 Found 102 hits with Last Name = 'nakao' and Initial = 'y'
Found 102 hits with Last Name = 'nakao' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50269601
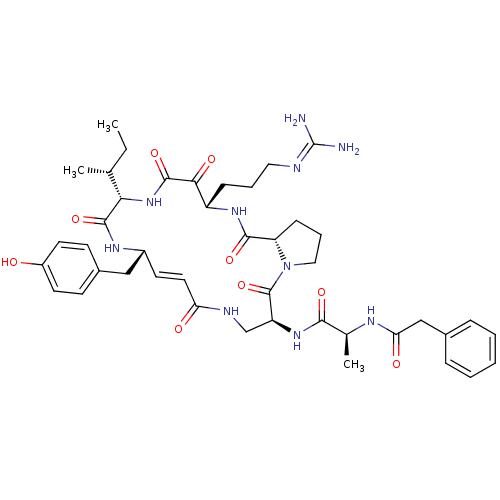
(CHEMBL507449 | cyclotheonamide E)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-c1ccccc1 |r,t:34| Show InChI InChI=1S/C43H58N10O9/c1-4-25(2)36-40(60)49-29(22-28-14-17-30(54)18-15-28)16-19-34(55)47-24-32(51-38(58)26(3)48-35(56)23-27-10-6-5-7-11-27)42(62)53-21-9-13-33(53)39(59)50-31(37(57)41(61)52-36)12-8-20-46-43(44)45/h5-7,10-11,14-19,25-26,29,31-33,36,54H,4,8-9,12-13,20-24H2,1-3H3,(H,47,55)(H,48,56)(H,49,60)(H,50,59)(H,51,58)(H,52,61)(H4,44,45,46)/b19-16+/t25-,26+,29-,31+,32+,33+,36+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Nat Prod 61: 667-70 (1998)
Article DOI: 10.1021/np970544n
BindingDB Entry DOI: 10.7270/Q2GB24XX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50259920
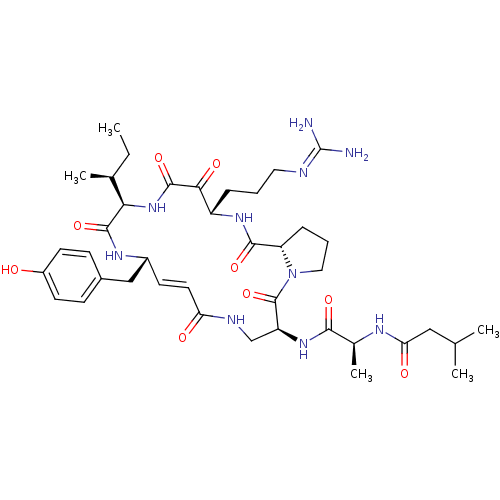
(CHEMBL448342 | Cyclotheonamide E3)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#6](-[#6])-[#6] |r,t:34| Show InChI InChI=1S/C40H60N10O9/c1-6-23(4)33-37(57)46-26(20-25-11-14-27(51)15-12-25)13-16-31(52)44-21-29(48-35(55)24(5)45-32(53)19-22(2)3)39(59)50-18-8-10-30(50)36(56)47-28(34(54)38(58)49-33)9-7-17-43-40(41)42/h11-16,22-24,26,28-30,33,51H,6-10,17-21H2,1-5H3,(H,44,52)(H,45,53)(H,46,57)(H,47,56)(H,48,55)(H,49,58)(H4,41,42,43)/b16-13+/t23-,24-,26+,28-,29-,30-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Nat Prod 61: 667-70 (1998)
Article DOI: 10.1021/np970544n
BindingDB Entry DOI: 10.7270/Q2GB24XX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50259922

(CHEMBL505589 | Cyclotheonamide E2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-c1ccccc1 |r,t:34| Show InChI InChI=1S/C42H56N10O9/c1-4-24(2)34-39(59)48-28(22-26-14-17-29(53)18-15-26)16-19-33(54)46-23-31(50-36(56)25(3)47-37(57)27-10-6-5-7-11-27)41(61)52-21-9-13-32(52)38(58)49-30(35(55)40(60)51-34)12-8-20-45-42(43)44/h5-7,10-11,14-19,24-25,28,30-32,34,53H,4,8-9,12-13,20-23H2,1-3H3,(H,46,54)(H,47,57)(H,48,59)(H,49,58)(H,50,56)(H,51,60)(H4,43,44,45)/b19-16+/t24-,25-,28+,30-,31-,32-,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Nat Prod 61: 667-70 (1998)
Article DOI: 10.1021/np970544n
BindingDB Entry DOI: 10.7270/Q2GB24XX |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cathepsin B was determined |
Bioorg Med Chem Lett 9: 3397-402 (1999)
BindingDB Entry DOI: 10.7270/Q2G44SGN |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50213272
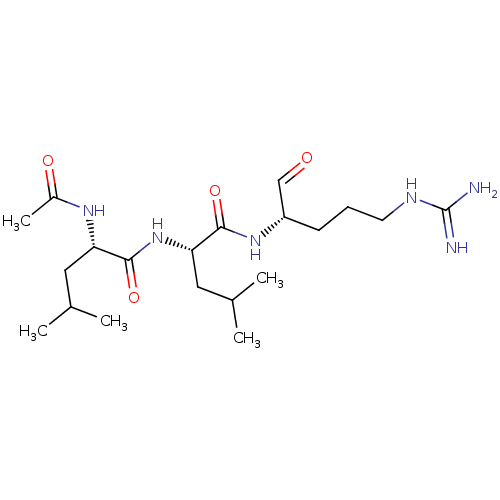
(CHEBI:6426 | Leupeptin)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O |r| Show InChI InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cathepsin B was determined |
Bioorg Med Chem Lett 9: 3397-402 (1999)
BindingDB Entry DOI: 10.7270/Q2G44SGN |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118723
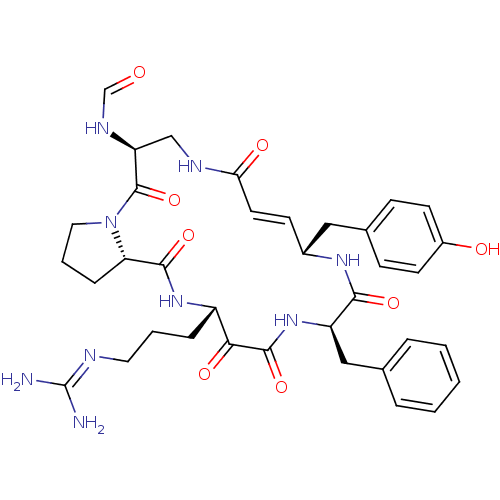
(CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-1=O)-[#7]-[#6]=O |r,t:24| Show InChI InChI=1S/C36H45N9O8/c37-36(38)39-16-4-8-26-31(49)34(52)44-27(19-22-6-2-1-3-7-22)32(50)42-24(18-23-10-13-25(47)14-11-23)12-15-30(48)40-20-28(41-21-46)35(53)45-17-5-9-29(45)33(51)43-26/h1-3,6-7,10-15,21,24,26-29,47H,4-5,8-9,16-20H2,(H,40,48)(H,41,46)(H,42,50)(H,43,51)(H,44,52)(H4,37,38,39)/b15-12+/t24-,26+,27-,28+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Nat Prod 61: 667-70 (1998)
Article DOI: 10.1021/np970544n
BindingDB Entry DOI: 10.7270/Q2GB24XX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50217331
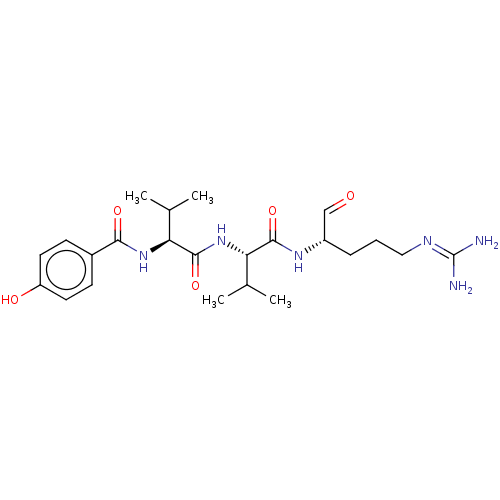
(TOKARAMIDE A)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-c1ccc(-[#8])cc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O Show InChI InChI=1S/C23H36N6O5/c1-13(2)18(21(33)27-16(12-30)6-5-11-26-23(24)25)29-22(34)19(14(3)4)28-20(32)15-7-9-17(31)10-8-15/h7-10,12-14,16,18-19,31H,5-6,11H2,1-4H3,(H,27,33)(H,28,32)(H,29,34)(H4,24,25,26)/t16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60.9 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cathepsin B was determined |
Bioorg Med Chem Lett 9: 3397-402 (1999)
BindingDB Entry DOI: 10.7270/Q2G44SGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 83.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50478556
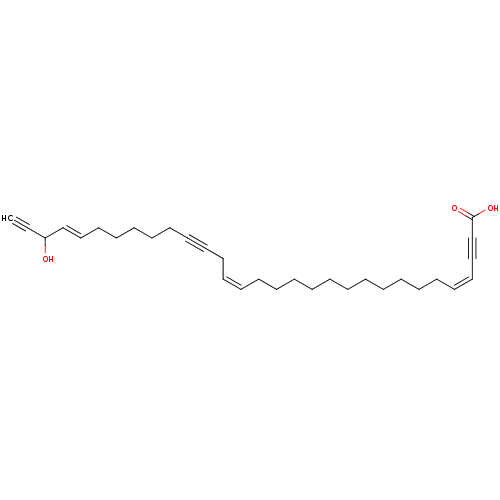
(CORTICATIC ACID A)Show SMILES OC(\C=C\CCCCCC#CC\C=C/CCCCCCCCCCC\C=C/C#CC(O)=O)C#C Show InChI InChI=1S/C31H44O3/c1-2-30(32)28-26-24-22-20-18-16-14-12-10-8-6-4-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31(33)34/h1,6,8,23,25-26,28,30,32H,3-5,7,9-11,13,15-22,24H2,(H,33,34)/b8-6-,25-23-,28-26+ | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of alpha-glucosidase |
J Nat Prod 65: 922-4 (2002)
Article DOI: 10.1021/np0106642
BindingDB Entry DOI: 10.7270/Q20004T1 |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50478507

(Nobiloside)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([#6](-[#8])=O)[#6]-3=[#6](-[#6]-[#6][C@]12[#6])[C@@]1([#6])[#6]-[#6]-[#6@H](-[#8][C@]2([H])[#8]-[#6@@H](-[#6@@H](-[#8][C@]4([H])[#8]-[#6@@H](-[#6@H](-[#8])-[#6@H](-[#8][C@]5([H])[#8]-[#6]-[#6@H](-[#8])-[#6@H](-[#8])-[#6@H]5-[#8])-[#6@H]4-[#8])-[#6](-[#8])=O)-[#6@H](-[#8])-[#6@H]2-[#8])-[#6](-[#8])=O)C([#6])([#6])[C@]1([H])[#6]-[#6]-3)[#6@H](-[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r,c:8| Show InChI InChI=1S/C47H72O19/c1-20(2)9-8-10-21(3)22-14-18-47(43(59)60)24-11-12-26-44(4,5)27(15-16-45(26,6)23(24)13-17-46(22,47)7)62-41-31(52)29(50)35(37(66-41)39(57)58)64-42-33(54)34(32(53)36(65-42)38(55)56)63-40-30(51)28(49)25(48)19-61-40/h9,21-22,25-37,40-42,48-54H,8,10-19H2,1-7H3,(H,55,56)(H,57,58)(H,59,60)/t21-,22-,25+,26+,27+,28+,29-,30-,31-,32-,33-,34+,35+,36+,37+,40+,41-,42-,45-,46-,47+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium perfringens neuraminidase |
J Nat Prod 65: 411-3 (2002)
Article DOI: 10.1021/np010480n
BindingDB Entry DOI: 10.7270/Q2280BDH |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50478555
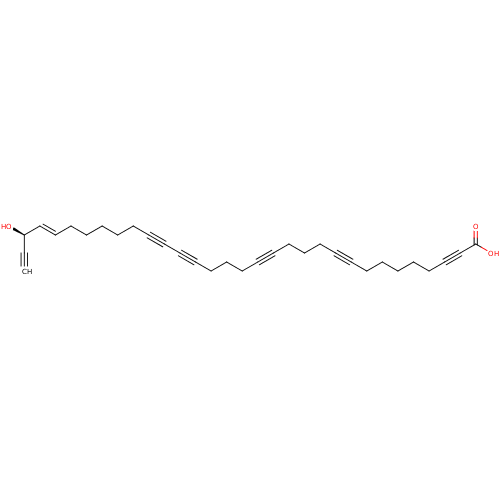
(Callyspongynic Acid)Show SMILES O[C@@H](\C=C\CCCCCC#CC#CCCCC#CCCCC#CCCCCCC#CC(O)=O)C#C |r| Show InChI InChI=1S/C32H38O3/c1-2-31(33)29-27-25-23-21-19-17-15-13-11-9-7-5-3-4-6-8-10-12-14-16-18-20-22-24-26-28-30-32(34)35/h1,27,29,31,33H,3,5,7-8,10,12,17-26H2,(H,34,35)/b29-27+/t31-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 531 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of alpha-glucosidase |
J Nat Prod 65: 922-4 (2002)
Article DOI: 10.1021/np0106642
BindingDB Entry DOI: 10.7270/Q20004T1 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50215926
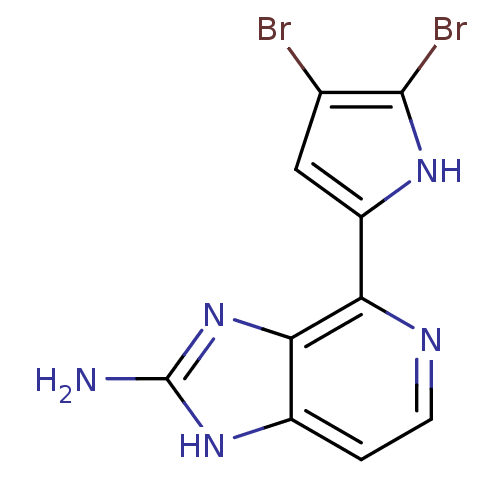
(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 924 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50377384
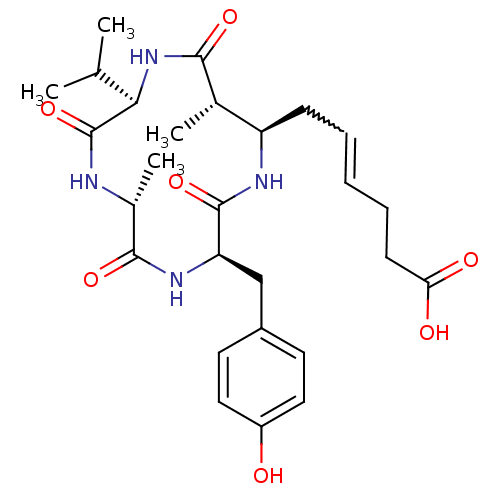
(AZUMAMIDE C)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H38N4O7/c1-15(2)23-27(38)28-17(4)25(36)30-21(14-18-10-12-19(32)13-11-18)26(37)29-20(16(3)24(35)31-23)8-6-5-7-9-22(33)34/h5-6,10-13,15-17,20-21,23,32H,7-9,14H2,1-4H3,(H,28,38)(H,29,37)(H,30,36)(H,31,35)(H,33,34)/t16-,17+,20+,21+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50372469
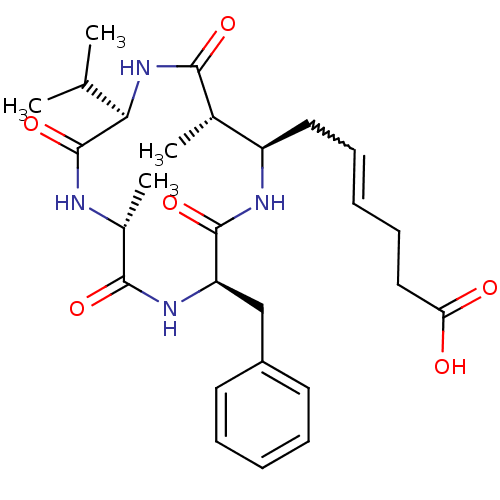
(AZUMAMIDE E)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H38N4O6/c1-16(2)23-27(37)28-18(4)25(35)30-21(15-19-11-7-5-8-12-19)26(36)29-20(17(3)24(34)31-23)13-9-6-10-14-22(32)33/h5-9,11-12,16-18,20-21,23H,10,13-15H2,1-4H3,(H,28,37)(H,29,36)(H,30,35)(H,31,34)(H,32,33)/t17-,18+,20+,21+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50377383
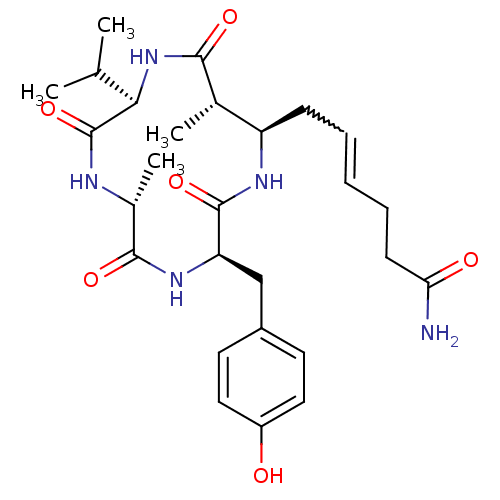
(AZUMAMIDE B)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(N)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H39N5O6/c1-15(2)23-27(38)29-17(4)25(36)31-21(14-18-10-12-19(33)13-11-18)26(37)30-20(16(3)24(35)32-23)8-6-5-7-9-22(28)34/h5-6,10-13,15-17,20-21,23,33H,7-9,14H2,1-4H3,(H2,28,34)(H,29,38)(H,30,37)(H,31,36)(H,32,35)/t16-,17+,20+,21+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50372469

(AZUMAMIDE E)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H38N4O6/c1-16(2)23-27(37)28-18(4)25(35)30-21(15-19-11-7-5-8-12-19)26(36)29-20(17(3)24(34)31-23)13-9-6-10-14-22(32)33/h5-9,11-12,16-18,20-21,23H,10,13-15H2,1-4H3,(H,28,37)(H,29,36)(H,30,35)(H,31,34)(H,32,33)/t17-,18+,20+,21+,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393111
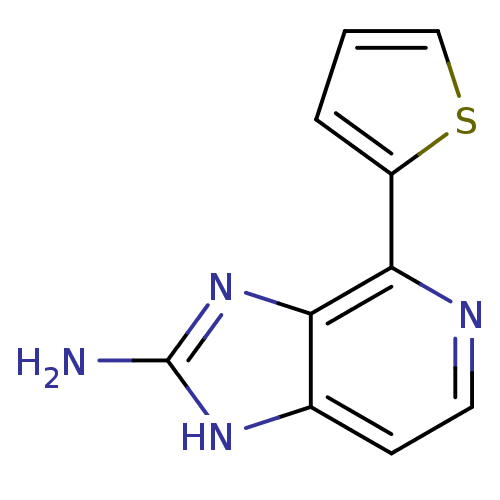
(CHEMBL2153261)Show InChI InChI=1S/C10H8N4S/c11-10-13-6-3-4-12-9(8(6)14-10)7-2-1-5-15-7/h1-5H,(H3,11,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50377384

(AZUMAMIDE C)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H38N4O7/c1-15(2)23-27(38)28-17(4)25(36)30-21(14-18-10-12-19(32)13-11-18)26(37)29-20(16(3)24(35)31-23)8-6-5-7-9-22(33)34/h5-6,10-13,15-17,20-21,23,32H,7-9,14H2,1-4H3,(H,28,38)(H,29,37)(H,30,36)(H,31,35)(H,33,34)/t16-,17+,20+,21+,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50377383

(AZUMAMIDE B)Show SMILES CC(C)[C@H]1NC(=O)[C@@H](C)[C@@H](CC=CCCC(N)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |w:11.10| Show InChI InChI=1S/C27H39N5O6/c1-15(2)23-27(38)29-17(4)25(36)31-21(14-18-10-12-19(33)13-11-18)26(37)30-20(16(3)24(35)32-23)8-6-5-7-9-22(28)34/h5-6,10-13,15-17,20-21,23,33H,7-9,14H2,1-4H3,(H2,28,34)(H,29,38)(H,30,37)(H,31,36)(H,32,35)/t16-,17+,20+,21+,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 expressed in 293T cells |
Bioorg Med Chem Lett 18: 2982-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.053
BindingDB Entry DOI: 10.7270/Q20G3M1G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50393109
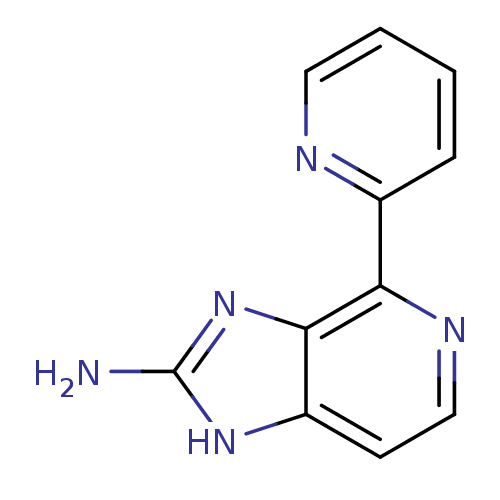
(CHEMBL2153264)Show InChI InChI=1S/C11H9N5/c12-11-15-8-4-6-14-9(10(8)16-11)7-3-1-2-5-13-7/h1-6H,(H3,12,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50393109

(CHEMBL2153264)Show InChI InChI=1S/C11H9N5/c12-11-15-8-4-6-14-9(10(8)16-11)7-3-1-2-5-13-7/h1-6H,(H3,12,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50484453
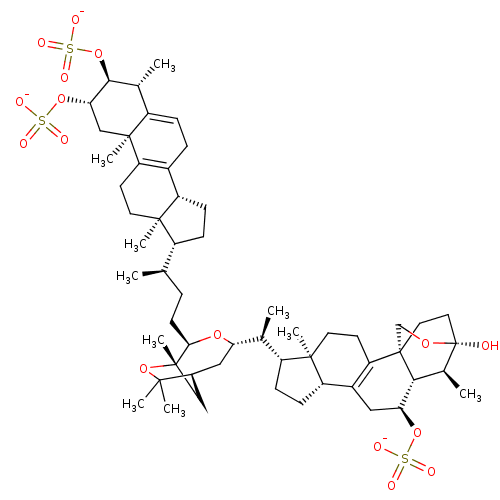
(Shishicrellastatin A)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[H][C@]12[#6][C@]([#6])([#8]C1([#6])[#6])[#6@@H](-[#6]-[#6]-[#6@@H](-[#6])[C@@]1([H])[#6]-[#6][C@@]3([H])[#6]-4=[#6](-[#6]-[#6][C@]13[#6])[C@@]1([#6])[#6]-[#6@H](-[#8]S([#8-])(=O)=O)-[#6@@H](-[#8]S([#8-])(=O)=O)-[#6@H](-[#6])-[#6]1=[#6]-[#6]-4)-[#8][C@@]([H])([#6]2)[#6@@H](-[#6])[C@@]1([H])[#6]-[#6][C@@]2([H])[#6]-3=[#6](-[#6]-[#6][C@]12[#6])[C@]12[#6]-[#6][C@]([#8])([#8]-[#6]1)[#6@@H](-[#6])[C@]2([H])[#6@H](-[#6]-3)-[#8]S([#8-])(=O)=O |r,c:21,47,64| Show InChI InChI=1S/C56H86O16S3.3Na/c1-30(37-14-16-40-35-12-13-39-32(3)49(71-75(64,65)66)46(70-74(61,62)63)28-53(39,9)42(35)19-21-51(37,40)7)11-18-47-54(10)27-34(50(5,6)72-54)25-44(68-47)31(2)38-15-17-41-36-26-45(69-73(58,59)60)48-33(4)56(57)24-23-55(48,29-67-56)43(36)20-22-52(38,41)8;;;/h13,30-34,37-38,40-41,44-49,57H,11-12,14-29H2,1-10H3,(H,58,59,60)(H,61,62,63)(H,64,65,66);;;/q;3*+1/p-3/t30-,31+,32-,33+,34+,37-,38-,40+,41+,44+,45+,46+,47-,48-,49+,51-,52-,53+,54+,55+,56+;;;/m1.../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of bovine cathepsin B after 1 hr by fluorescence assay |
Bioorg Med Chem 19: 6594-8 (2011)
Article DOI: 10.1016/j.bmc.2011.06.052
BindingDB Entry DOI: 10.7270/Q2PN98GC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50484452
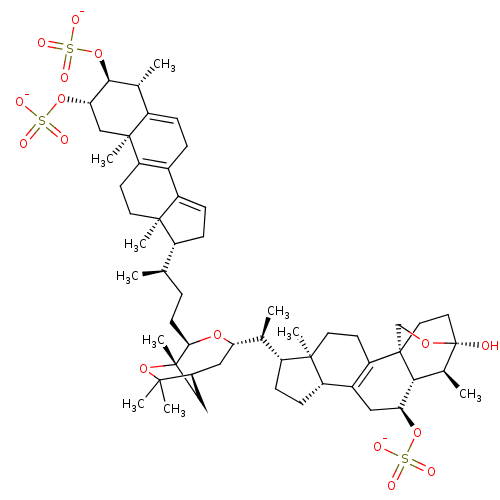
(Shishicrellastatin B)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[H][C@]12[#6][C@]([#6])([#8]C1([#6])[#6])[#6@@H](-[#6]-[#6]-[#6@@H](-[#6])[C@@]1([H])[#6]-[#6]=[#6]3-[#6]-4=[#6](-[#6]-[#6][C@]13[#6])[C@@]1([#6])[#6]-[#6@H](-[#8]S([#8-])(=O)=O)-[#6@@H](-[#8]S([#8-])(=O)=O)-[#6@H](-[#6])-[#6]1=[#6]-[#6]-4)-[#8][C@@]([H])([#6]2)[#6@@H](-[#6])[C@@]1([H])[#6]-[#6][C@@]2([H])[#6]-3=[#6](-[#6]-[#6][C@]12[#6])[C@]12[#6]-[#6][C@]([#8])([#8]-[#6]1)[#6@@H](-[#6])[C@]2([H])[#6@H](-[#6]-3)-[#8]S([#8-])(=O)=O |r,c:20,46,63,t:18| Show InChI InChI=1S/C56H84O16S3.3Na/c1-30(37-14-16-40-35-12-13-39-32(3)49(71-75(64,65)66)46(70-74(61,62)63)28-53(39,9)42(35)19-21-51(37,40)7)11-18-47-54(10)27-34(50(5,6)72-54)25-44(68-47)31(2)38-15-17-41-36-26-45(69-73(58,59)60)48-33(4)56(57)24-23-55(48,29-67-56)43(36)20-22-52(38,41)8;;;/h13,16,30-34,37-38,41,44-49,57H,11-12,14-15,17-29H2,1-10H3,(H,58,59,60)(H,61,62,63)(H,64,65,66);;;/q;3*+1/p-3/t30-,31+,32-,33+,34+,37-,38-,41+,44+,45+,46+,47-,48-,49+,51-,52-,53+,54+,55+,56+;;;/m1.../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of bovine cathepsin B after 1 hr by fluorescence assay |
Bioorg Med Chem 19: 6594-8 (2011)
Article DOI: 10.1016/j.bmc.2011.06.052
BindingDB Entry DOI: 10.7270/Q2PN98GC |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50393108
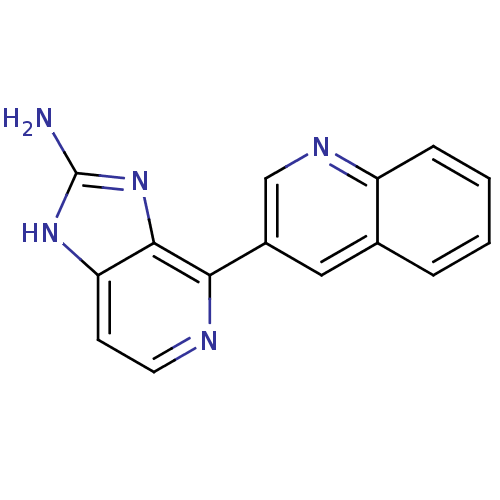
(CHEMBL2153266)Show InChI InChI=1S/C15H11N5/c16-15-19-12-5-6-17-13(14(12)20-15)10-7-9-3-1-2-4-11(9)18-8-10/h1-8H,(H3,16,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50215927
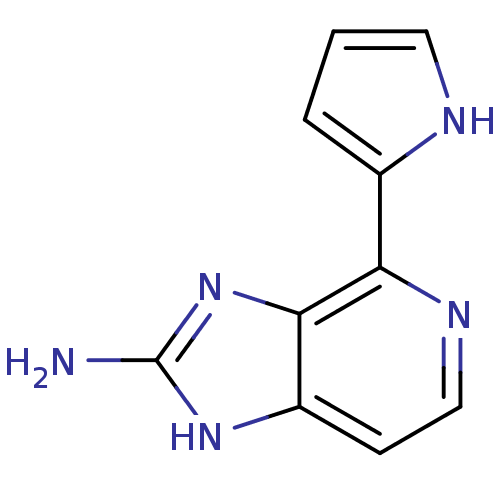
(4-(1H-pyrrol-2-yl)-1H-imidazo[4,5-c]pyridin-2-amin...)Show InChI InChI=1S/C10H9N5/c11-10-14-7-3-5-13-8(9(7)15-10)6-2-1-4-12-6/h1-5,12H,(H3,11,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50478557
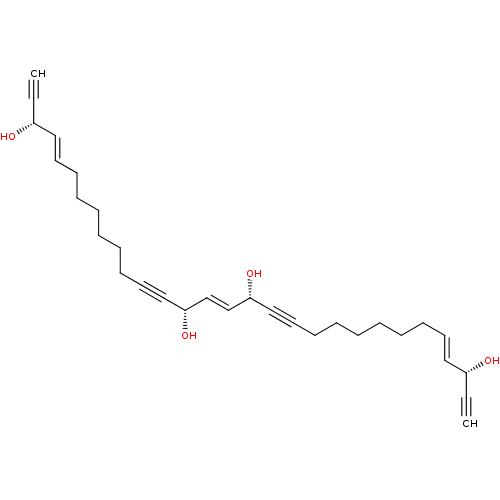
(Petrosynol)Show SMILES O[C@@H](\C=C\CCCCCCC#C[C@@H](O)\C=C\[C@H](O)C#CCCCCCC\C=C\[C@H](O)C#C)C#C |r| Show InChI InChI=1S/C30H40O4/c1-3-27(31)21-17-13-9-5-7-11-15-19-23-29(33)25-26-30(34)24-20-16-12-8-6-10-14-18-22-28(32)4-2/h1-2,17-18,21-22,25-34H,5-16H2/b21-17+,22-18+,26-25+/t27-,28-,29-,30-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of alpha-glucosidase |
J Nat Prod 65: 922-4 (2002)
Article DOI: 10.1021/np0106642
BindingDB Entry DOI: 10.7270/Q20004T1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PIM1 using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Sus scrofa) | BDBM50393109

(CHEMBL2153264)Show InChI InChI=1S/C11H9N5/c12-11-15-8-4-6-14-9(10(8)16-11)7-3-1-2-5-13-7/h1-6H,(H3,12,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of porcine brain CK1 using RRKHAAIGpSAYSITA as substrate |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50393113
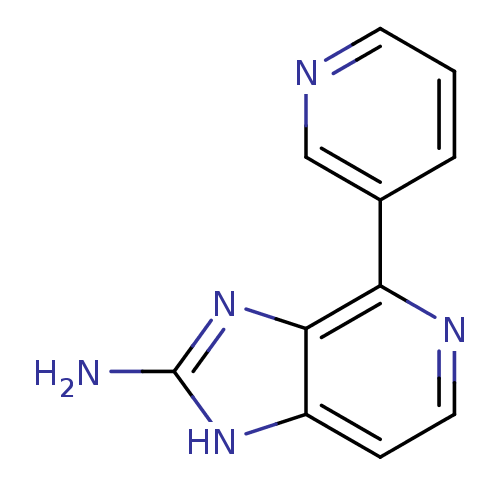
(CHEMBL2153265)Show InChI InChI=1S/C11H9N5/c12-11-15-8-3-5-14-9(10(8)16-11)7-2-1-4-13-6-7/h1-6H,(H3,12,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50393112
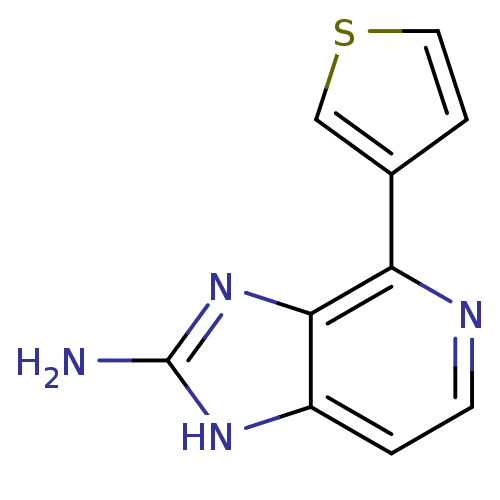
(CHEMBL2153263)Show InChI InChI=1S/C10H8N4S/c11-10-13-7-1-3-12-8(9(7)14-10)6-2-4-15-5-6/h1-5H,(H3,11,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50393112

(CHEMBL2153263)Show InChI InChI=1S/C10H8N4S/c11-10-13-7-1-3-12-8(9(7)14-10)6-2-4-15-5-6/h1-5H,(H3,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393115
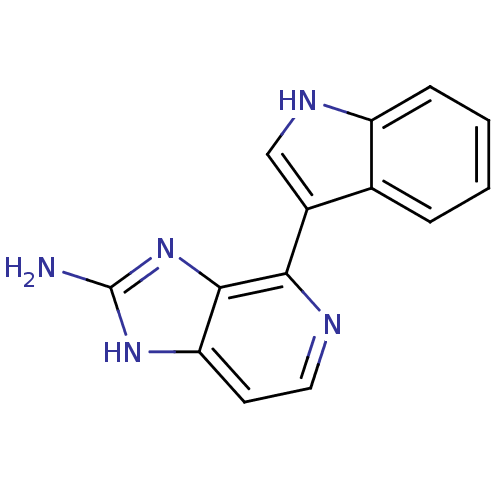
(CHEMBL575945)Show InChI InChI=1S/C14H11N5/c15-14-18-11-5-6-16-12(13(11)19-14)9-7-17-10-4-2-1-3-8(9)10/h1-7,17H,(H3,15,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50393111

(CHEMBL2153261)Show InChI InChI=1S/C10H8N4S/c11-10-13-6-3-4-12-9(8(6)14-10)7-2-1-5-15-7/h1-5H,(H3,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393110
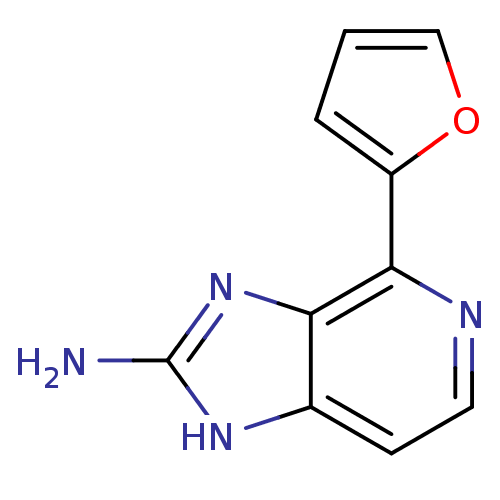
(CHEMBL2153260)Show InChI InChI=1S/C10H8N4O/c11-10-13-6-3-4-12-9(8(6)14-10)7-2-1-5-15-7/h1-5H,(H3,11,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50003299

(CHEMBL469919)Show SMILES [Na+].[H][C@]12CCCC(C)(C)[C@]1([H])CC[C@]1(C)[C@@H](CCc3ccoc3)\C(CC[C@]21[H])=C\OS([O-])(=O)=O |r| Show InChI InChI=1S/C24H36O5S.Na/c1-23(2)12-4-5-19-21(23)10-13-24(3)20(8-6-17-11-14-28-15-17)18(7-9-22(19)24)16-29-30(25,26)27;/h11,14-16,19-22H,4-10,12-13H2,1-3H3,(H,25,26,27);/q;+1/p-1/b18-16+;/t19-,20-,21+,22+,24+;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aoyama Gakuin University
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Nat Prod 61: 248-50 (1998)
Article DOI: 10.1021/np970376z
BindingDB Entry DOI: 10.7270/Q2P55Q04 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50478551
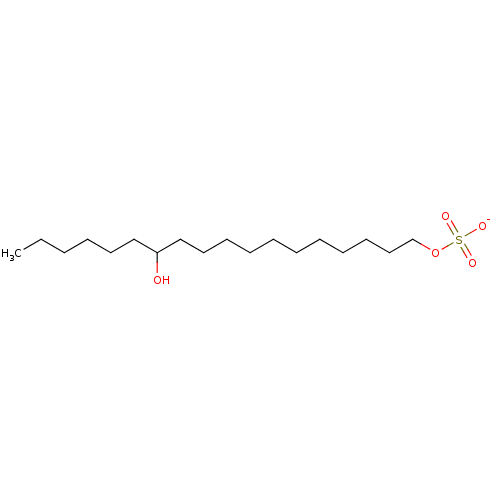
(CHEMBL462863 | Sodium 1-(12-Hydroxy)Octadecanyl Su...)Show SMILES [Na;v0+].[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](-[#8])-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#8]S([#8-])(=O)=O Show InChI InChI=1S/C18H38O5S.Na/c1-2-3-4-12-15-18(19)16-13-10-8-6-5-7-9-11-14-17-23-24(20,21)22;/h18-19H,2-17H2,1H3,(H,20,21,22);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MMP2 |
J Nat Prod 65: 1936-8 (2002)
Article DOI: 10.1021/np020250o
BindingDB Entry DOI: 10.7270/Q27S7RMJ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50478536

(Halistanol Sulfate)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[C@]3([H])[#6]-[#6@H](-[#8]S([#8-])(=O)=O)[C@@]4([H])[#6]-[#6@H](-[#8]S([#8-])(=O)=O)-[#6@H](-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])-[#8]S([#8-])(=O)=O)[#6@H](-[#6])-[#6]-[#6]-[#6](-[#6])C([#6])([#6])[#6] |r| Show InChI InChI=1S/C29H52O12S3.3Na/c1-17(8-9-18(2)27(3,4)5)20-10-11-21-19-14-24(39-42(30,31)32)23-15-25(40-43(33,34)35)26(41-44(36,37)38)16-29(23,7)22(19)12-13-28(20,21)6;;;/h17-26H,8-16H2,1-7H3,(H,30,31,32)(H,33,34,35)(H,36,37,38);;;/q;3*+1/p-3/t17-,18?,19+,20-,21+,22+,23-,24+,25+,26+,28-,29-;;;/m1.../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of truncated recombinant MT1-MMP expressed in Escherichia coli JM109 after 1 hr by fluorescence assay |
J Nat Prod 66: 569-71 (2003)
Article DOI: 10.1021/np020572s
BindingDB Entry DOI: 10.7270/Q2WH2SSF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50393112

(CHEMBL2153263)Show InChI InChI=1S/C10H8N4S/c11-10-13-7-1-3-12-8(9(7)14-10)6-2-4-15-5-6/h1-5H,(H3,11,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg- NH2 as substrate preincubated for 10 mins measured after 3 hrs by fluorescence ass... |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(RAT) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50478535
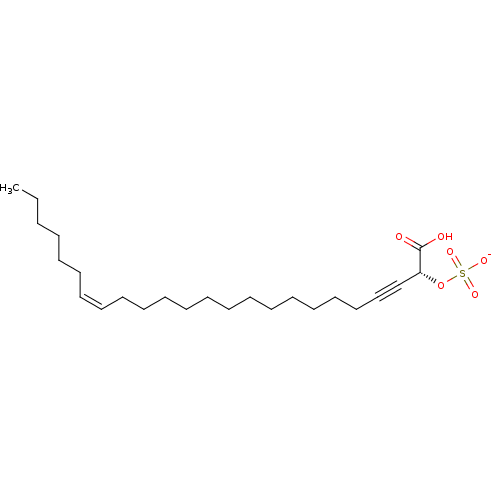
(CALLYSPONGINOL SULFATE A | CHEBI:65567)Show SMILES [Na;v0+].[#6]-[#6]-[#6]-[#6]-[#6]-[#6]\[#6]=[#6]/[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]C#C[#6@@H](-[#8]S([#8-])(=O)=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C24H42O6S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23(24(25)26)30-31(27,28)29;/h7-8,23H,2-6,9-20H2,1H3,(H,25,26)(H,27,28,29);/q;+1/p-1/b8-7-;/t23-;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of truncated recombinant MT1-MMP expressed in Escherichia coli JM109 after 1 hr by fluorescence assay |
J Nat Prod 66: 569-71 (2003)
Article DOI: 10.1021/np020572s
BindingDB Entry DOI: 10.7270/Q2WH2SSF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50393110

(CHEMBL2153260)Show InChI InChI=1S/C10H8N4O/c11-10-13-6-3-4-12-9(8(6)14-10)7-2-1-5-15-7/h1-5H,(H3,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using [gamma-32P] ATP after 30 mins by scintillation counting |
J Med Chem 54: 2492-503 (2011)
Article DOI: 10.1021/jm200039m
BindingDB Entry DOI: 10.7270/Q2QV3NMT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data