)36)23(31)13-18)33-24-12-17(16-32)6-9-25(24)34(26)5/h6-9,12-15H,10-11H2,1-5H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 9-cis-retinoic acid-induced RXRalpha transactivation |
Bioorg Med Chem Lett 17: 4804-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.080
BindingDB Entry DOI: 10.7270/Q2ZS2W7W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data