

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474150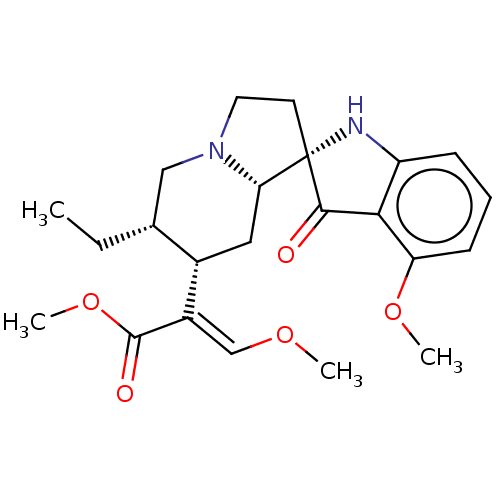 (CHEMBL58362) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50292847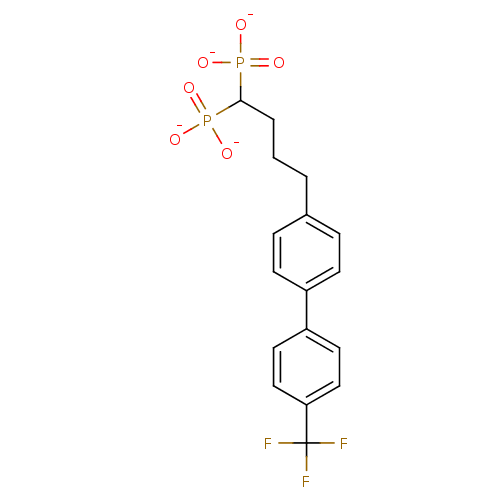 (4-[4-(4-Trifluoromethylphenyl)phenyl)]butyldiphosp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay | J Med Chem 52: 976-88 (2009) Article DOI: 10.1021/jm801023u BindingDB Entry DOI: 10.7270/Q2VM4C87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50292846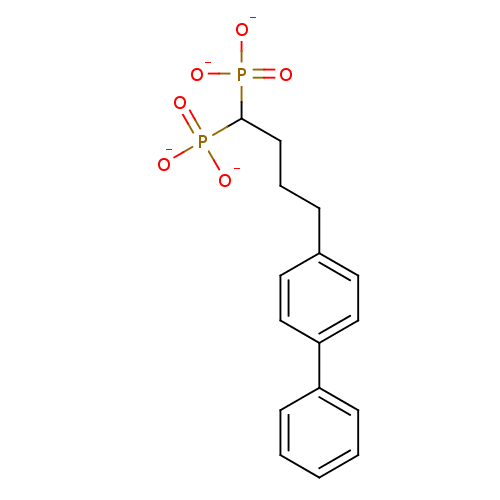 (4-(4-Biphenyl)butyldiphosphonic Acid Tetrapotassiu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay | J Med Chem 52: 976-88 (2009) Article DOI: 10.1021/jm801023u BindingDB Entry DOI: 10.7270/Q2VM4C87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474150 (CHEMBL58362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50008781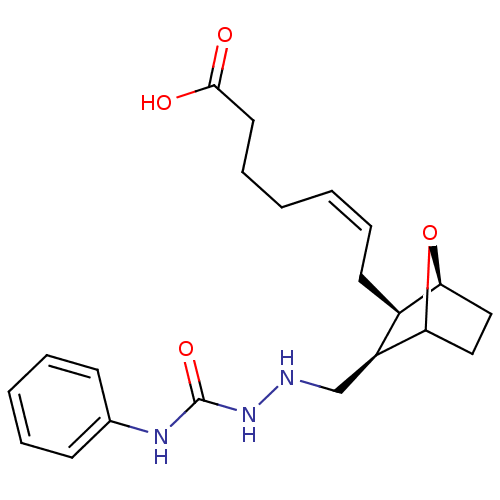 (7-(3-(2-ethyl-N-phenylhydrazinecarboxamide)-7-oxa-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 557-62 (1989) BindingDB Entry DOI: 10.7270/Q24Q7SGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50049222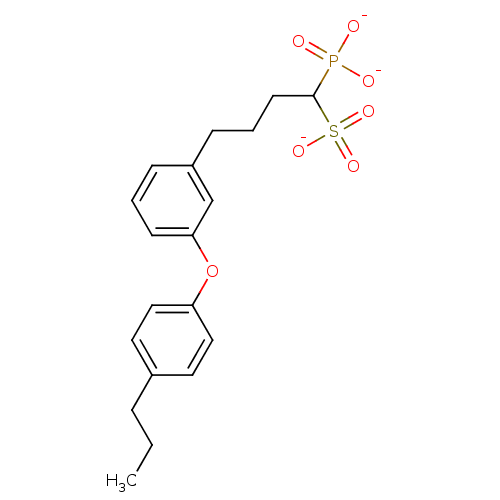 (1-Phosphono-4-[3-(4-propylphenoxy)phenyl]butylsulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay | J Med Chem 52: 976-88 (2009) Article DOI: 10.1021/jm801023u BindingDB Entry DOI: 10.7270/Q2VM4C87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50292848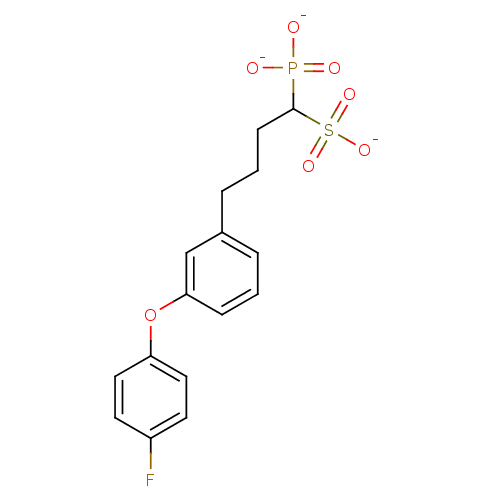 (1-Phosphono-4-[3-(4-fluorophenoxy)phenyl]butylsulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay | J Med Chem 52: 976-88 (2009) Article DOI: 10.1021/jm801023u BindingDB Entry DOI: 10.7270/Q2VM4C87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474152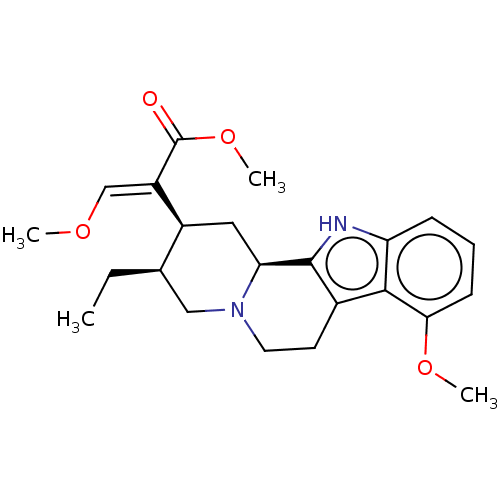 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25299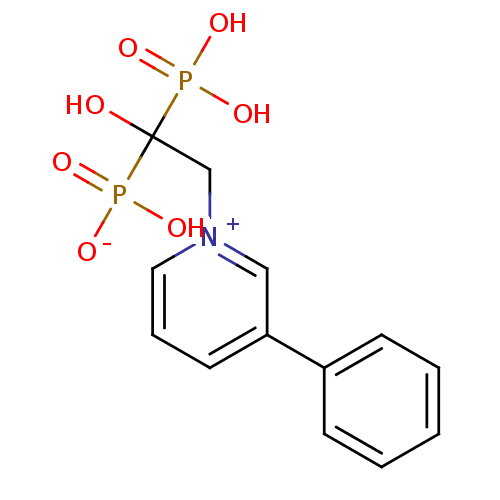 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12578 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474151 (CHEMBL292521) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474153 (CHEMBL61630) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM81948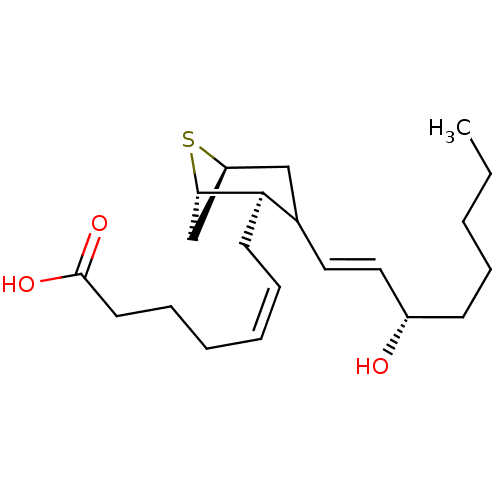 (CAS_89617-02-7 | ONO 11,113 | ONO-11113 | STA2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 557-62 (1989) BindingDB Entry DOI: 10.7270/Q24Q7SGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25310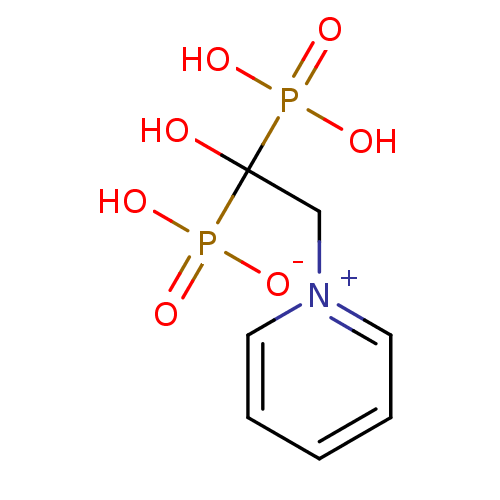 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165352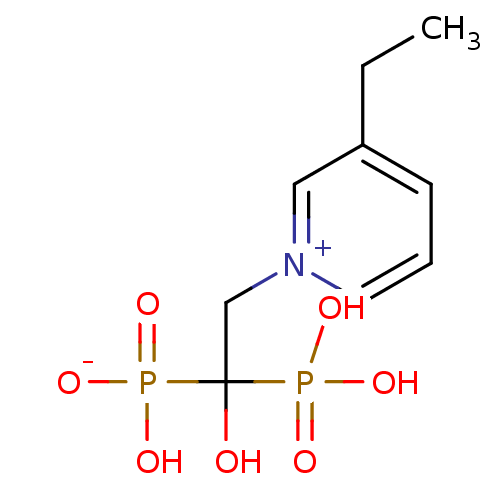 (CHEMBL192043 | hydrogen 2-(3-ethylpyridinium-1-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165353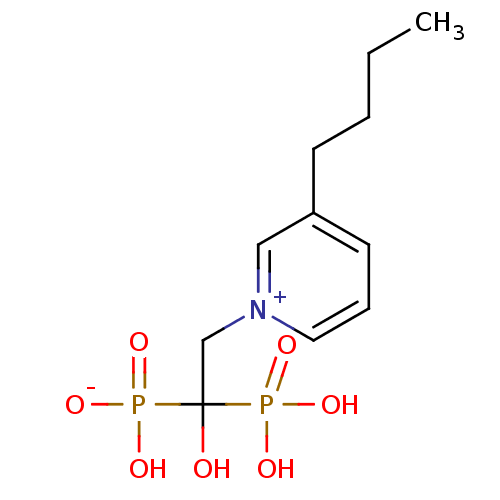 (CHEMBL373332 | hydrogen 2-(3-butylpyridinium-1-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alpha | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165349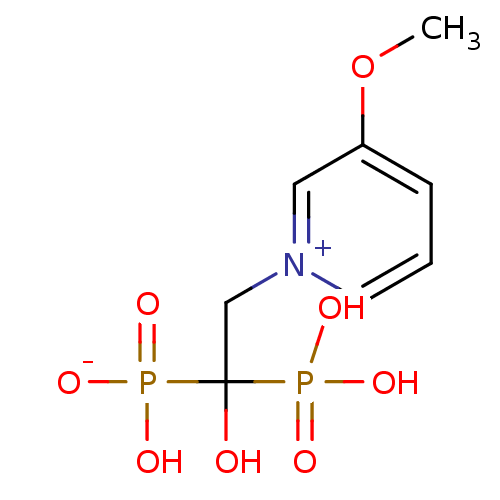 (CHEMBL363145 | hydrogen 1-hydroxy-2-(3-methoxypyri...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards Vasopressin V1a receptor in rat liver | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165342 (CHEMBL193356 | hydrogen 1-hydroxy-2-(3-methylpyrid...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM81949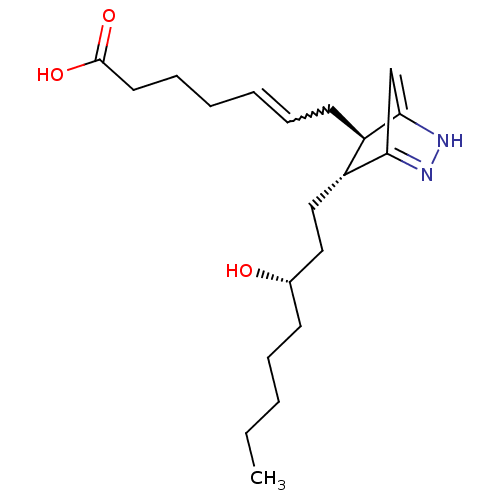 (9,11-AzoPGH2 | 9,11-azo PGH2 | J389.306E | PGH2, 9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 557-62 (1989) BindingDB Entry DOI: 10.7270/Q24Q7SGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165340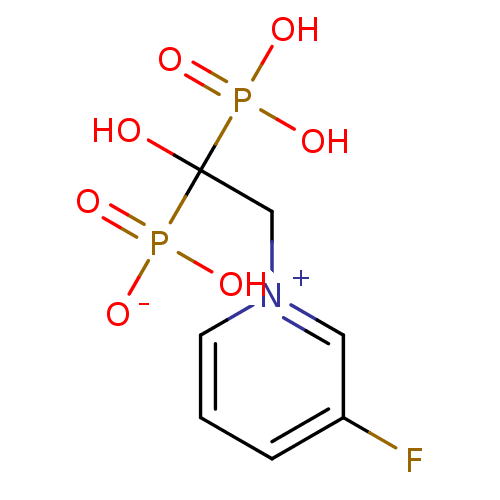 (BPH-461 | CHEMBL193722 | hydrogen 2-(3-fluoropyrid...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50018531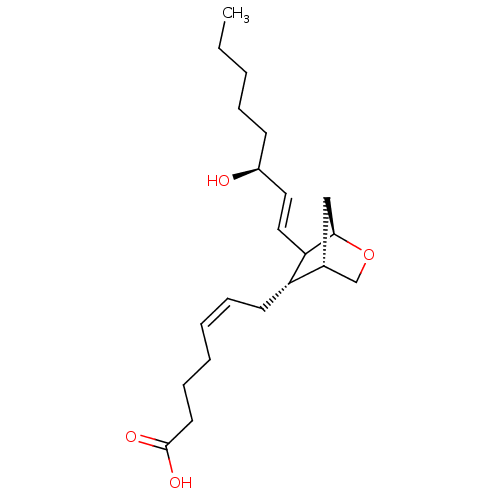 ((Z)-7-[(S)-6-((E)-(S)-3-Hydroxy-oct-1-enyl)-2-oxa-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by PDSP Ki Database | J Pharmacol Exp Ther 251: 557-62 (1989) BindingDB Entry DOI: 10.7270/Q24Q7SGJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165350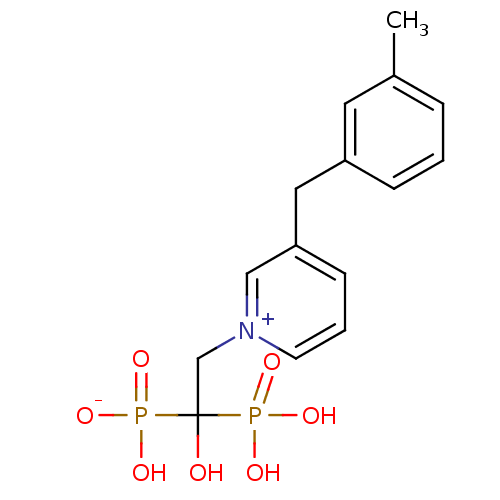 (CHEMBL192938 | hydrogen 1-hydroxy-2-[3-(3-methylbe...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474149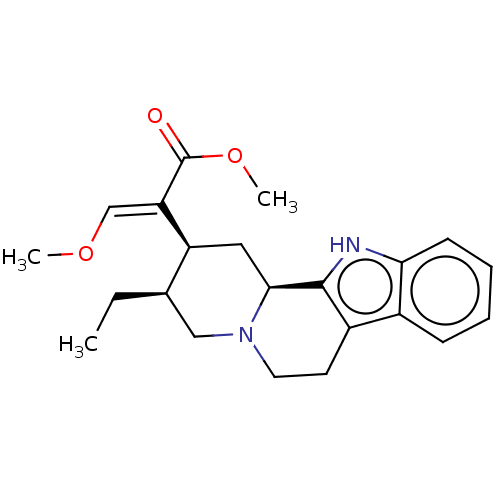 (CHEBI:70073 | Corynanrheidine) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165351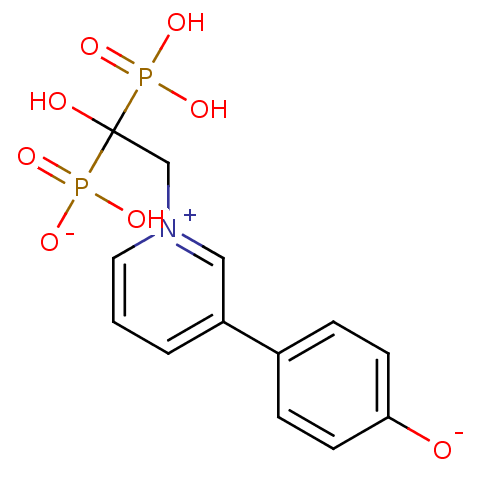 (CHEMBL193619 | sodium hydrogen 1-hydroxy-2-[3-(4-o...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50474150 (CHEMBL58362) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor kappa 1 using [3H]- U-69,593 as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165348 (CHEMBL425896 | hydrogen 1-hydroxy-2-isoquinolinium...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25313 ((4-amino-1-hydroxy-1-phosphonobutyl)phosphonic aci...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165338 (CHEMBL190258 | hydrogen 2-(4-benzylpyridinium-1-yl...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50474153 (CHEMBL61630) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor kappa 1 using [3H]- U-69,593 as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474153 (CHEMBL61630) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand | J Med Chem 45: 1949-56 (2002) Article DOI: 10.1021/jm010576e BindingDB Entry DOI: 10.7270/Q2WS8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50165339 (CHEMBL363434 | hydrogen 2-(3-benzylpyridinium-1-yl...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12581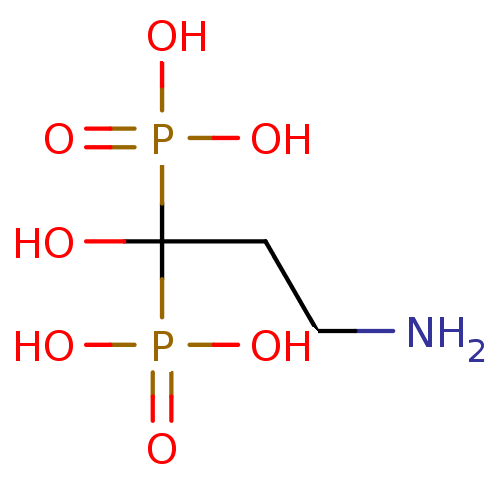 ((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards Farnesyl diphosphate synthase from leishmania major | J Med Chem 48: 2957-63 (2005) Article DOI: 10.1021/jm040209d BindingDB Entry DOI: 10.7270/Q2Z89D7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330955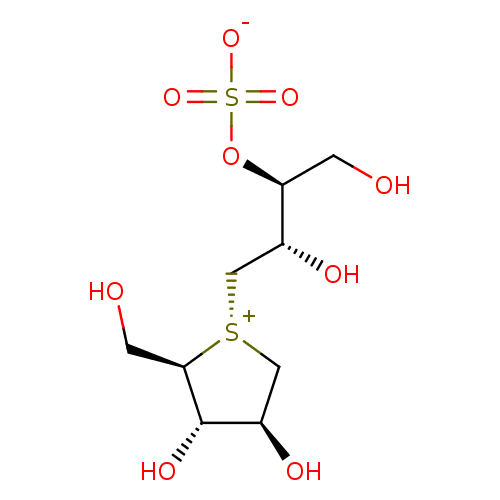 ((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2464 total ) | Next | Last >> |