

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073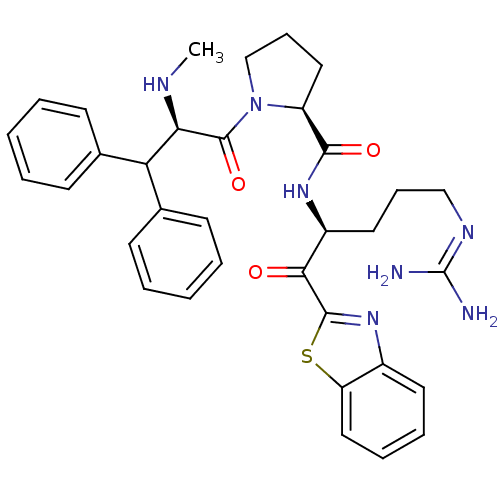 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930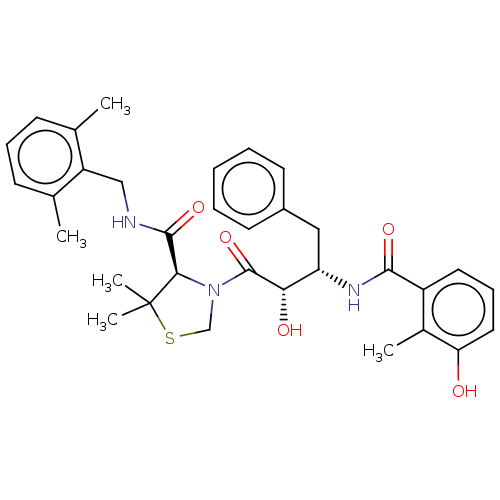 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033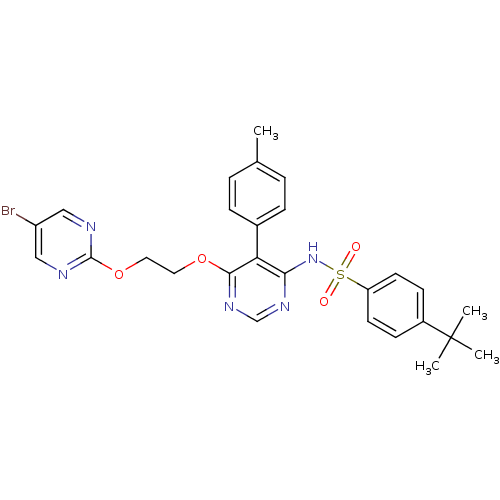 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065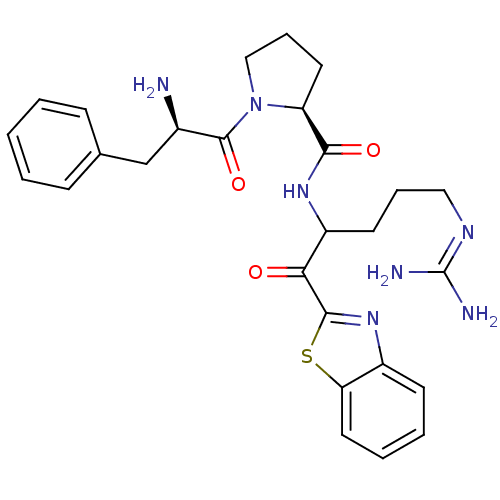 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127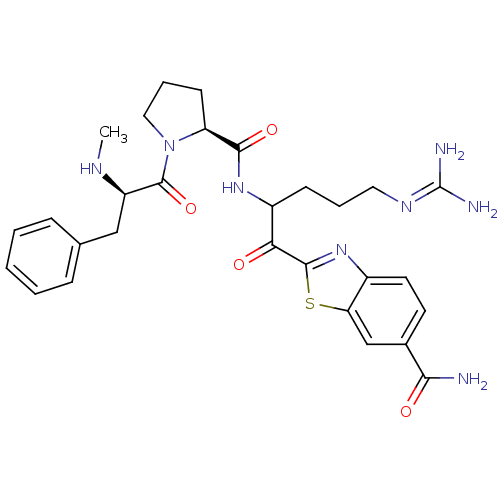 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133884 (CHEMBL3634251) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50085683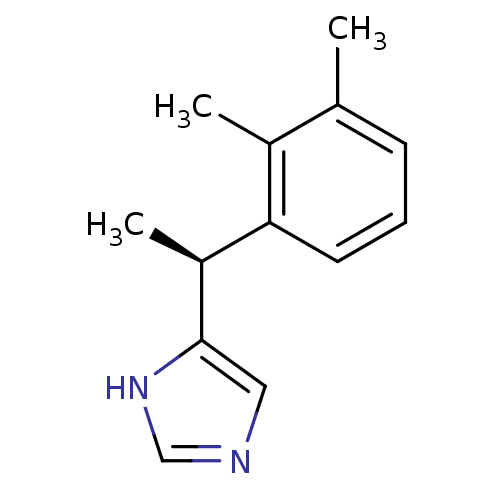 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953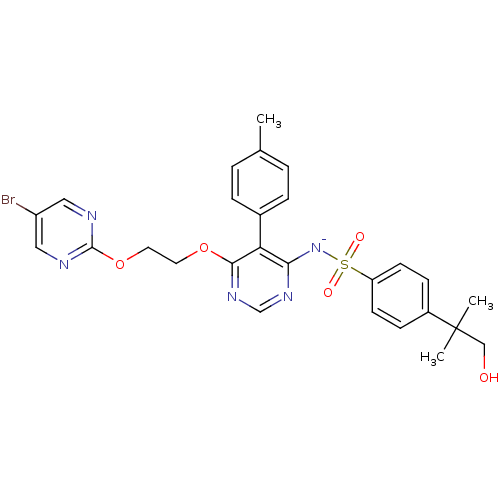 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50299217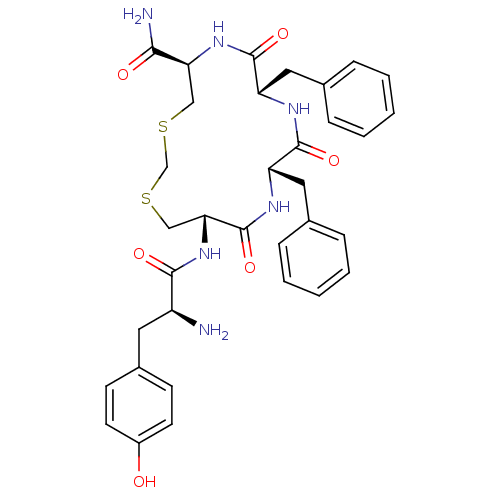 ((5R,8S,11S,14S)-14-((S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cells after 1 hr by liquid scintillation counting | J Med Chem 52: 7724-31 (2009) Article DOI: 10.1021/jm9007483 BindingDB Entry DOI: 10.7270/Q2GQ6XTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14076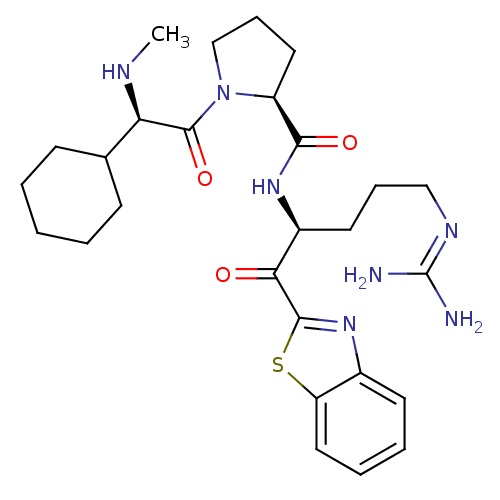 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133890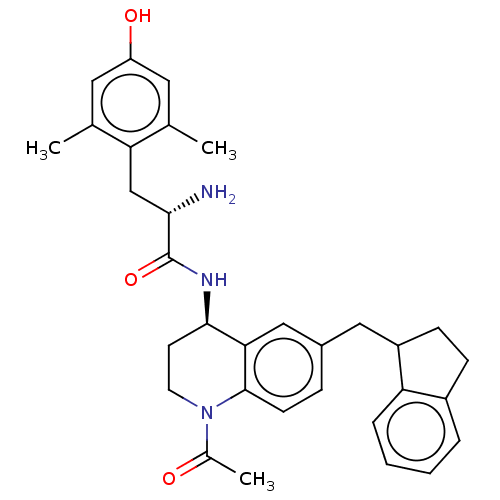 (CHEMBL3634258) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133885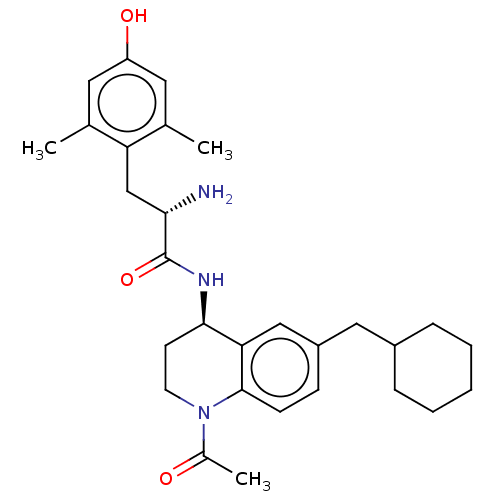 (CHEMBL3634250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50347177 (CHEMBL1797687) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum homogenate by scintillation counting | Bioorg Med Chem Lett 21: 4104-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.147 BindingDB Entry DOI: 10.7270/Q25M66P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535334 (CHEMBL4467875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442965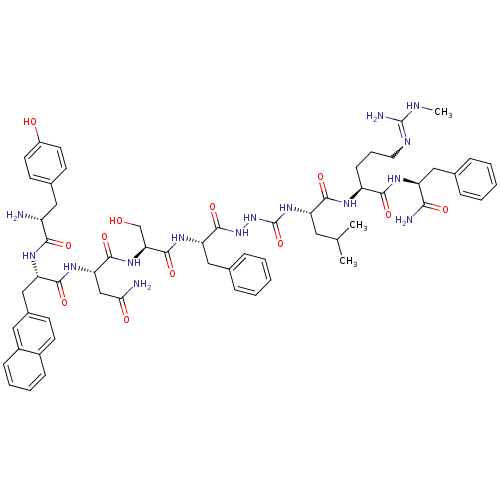 (CHEMBL3086282) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442966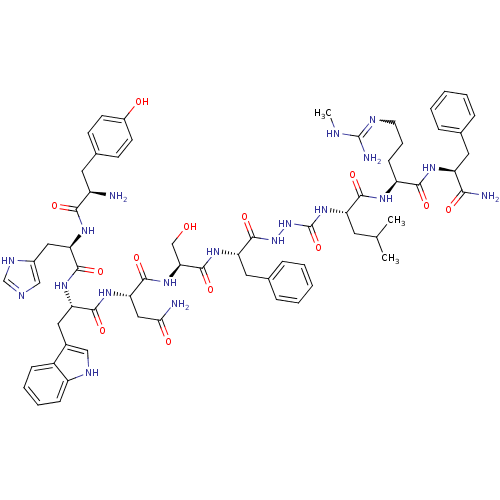 (CHEMBL3087927) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50347179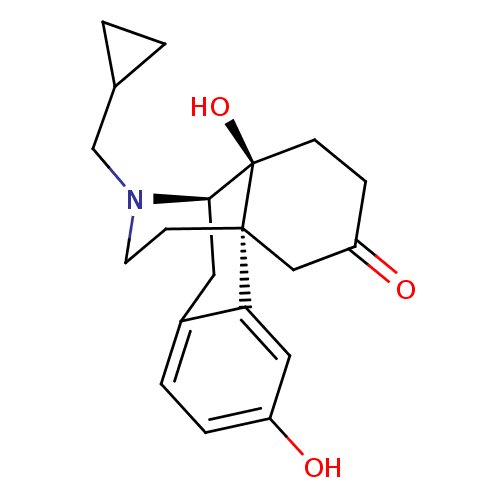 (CHEMBL1797689) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cerebellum homogenate by scintillation counting | Bioorg Med Chem Lett 21: 4104-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.147 BindingDB Entry DOI: 10.7270/Q25M66P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM26349 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535342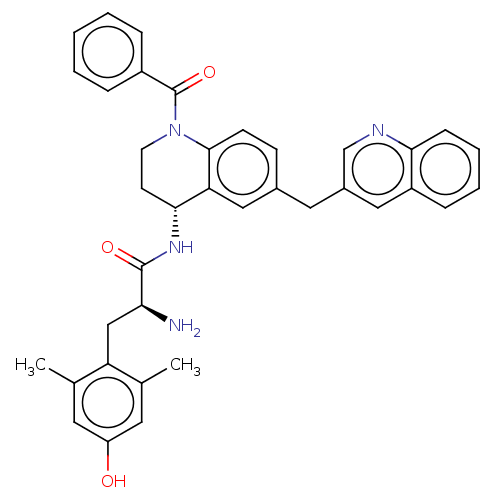 (CHEMBL4569709) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133896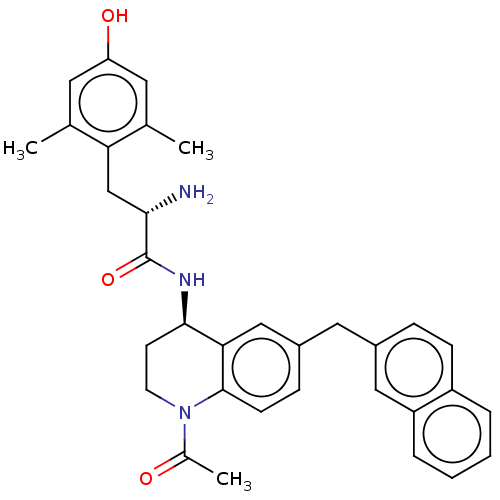 (CHEMBL3634252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50442968 (CHEMBL3087793) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to rat KISS1R | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535333 (CHEMBL4588807) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50195673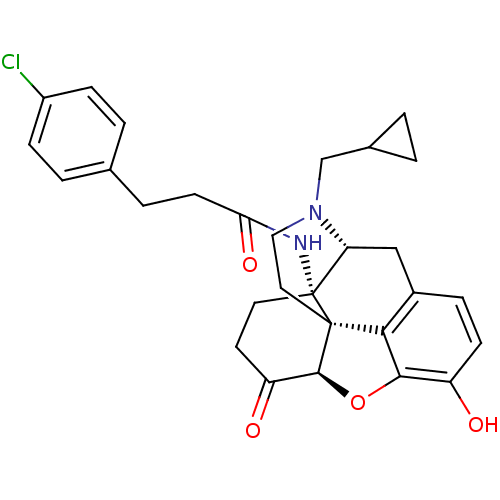 (CHEMBL267027 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in guinea pig brain homogenate | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50442967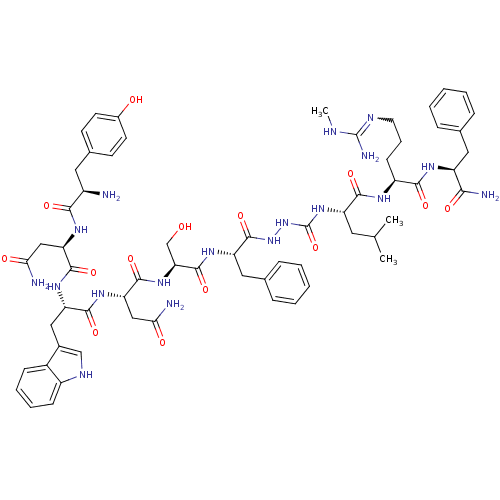 (CHEMBL3087925) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to rat KISS1R | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133886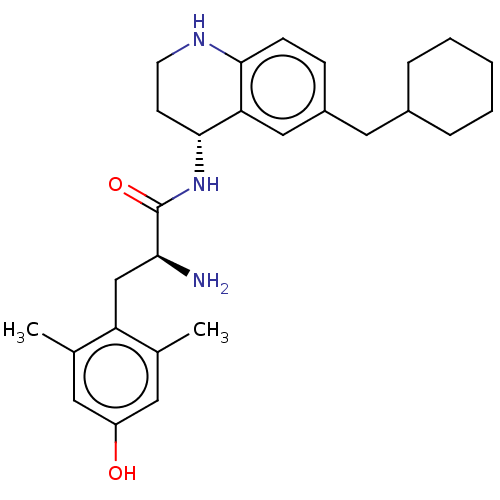 (CHEMBL3634249) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50442966 (CHEMBL3087927) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to rat KISS1R | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442964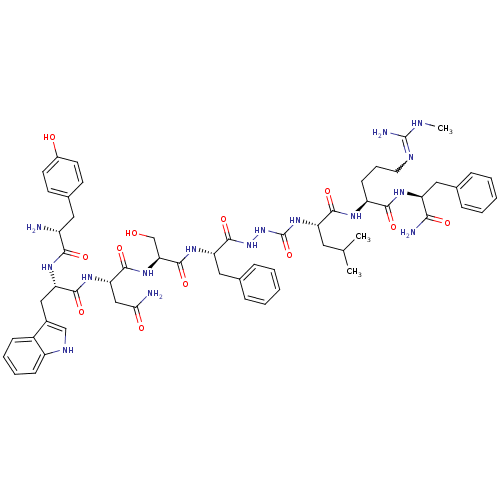 (CHEMBL3085809) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from recombinant human kappa opioid receptor expressed in CHO cells | J Med Chem 57: 4049-57 (2014) Article DOI: 10.1021/jm401964y BindingDB Entry DOI: 10.7270/Q2NG4S62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133887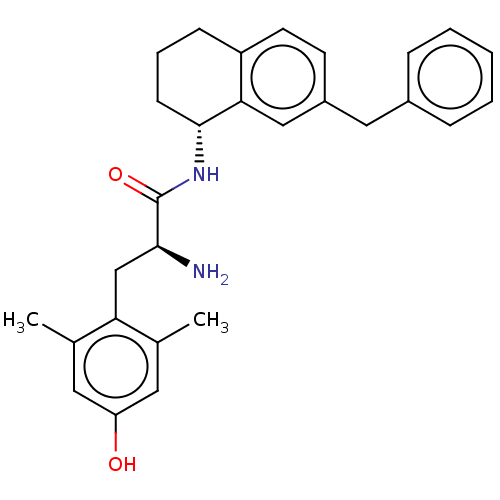 (CHEMBL3634248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442968 (CHEMBL3087793) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50392401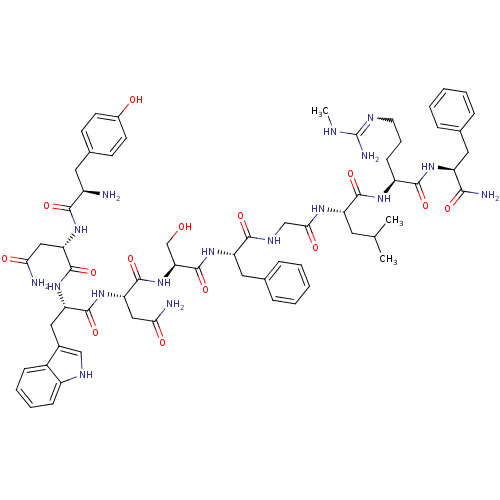 (CHEMBL2151642) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133891 (CHEMBL3634257) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133892 (CHEMBL3634256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu-opioid receptor expressed in C6 cell membrane for 1 hr by liquid scintillation counting analysis | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535347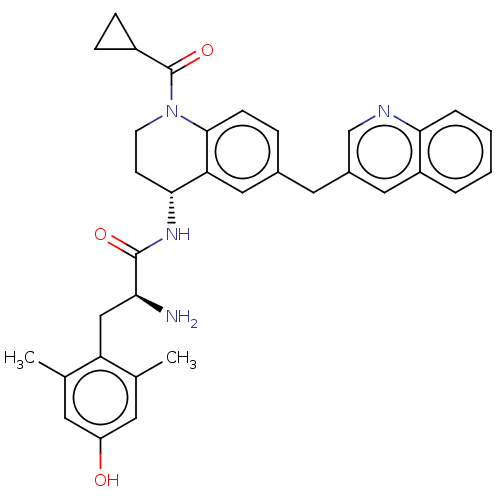 (CHEMBL4476818) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535351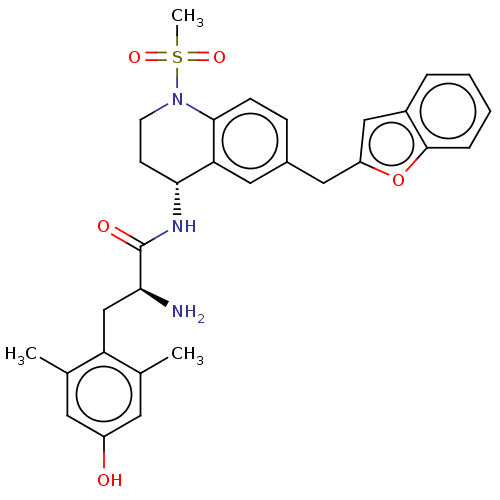 (CHEMBL4459271) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535337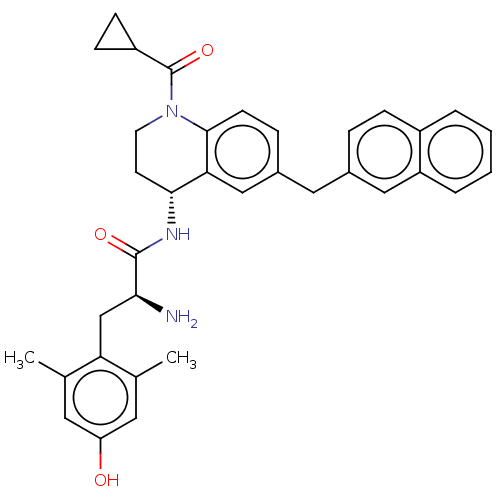 (CHEMBL4446945) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535349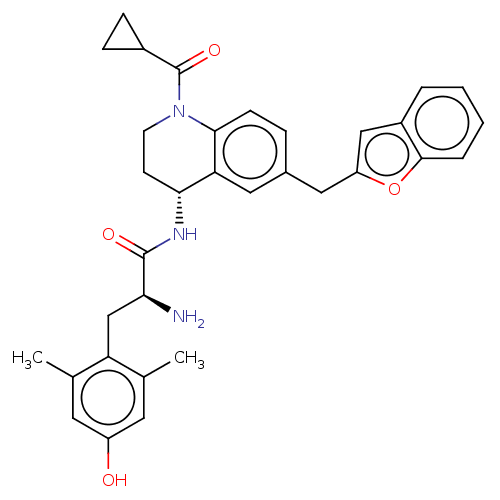 (CHEMBL4451910) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes measured after 1 hr by liquid scintillation counting... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50442965 (CHEMBL3086282) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to rat KISS1R | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50392405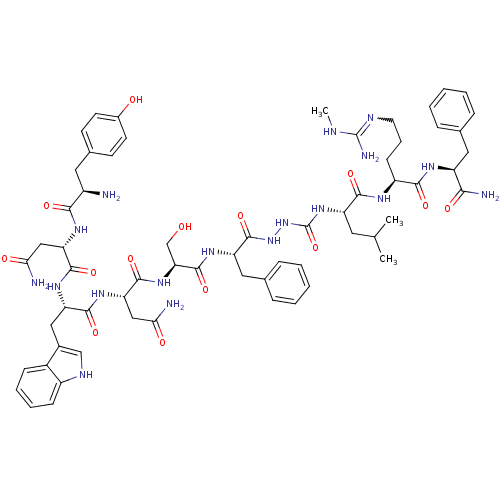 (CHEMBL2151646) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442963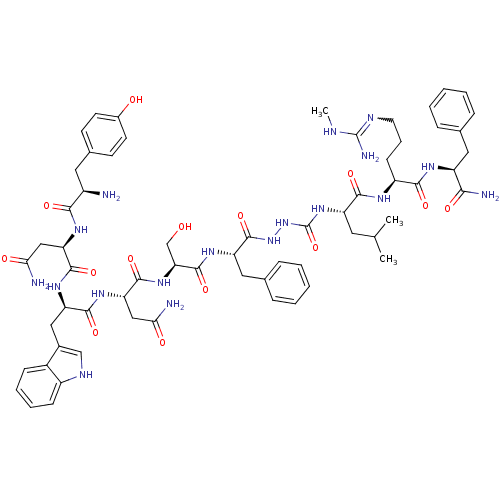 (CHEMBL3085804) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50336669 (CHEMBL1672477 | N-[(6beta,14beta-Epoxy-3-hydroxy-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in guinea pig forebrain | Bioorg Med Chem 19: 1205-21 (2011) Article DOI: 10.1016/j.bmc.2010.12.035 BindingDB Entry DOI: 10.7270/Q23N23P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50442962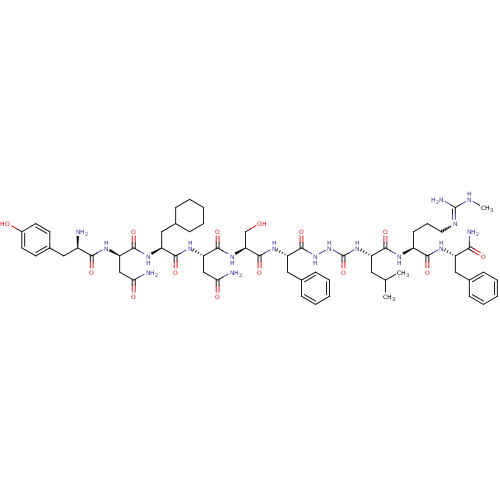 (CHEMBL3087929) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to human KISS1R expressed in CHO cell membranes | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM26349 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to rat KISS1R | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Rattus norvegicus) | BDBM50442964 (CHEMBL3085809) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Binding affinity to rat KISS1R | J Med Chem 56: 8298-307 (2013) Article DOI: 10.1021/jm401056w BindingDB Entry DOI: 10.7270/Q25M675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50131385 ((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center Curated by ChEMBL | Assay Description Inhibition of recombinant human matrix metalloproteinase-9 | Bioorg Med Chem Lett 13: 2737-40 (2003) BindingDB Entry DOI: 10.7270/Q2PC31SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50131385 ((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21 Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloprotease 9 | J Med Chem 47: 1930-8 (2004) Article DOI: 10.1021/jm0304313 BindingDB Entry DOI: 10.7270/Q2ZS2VXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 30754 total ) | Next | Last >> |